Abstract
Perilipin 1, unlike the other perilipins, is thought to be restricted to the fat droplet. We reassessed its cellular distribution using the fat droplet marker CGI-58 in OP9 and 3T3-L1 adipocyte lines and in brown adipose tissue (BAT). As expected, we found perilipin 1 in the fat droplet-enriched floating fraction from centrifuged adipocyte or BAT homogenates. However, about half of perilipin 1 was suspended in the cytosol/infranate or pelleted with cellular membranes. In these fractionations, most of the fat droplet-associated protein CGI-58 was in the floating fraction. In BAT and OP9 adipocytes about a third of perilipin 1 pellets, compared with a much smaller fraction of CGI-58. Co-imaging perilipin 1 and smooth endoplasmic reticulum (ER) markers reveals both ER and fat droplet associated perilipin 1 in OP9 adipocytes. Consistent with these observations, perilipin 1 overexpressed in COS7 cells mostly fractionates with cellular membranes and imaging shows it on the ER. In 3T3-L1 adipocytes almost half of perilipin 1 floats, half is suspended as infranate and small amounts pellet. Finally, driving rapid fat droplet synthesis in OP9 adipocytes increases the intensity of perilipin 1 on fat droplets, while decreasing non-fat droplet immunolabeling. Confirming the morphological findings, fractionation shows perilipin 1 moving from the pelleted to the floated fractions. In conclusion, this study documents an expanded intracellular distribution for perilipin 1 and its movement from ER to fat droplet during lipid synthesis.
Keywords: lipid droplets, diacylglycerol, 3T3-L1, fractionation, OP9, brown fat, CGI-58
Introduction
When food is scarce, animals depend on stored fatty acids for energy. These stores are in the form of fats (triacylglycerols and other neutral lipids) surrounded by amphipathic lipids and proteins. The amphipathic perilipins are found in animals which are specialized to survive sporadic food availability and whose survival hinges on storing and protecting fat when food is available and mobilizing fat when needed. Perilipin 1 was the first protein shown to be on animal fat droplets.1 In adipocytes, when fat is not needed for energy, basally phosphorylated perilipin 1 coats the fat, where it protects the energy stores by blocking fat hydrolysis. When stored energy is needed, perilipin 1 is phosphorylated by protein kinase A (PKA) and the hyper-phosphorylated perilipin recruits and organizes the activation of the lipolytic machinery.2
The name perilipin describes the location of the protein at the perimeter of fat droplets. In addition to perilipin 1, other proteins with sequence similarity,3 encoded by four different genes (perilipins 2, 3, 4 and 5), also can be peri-lipid.4-7 Protein sequence and experimental data suggest that all five perilipins are translated on free ribosomes, and thus it is likely that all are at least transiently cytosolic.8 Perilipins have two motifs characteristic of amphipathic helices that reversibly bind lipid. The first is an 11-mer repeat found in many exchangeable lipid binding proteins including perilipins, apolipoproteins and cytidylyltransferase.9 The second was discovered when the carboxyl 2/3 of perilipin 3 was crystallized and analysis of the crystal revealed a 4-helix bundle.10 This same structure in lipoproteins can reversibly bind lipid by splaying out on its surface or can be stably surrounded by aqueous solution by folding its hydrophobic surfaces inward. In contrast, oleosins use the plant ER translocation machinery to embed and irreversibly anchor long hydrophobic domains into cellular lipids. This results in oleosins being stably embedded in fat (oil) droplets and protecting the structural integrity of oil droplets in seeds during desiccation or freezing.11 In contrast perilipins are reported to be targeted post-translationally to membrane leaflets by both the composition of leaflet lipids12 and the underlying lipids.13 As a result, and in contrast to oleosins, intracellular membrane trafficking and changing nutrient-flux control the heterogeneous and dynamic intracellular distribution of the perilipins.5-7,14 Perilipins have been reported to link to a host of cytosolic proteins and organelles.15-19 Thus, it is proposed that different perilipins link distinct lipid pools to specific cytosolic machinery according to cell type and metabolic state.20,21
To date, most studies report perilipin 1 as only coating fat droplets,1 and thus it is used as a fat droplet marker protein.22 The presumption that perilipin 1 is a fat droplet marker, together with the lack of other fat droplet marker proteins, previously caused us to dismiss non-fat droplet perilipin 1 as contamination. Since other perilipins were found in soluble fractions and on the ER5-7,12,14 and supported by the recent identification of other specific fat droplet marker proteins,19,23 we reexamined the distribution of perilipin 1 using the fat droplet protein marker CGI-58, hypothesizing that like other perilipins, it also extends beyond the fat/cytosol interface. In this work we show that perilipin 1 staining overlaps with smooth ER markers and that in adipocytes perilipin 1 fractionates about equally between floating, pelleting and soluble fractions, whereas CGI-58 is concentrated in the floating fractions. The data reveal that in addition to the previously identified pool of perilipin 1 coating fat droplets, there are ER and soluble pools of the protein that are influenced by nutritional changes.
Results
Evidence that perilipin 1 can coat the smooth ER
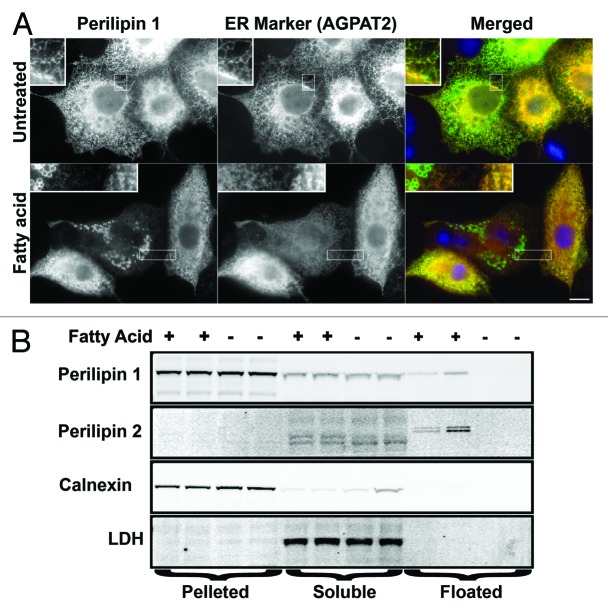
The ER is the main site of glycerol acylation to triacylglycerol (TAG) and cholesteryl ester (CE) formation, and for fat droplet emergence.24 Further, there are multiple lines of evidence for bidirectional traffic between the ER and fat droplets.12,25 Perilipins 2, 3, 4 and 5 partition to the ER with various treatments (Fig. S2).12 These data led us to hypothesize that perilipin 1 also coats the cytosolic leaflet of the ER under basal conditions. To test this we co-transfected COS7 cells with perilipin 1 and the ER marker acyl-glycerol-3-phosphate acyltransferase 2 (AGPAT2). The results show that perilipin 1 almost completely co-localized with the ER marker (Fig. 1A). This ectopically expressed perilipin 1 is still capable of binding fat droplets however, since when fat droplet formation is driven by fatty acid supplementation, the perilipin 1 antibody labels an ER-fat droplet continuum in COS7 cells (Fig. 1A and B). The same ER-fat droplet perilipin 1 staining is seen in COS7 transfected with only perilipin 1 (data not shown) indicating it is not related to AGPAT2 expression. Interestingly, given perilipin 1’s association with lipid, its staining is more similar to the lipid synthetic enzyme AGPAT2 than to the ER marker PDI which processes proteins entering the secretory pathway (Fig. S1). Fractionation of COS7 cells (Fig. 1B) confirms that there is more perilipin 1 in the pelleted material than can be accounted for by fat droplet contamination evaluated by the fat droplet marker perilipin 2. These data indicate that perilipin 1 (Fig. 1) and perilipin 2 (Fig. S2) can coat the ER. In previous work perilipins 3, 4, and 5 were shown on the ER.12 Consistent with these findings, endogenous perilipin 1 coats the ER of cultured adipocytes lacking fat droplets due to the ablation of fat synthesis genes.26 Thus, all five perilipins can coat the smooth ER.
Figure 1. Most of the ectopically expressed perilipin 1 in COS7 is found on the ER. COS7 were cotransfected with cDNAs that express perilipin 1 and AGPAT2-HA. Then cells were cultured in standard media or media supplemented with 900 μM of the fatty acid oleate for 18 h. (A) Shows cells stained with the guinea pig antiserum against perilipin 1 (1:5,000) and the mouse monoclonal antibody against HA (2 μg/ml) revealing the ER marker AGPAT2. Note DAPI stained blue nucleus of untransfected cell in the lower right corner of untreated merged panel where no red or green signals are observed. Bar = 10 μm. (B) Shows four fractionations. Fractionations 1 and 2 show cells supplemented with fatty acid and fractionations 3 and 4 show untreated cells. Equal percents of each fraction (pelleted, soluble, or floated) were immunoblotted and probed with guinea pig antiserum against perilipin 1 at 1:10,000, guinea pig anti perilipin 2 at 1:1,000, rabbit anti calnexin at 500 ng/ml and anti LDH (lactate dehydrogenase) was diluted 1:5,000.
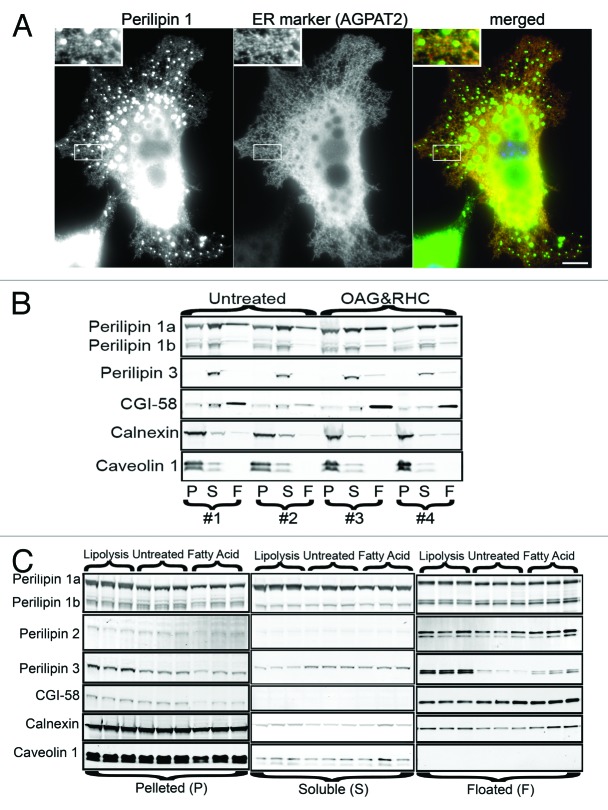
Similar to the data in COS cells expressing ectopic perilipin 1, a portion of endogenous perilipin 1 in OP9 adipocytes coats the ER (Fig. 2A–C). Also, as previously seen, treatments with the fatty acid oleate or increasing intracellular DAG (OAG + RHC) redistributed perilipin 3 (Fig. 2B).5-7,12 The work presented here is the first report that a lipolytic agonist (forskolin) partitions perilipin 3 to the floating fraction (Fig. 3C). Immuno-imaging lipolytic adipocytes also shows perilipin 3 on peripheral droplets, similar to what is seen during fatty acid treatment (data not shown). Consistent with these observations lipolytically induced microdroplets are reported to be nascent fat droplets synthesized from reesterified fatty acids.27,28
Figure 2. A portion of endogenous perilipin 1 is found on the ER in OP9 adipocytes. (A) OP9 adipocytes were transfected to express the ER marker AGPAT2-HA, and then immunolabeled for endogenous perilipin 1 and HA. This adipocyte was chosen because the ER is unobscured by large fat droplets and the flat processes are obvious (Bar = 10 μm). (B) Shows untreated OP9 adipocytes in fractionations 1 and 2 and adipocytes treated with 500 μM OAG and 20 μM RHC80267 for 30 min to increase intracellular DAG levels in fractionations 3 and 4. (C) Shows OP9 adipocyte fractionations. Adipocytes were untreated, lipolytically stimulated, or given oleate to drive fat droplet genesis and storage. Treatments were done in triplicate and nine separate dishes of adipocytes are represented. For (B and C), the pelleted, floated and soluble fractions were immunoblotted and probed with rabbit antibodies against perilipin 3 at 400 ng/ml, caveolin-1 at 200 ng/ml or rabbit CGI-58 antisera diluted 1:2,000. Other antibody dilutions were as described in Figure 1.
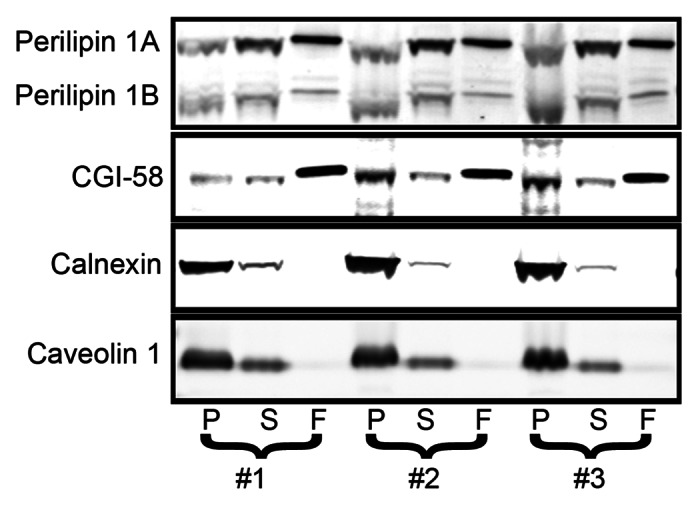
Figure 3. 3T3-L1 adipocytes have a large pool of soluble perilipin 1. 3T3-L1 adipocytes were fractionated and the fractions analyzed by immunoblotting as described in Figure 2.
These perilipin 3 perturbing conditions did not alter perilipin 1 distribution, suggesting that under most metabolic conditions perilipin 1 coats the ER. Finally, perilipin 1 coating the ER is likely not unique to OP9 adipocytes, because in brown adipose tissue (BAT) it is similarly distributed across fractions (Fig. 4). The observation that a greater fraction of perilipin 1 pellets as compared with the fat droplet marker CGI-58 indicates that perilipin 1 in the pelleted fraction is not attributable to fat droplet contamination (Figs. 2, 3 and 4). These findings provide the first evidence that endogenous members of the perilipin family coat the ER under standard culture conditions.
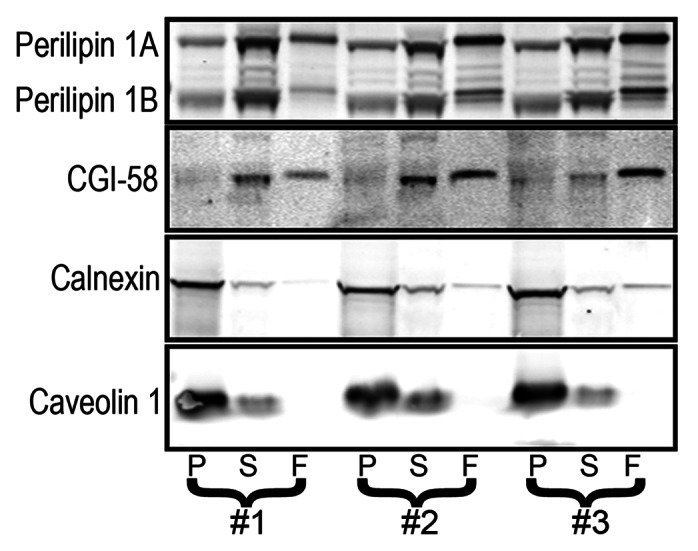
Figure 4. BAT has soluble and pelleting pools of perilipin 1. BAT was fractionated and fractions were analyzed by immunoblotting as described in the previous figures.
In these fractionations, we found more perilipin 1 in soluble fractions than is explainable by fat droplet contamination (Figs. 1–5). It is possible that monomeric perilipin 1 assumes a stable and soluble conformation. However, three considerations favor perilipin 1 being part of a lipid containing particle. First, it is difficult to imagine a stable conformation for perilipin 1 in aqueous solution. It anchors to fat droplets through multiple long hydrophobic sequences29,30 Second, perilipin 1 instability in the absence of TAG8 is consistent with the perilipin 1 in the soluble fraction being associated with a small high density lipid particle. Third, there is precedence for such perilipin coated particles, since perilipins 3 and 5 are reported to coat a high density TAG-rich particle in the cytosol. These particles likely remain in our soluble fraction.31 It is also possible that the perilipin 1 in the soluble fractions reflects heterogeneity of the ER markers (Fig. S1), and that perilipin 1 in these fractions is coating vesiculated TAG-laden and calnexin-poor ER. Consistent with this possibility, there is a small amount of ER contamination (calnexin) of the soluble fractions (Figs. 1–5).
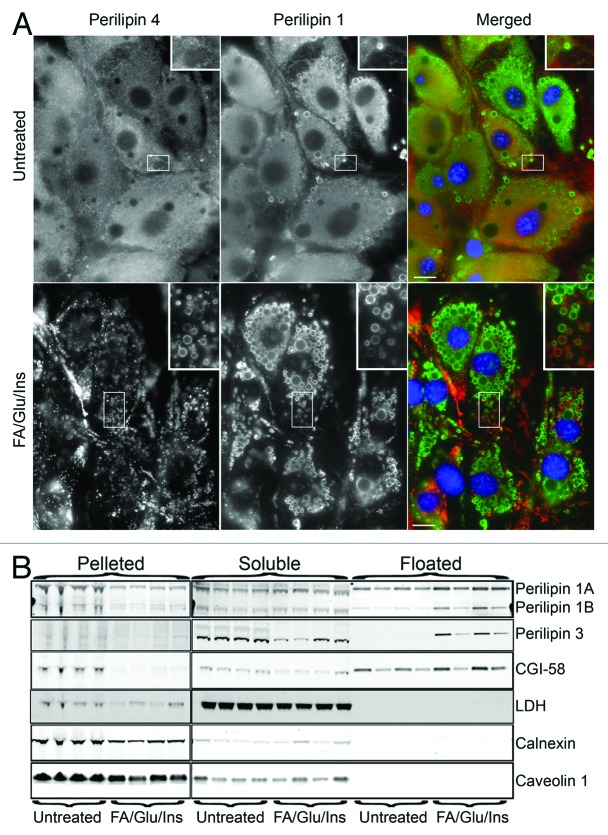
Figure 5. During rapid fat droplet accumulation perilipin 1 moves onto fat droplets. (A) OP9 adipocytes were untreated or treated with 1.8 mM oleate, 25 mM glucose and 100 nM insulin (FA/Glu/Ins) as indicated. Bar = 10 μm. Adipocytes were labeled with perilipin 1 antiserum (1:10,000) and perilipin 4 antibody at 1 μg/ml. (B) Four dishes of adipocytes were untreated and four dishes were FA/Glu/Ins treated. Each dish was fractioned, immunoblotted and probed with the antibodies indicated. See previous figures for dilutions. The goat anti-CGI-58 was used at 1 μg/ml.
Perilipin 1 moves between the ER and fat droplets
As previously seen in 3T3-L1 adipocytes, fatty acid with glucose and insulin (FA/Glu/Ins) is more effective at driving accumulation of perilipin 4 coated fat droplets than fatty acid alone.14 This massive accumulation of perilipin 4 coated fat droplets also is seen in OP9 adipocytes (Fig. 5A). Treatment of OP9 cells with FA in combination with glucose and insulin (FA/Glu/ins) changed immunolabeling of both perilipin 1 and 4 from hazy reticular to much more fat droplet focused (Fig. 5A). Perilipin 1 droplets were more central as compared with the peripheral perilipin 4 droplets (Fig. 5A). A change in perilipin 1 distribution is also seen by fractionation (Fig. 5B); the perilipin 1 signals, especially evident with perilipin 1B, are stronger in the floated fractions of the FA/Glu/insulin treated cells than in the parallel fractions from untreated cells. There is also a corresponding loss from the pelleted fraction after treatment (Fig. 5B). These data indicate that like other perilipins, perilipin 1 moves between the outer membrane leaflet of the ER and fat droplets (Figs. 1–5), albeit under somewhat different control than perilipin 3 (Figs. 3 and 5).
Discussion
The perilipins are a family of proteins synthesized on free ribosomes and largely composed of sequences associated with amphipathic helices that reversibly bind lipid monolayers.8-10,32 Under various conditions, proteins of the perilipin family have been reported coating fat droplets, the ER, and in the cytosol while perilipin 1 was assumed to be exclusively fat droplet associated. Here we show that ectopic or endogenous perilipin 1, in addition to being highly concentrated on fat droplets, can be found in cytosolic and membrane fractions in amounts not attributable to fat droplet contamination. We further show that a portion of perilipin 1 staining overlaps with the ER marker AGPAT2. Treatment with fatty acids, glucose and insulin, which promotes fat droplet formation, recruits perilipin 1 to fat droplets reducing reticular staining and the percent of perilipin 1 recovered in the cellular pellet fraction.
Almost all reports localize perilipin 1 on fat droplets, as does this study. Why then do we find perilipin 1 on the ER and cytosol while previous studies did not? A number of issues may help explain this difference. Key tools were lacking in previous studies, notably the availability of a perilipin independent fat droplet marker. In the present study CGI-58 served as such a marker, allowing us to make a clearer distinction between fat droplet and non-fat droplet structures. Further, having previously treated perilipin 1 itself as a fat droplet marker, we attributed any perilipin 1 recovered in non-fat droplet fractions to faulty fractionation or detection. Given this bias, and the lack of a true fat droplet marker and the frailties of chemiluminescence quantification of immunoblotted proteins, it is not surprising that non-fat droplet perilipin 1 was previously unreported in fractionation studies. It is also worth noting that this omission may also reflect the common use of 3T3-L1 cells for the earlier studies. We are able to microscopically image perilipin 1 on OP9 adipocyte ER. However, this is more difficult in 3T3-L1 adipocytes likely due to two factors. First, OP9 adipocytes have large flat processes with easily discernible ER, while 3T3-L1 adipocytes lack them and the ER consists of a fine reticulum throughout the cytosol of these thick cells. Second, the fractions show more perilipin 1 in the pelleted material from OP9 than from 3T3-L1 (Figs. 2 and 3), suggesting there is more ER perilipin 1 in OP9 adipocytes. Previous morphologic localization of perilipin 1 used 3T3-L1 adipocytes and the rabbit anti-perilipin 1 antibody, which poorly binds ER perilipin 1 (Fig. S3).
Perilipin 1 is reported to be degraded in proteasomes.33 This degradation requires entry into proteasomes, and because fat droplets are larger than proteasomes, it is difficult to reconcile this with perilipin 1 being constitutively bound to large fat droplets and being degraded at that location. However, proteasomal degradation would be consistent with soluble perilipin 1 or with perilipin 1 on smaller lipid particles.
Why is adipocyte perilipin 1 the only perilipin coating the ER under physiological conditions while pharmacological treatments were needed to recruit other perilipins to the ER? In many cell types without perilipin 1, fat droplets are functionally connected to the endoplasmic reticulum and presence of perilipin 1 may highlight this link.12,25 Many fat processing enzymes including fatty acid elongating and desaturating enzymes,34,35 TAG lipase36 and acylating enzymes37-39 reside in the ER. Further, the unilocular fat droplet structure of primary white adipocytes minimizes the fat droplet/cytosol interface. We speculate that in adipocytes, which are specialized to flux fat, perilipin 1 coating the ER increases the capacity to flux fat by expanding the fat/cytosol interface. Consistent with this notion, perilipin 1 ablation in mice lessens fatty acid release during hormone stimulated lipolysis.
Materials and Methods
Reagents
1-Oleoyl-2-acetyl-sn-glycerol (OAG) was from Cayman Chemical (62600). Diacylglycerol lipase inhibitor RHC 80267 was from Tocris (1842) and bovine serum albumin (BSA) from Equitech-Bio (BAH66). TransIT®-LT1 Transfection reagent was from Mirus (MIR 2300). Unless otherwise stated, reagents were from Sigma-Aldrich. Oleic acid was solubilized with sodium hydroxide and bound to BSA at a ratio of 5.5 oleate/BSA.
Cell culture
3T3-L1 cells were cultured and differentiated as described previously.14 OP9 mouse stromal cells, which have the potential to rapidly differentiate into adipocytes, were cultured as described previously.40 For adipocytes, a clone of these cells was used. This clone was differentiated by culturing in 3T3-L1 differentiation media 1 with 0.9 mM oleate for 3 d, then returned to OP9 media. Adipocytes were used 24 to 48 h after being returned to OP9 media. COS7 and HeLa cells were cultured in DMEM with 10% fetal bovine serum and antibiotic as described previously.5,6
Transfection of OP9, COS7 and HeLa cells
TransIT®-LT1 transfection reagent was used to transfect cells according to the manufacturer’s protocol. Per 10 cm2 plate area of OP9 cells, 2 μg of DNA and 6 μl TransIT-LT1 were used and for HeLa and COS7 cells 1 μg of DNA and 3 μl TransIT-LT1 were used. OP9 cells were ~30% confluent and COS7 and HeLa were ~60% confluent when transfected. OP9 adipocytes were electroporated using the Lonza Nucleofector® II with solution V and program T-030.
Expression constructs
The HA-tagged Acyl-glycerol-3-phosphate acyltransferase 2 (AGPAT2) and FLAG tagged-diacylglycerol O-acyltransferase 1 (DGAT1) expression was driven by constructs described previously.12,38 The mouse perilipin 1A coding sequence was inserted into the mammalian expression vector pCAGGS.41
Antibodies
Affinity isolated rabbit antibodies against the N-terminus of perilipin 3 and 4 were described previously.5,14 The perilipin 1 antiserum was raised in rabbit against the 105 amino acid N-terminal peptide of perilipin 14 (used in Fig. S3) and CGI-58 antiserum was raised in rabbits against recombinant CGI-5815 (used in Figs. 2–4) (gifts from Dr. Dawn Brasaemle). Antigen-affinity purified CGI-58 antibody raised in goat was purchased from Aviva Systems Biology Corp. (used in Fig. 5) (OAEB01343). Guinea pig antisera against perilipin 1 and 2 were from Fitzgerald Industries (RDIPROGP29 and RDIPROGP40, respectively). Mouse monoclonal antibody against Protein Disulfide Isomerase and protein A isolated antibodies from anti-calnexin rabbit antiserum were from Enzo Life Sciences (SPA-891 and SPA-860 respectively). Antibodies antigen-affinity isolated out of rabbit antiserum against the Influenza Hemagglutinin (HA) epitope and anti-HA mouse ascites were from Sigma (H9658 and H6908). Goat antigen-affinity isolated anti-FLAG antibody was from Abcam (ab1257). Secondary antibodies for immunofluorescence microscopy were Alexa conjugated anti-IgG antibodies from Invitrogen. Lactate dehydrogenase (LDH) antibody was from Abcam Inc. (ab52488).
FA/Glu/insulin treatment
Adipocyte media was replaced with 1.8 mM oleate, 25 mM glucose and 100 nM insulin in phosphate buffered saline and incubated at 37°C while rotating at 120 rpm for 100 min.
Fractionation of brown adipose tissue (BAT), COS7 cells, OP9 preadipocytes, OP9 adipocytes and 3T3-L1 adipocytes
Cells and BAT were fractionated into pelleting, soluble and floating fractions as described previously.42 The experimental protocol for mouse tissue harvesting was reviewed and approved by the Washington University Animal Studies Committee. COS7 cells and OP9 preadipocytes were disrupted by 5 passes through a 27 gauge needle. Adipocytes were disrupted by 5 passes through a 25 gauge needle. Floating, soluble and pelleted fractions were brought to the same volume by weighing and addition of buffer, and thus equal percentages of each fraction were analyzed. To validate our cell disruption technique we compared with disruption using five passes through a Teflon pestle glass homogenizer as previously described Fig. S4.23
Immunofluorescence microscopy
Cells were fixed and stained as described previously.12 Slides were imaged on a Nikon Eclipse TE2000-U microscope. Images were captured using a Photometrics Coolsnap cf camera driven by MetaMorph version 6.2r6 software (Molecular Devices). For adipocytes, day 4 adipocytes were trypsinized and replated at 50% original density into 6-well dishes with coverslips.
Thin layer chromatography (TLC)
Two hundred microliters of each cell fraction was extracted as described previously.12 The extracted material was resuspended in hexane:ether (1:1) and spotted on Whatman Partisil LK6D Silica Gel TLC plates. Lipids were resolved in hexane:ether:acetic acid (70:30:1.2) and lipids were stained with molecular iodine.
Immunoblotting
SDS-PAGE, transfer to membranes and probing of membranes were done as described previously.12 Membranes were imaged using the LI-COR Odyssey system and infrared fluorescing antibodies (LI-COR Biotechnology).
Supplementary Material
Acknowledgments
The authors acknowledge the help of Terri Pietka. The authors thank Anatoly Tzekov for supplying 3T3-L1 adipocytes and Dawn Brasaemle for the CGI-58 antibody. This work is supported in whole or in part by the National Institutes of Health Grants R01 DK088206–01A1 (N.E.W) and DK33301 (N.A.A.). Postgraduate support (to J.R.S.) was from the Training Program in Cardiovascular Biology NHLBI T32HL007275-33 (Jeanne Nerbonne). We also thank the Washington University Nutrition Obesity Research Center supported by grant number P30DK056341 NORC.
Glossary
Abbreviations:
- TAG
triacylglycerol
- DGAT
diacylglycerol acyltransferase
- OAG
1-Oleoyl-2-acetyl-sn-glycerol
- GFP
green fluorescent protein
- ER
endoplasmic reticulum
- TLC
thin layer chromatography
- PDI
protein disulfide isomerase
- BAT
brown adipose tissue
- LDH
lactate dehydrogenase
- AGPAT2
Acyl-glycerol-3-phosphate acyltransferase 2
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/22864
References
- 1.Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–26. [PubMed] [Google Scholar]
- 2.Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem. 2009;326:15–21. doi: 10.1007/s11010-008-9998-8. [DOI] [PubMed] [Google Scholar]
- 3.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–71. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–63. [PubMed] [Google Scholar]
- 5.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–55. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 6.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–28. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 7.Wolins NE, Rubin B, Brasaemle DL. TIP47 associates with lipid droplets. J Biol Chem. 2001;276:5101–8. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- 8.Brasaemle DL, Barber T, Kimmel AR, Londos C. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem. 1997;272:9378–87. doi: 10.1074/jbc.272.14.9378. [DOI] [PubMed] [Google Scholar]
- 9.Bussell R, Jr., Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–78. doi: 10.1016/S0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 10.Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein; the PAT family member TIP47. Structure. 2004;12:1199–207. doi: 10.1016/j.str.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Hills MJ, Watson MD, Murphy DJ. Targeting of oleosins to the oil bodies of oilseed rape (Brassica napus L.) Planta. 1993;189:24–9. doi: 10.1007/BF00201339. [DOI] [PubMed] [Google Scholar]
- 12.Skinner JR, Shew TM, Schwartz DM, Tzekov A, Lepus CM, Abumrad NA, et al. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J Biol Chem. 2009;284:30941–8. doi: 10.1074/jbc.M109.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh K, Lee YK, Londos C, Raaka BM, Dalen KT, Kimmel AR. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. J Cell Sci. 2012;125:4067–76. doi: 10.1242/jcs.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem. 2003;278:37713–21. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–71. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 16.Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, et al. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Sreenevasan U, Hu H, Saladino A, Polster BM, Lund LM, et al. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011;52:2159–68. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welte MA, Cermelli S, Griner J, Viera A, Guo Y, Kim DH, et al. Regulation of lipid-droplet transport by the perilipin homolog LSD2. Curr Biol. 2005;15:1266–75. doi: 10.1016/j.cub.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, et al. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–89. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–59. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–91. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Yang RY, Yu L, Graham JL, Hsu DK, Lloyd KC, Havel PJ, et al. Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. Proc Natl Acad Sci U S A. 2011;108:18696–701. doi: 10.1073/pnas.1109065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–42. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 24.Brasaemle DL, Wolins NE. Packaging of fat: an evolving model of lipid droplet assembly and expansion. J Biol Chem. 2012;287:2273–9. doi: 10.1074/jbc.R111.309088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, Schneiter R. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci. 2011;124:2424–37. doi: 10.1242/jcs.076836. [DOI] [PubMed] [Google Scholar]
- 26.Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, et al. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 2011;52:657–67. doi: 10.1194/jlr.M013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ariotti N, Murphy S, Hamilton NA, Wu L, Green K, Schieber NL, et al. Postlipolytic insulin-dependent remodeling of micro lipid droplets in adipocytes. Mol Biol Cell. 2012;23:1826–37. doi: 10.1091/mbc.E11-10-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paar M, Jüngst C, Steiner NA, Magnes C, Sinner F, Kolb D, et al. Remodeling of lipid droplets during lipolysis and growth in adipocytes. J Biol Chem. 2012;287:11164–73. doi: 10.1074/jbc.M111.316794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia A, Sekowski A, Subramanian V, Brasaemle DL. The central domain is required to target and anchor perilipin A to lipid droplets. J Biol Chem. 2003;278:625–35. doi: 10.1074/jbc.M206602200. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian V, Garcia A, Sekowski A, Brasaemle DL. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–91. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Bartholomew SR, Bell EH, Summerfield T, Newman LC, Miller EL, Patterson B, et al. Distinct cellular pools of perilipin 5 point to roles in lipid trafficking. Biochim Biophys Acta. 2012;1821:268–78. doi: 10.1016/j.bbalip.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong BM, Russell TD, Schaack J, Orlicky DJ, Reigan P, Ladinsky M, et al. The adipophilin C terminus is a self-folding membrane-binding domain that is important for milk lipid secretion. J Biol Chem. 2011;286:23254–65. doi: 10.1074/jbc.M110.217091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu G, Sztalryd C, Londos C. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta. 2006;1761:83–90. doi: 10.1016/j.bbalip.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–49. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Ozols J. Degradation of hepatic stearyl CoA delta 9-desaturase. Mol Biol Cell. 1997;8:2281–90. doi: 10.1091/mbc.8.11.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Wei E, Quiroga AD, Sun X, Touret N, Lehner R. Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Mol Biol Cell. 2010;21:1991–2000. doi: 10.1091/mbc.E09-05-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1–9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gale SE, Frolov A, Han X, Bickel PE, Cao L, Bowcock A, et al. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J Biol Chem. 2006;281:11082–9. doi: 10.1074/jbc.M509612200. [DOI] [PubMed] [Google Scholar]
- 39.Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, et al. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 2006;18:2294–313. doi: 10.1105/tpc.106.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, et al. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–60. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- 42.Harris LA, Shew TM, Skinner JR, Wolins NE. A single centrifugation method for isolating fat droplets from cells and tissues. J Lipid Res. 2012;53:1021–5. doi: 10.1194/jlr.D023598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.