Abstract
Obesity is currently a global pandemic, and is associated with increased mortality and co-morbidities including many metabolic diseases. Obesity is characterized by an increase in adipose mass due to increased energy intake, decreased energy expenditure, or both. While white adipose tissue is specialized for energy storage, brown adipose tissue has a high concentration of mitochondria and uniquely expresses uncoupling protein 1, enabling it to be specialized for energy expenditure and thermogenesis. Although brown fat was once considered only necessary in babies, recent morphological and imaging studies have provided evidence that, contrary to prior belief, this tissue is present and active in adult humans. In recent years, the topic of brown adipose tissue has been reinvigorated with many new studies regarding brown adipose tissue differentiation, function and therapeutic promise. This review summarizes the recent advances, discusses the emerging questions and offers perspective on the potential therapeutic applications targeting this tissue.
Keywords: adipogenesis, BATokine, brown adipose tissue, browning, mitochondria, thermogenesis, UCP1
Obesity has now reached pandemic levels globally,1 and while the etiology and physiology are complex, the majority of weight gain in obese humans is characterized by an increase in adipose mass, and adipose tissue hypertrophy and lipid overload is believed to eventually precipitate other morbidities such as cardiovascular disease and type 2 diabetes.2 In contrast to white adipose tissue (WAT), which not only stores energy in the form of triglycerides but also is recognized as an important endocrine and immune organ, brown adipose tissue (BAT) is specialized for energy expenditure. While WAT structure is characterized by a single, large lipid droplet and few mitochondria, BAT contains several small lipid droplets (multilocular), many mitochondria, and uniquely expresses uncoupling protein 1 (UCP1).3-8 UCP1 is localized to the inner mitochondrial membrane and acts to uncouple oxidative phosphorylation from ATP production, thereby releasing energy as heat (termed thermogenesis). BAT plays a pivotal role in adaptive thermogenesis, a physiological process during which energy is dissipated in response to environmental changes, such as cold temperature and diet.9,10 BAT is also able to utilize both glucose and fatty acids in mitochondrial metabolism, however the thermogenic capacity of BAT is enormous. In humans, it has been estimated that as little as 50 g of BAT (less than 0.1% of body weight) could utilize up to 20% of basal caloric needs if maximally stimulated.11 This energy expending role makes BAT an important potential tool for combating the complications of human obesity.
BAT is important for temperature regulation in small mammals. In humans, it is present in abundant quantity in newborns,12 but it was traditionally believed that BAT was nonexistent or nonfunctional in adult humans. However, this dogma was recently reversed by evidence from nuclear medicine,13-20 which showed active BAT in adult humans. Since then, there has been a flurry of new data surrounding BAT function and therapeutic potential.21-23 The goal of this review is to summarize and offer perspective on these recent advancements in knowledge about BAT, from studies conducted in humans to rodent or in vitro models, with a special focus on recently published papers.
The Importance of BAT with Cold-Exposure and for Seasonal Hibernating Mammals
The physiological importance of BAT, previously referred to as the ‘hibernating gland,’ is most strikingly observed in seasonal mammals, which require BAT’s thermogenic properties to maintain body temperature during periods of hibernation or torpor, and to mediate periods of arousal and re-warming from these decreased metabolic states. Hibernation is a period of heterothermia, where body temperature may drop from 35–37°C to 0°C, accompanied by a period of metabolic reduction.24 The onset of hibernation is often triggered by shortening daylight cues reaching the brain, in conjunction with the brain’s own circadian rhythms. Torpor, on the other hand, is a short-term state of reduced physical activity and metabolism, and may be induced by reductions in environmental temperature or caloric restriction (or both).
A recent study measured liver and BAT gene expression in arctic ground squirrels during torpor, a hibernatory period of reduced ambient temperature which requires an 8-fold increase in energetic demand in order to maintain body temperature.25 This study showed that in comparison to squirrels during warm summer months (i.e., not during torpor), hibernators had increased gene expression in pathways regulating fatty acid catabolism, ketogenesis and gluconeogenesis. By contrast, genes for fatty acid synthesis, amino acid metabolism, the urea cycle, glycolysis and lipid metabolism were decreased. Whether or not similar metabolic pathways are regulated in non-hibernation conditions of increased BAT thermogenesis remains to be determined.
BAT UCP1 also plays an important role in arousal from hibernation or torpor. Despite the importance of UCP1 for thermogenesis and energy expenditure, it has previously been shown that UCP1 deficient mice (UCP1−/−) are cold sensitive, but do not become obese on a high-fat diet at room temperature, although they do have an impaired thermogenic response after cold or β3-adrenergic stimulation.26 However, when room temperature is increased (to 27°C or 30°C, the latter of which is thermoneutral for mice), the resistance to diet-induced obesity is abolished.27,28 In a recent study utilizing this UCP1−/− model, bouts of torpor under conditions of 48 h food deprivation and cold-exposure lasted significantly longer if induced during the dark-phase. Mice without UCP1 also had fewer daily bouts of torpor, took longer to arouse from torpor and required 60% more energy to do so.29 During hibernation, BAT increases in mass and displays higher UCP1 expression. Stimulation of BAT β3-adrenergic receptors with CL316,243 resulted in faster arousal from hibernation, while a β3-adrenergic receptor antagonist produced the opposite effect.30 These studies indicate that UCP1 and β3-adrenergic signaling are required for changes in energy metabolism with diet and cold, a mechanism that is potentially similar in seasonal and non-seasonal mammals.
Melatonin may offer one insight into this similarity. Melatonin influences recruitment of brown adipocytes as well as their metabolic activity. In response to light cues received by the retina and pineal gland, melatonin production is upregulated, and short photoperiods (less light) have similar effects on BAT as cold environmental temperature.31 It is now appreciated that melatonin may not only transmit information about photoperiods, but also about temperature and food availability, suggesting that rodent hibernatory models of seasonal changes in BAT thermogenesis may be indicative of BAT physiology in situations of cold or diet-induced thermogenesis as well (reviewed in ref. 31). Additionally, it is clear from rodent models of seasonally activated BAT that inputs via the central nervous system (CNS) are of utmost importance. Similarly, cold-exposure and other situations that stimulate BAT in non-seasonal models also involve pathways which originate in the CNS.
Developmental Origin of Brown Fat
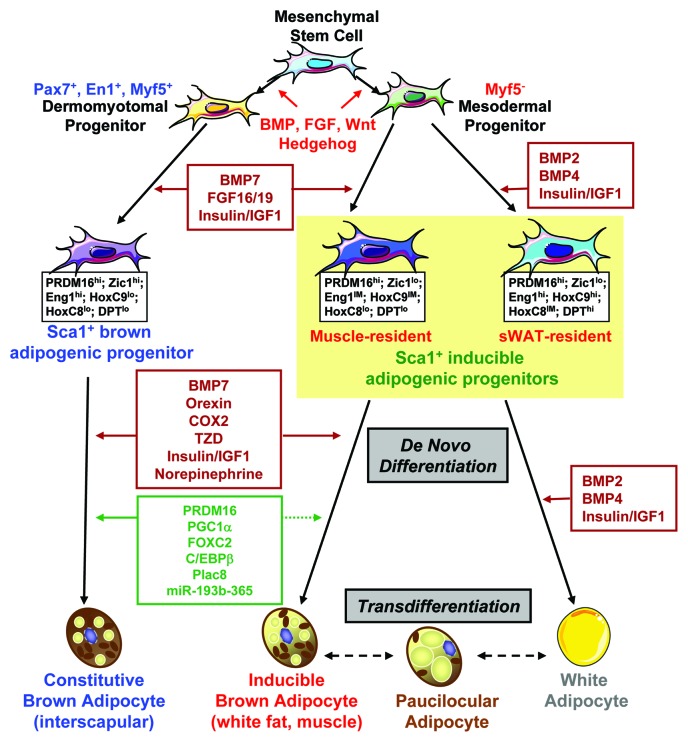
In rodents, brown fat is located in an interscapular fat pad, as well as smaller pads in other anatomical regions (such as perirenal and perivascular), and brown adipocytes are also dispersed throughout skeletal muscle and white fat. Recent fate-mapping studies combined with cell sorting analysis revealed that in rodents there are distinct progenitors that give rise to fat cells located in different anatomical locations of the body (Fig. 1). The myf5-expressing progenitors give rise to skeletal muscle and preformed brown adipocytes, which are found in the interscapular and perirenal regions,32 and brown fat precursor cells express a myogenic gene signature,33 suggesting that brown fat and skeletal muscle share a common developmental ancestry. Indeed, during embryonic development, cells expressing transcription factors that are known to mark dermomyotome are found to give rise to the interscapular BAT. Homeobox transcription factor engrailed 1 (EN1)-expressing cells give rise to dermis, muscle and brown fat.34 Selectively marking the somatic Pax7-expressing cells at embryonic day 9.5 (E9.5) demonstrates that Pax7-expressing cells can give rise to dorsal dermis, some muscle and brown fat. After E12.5, marked Pax7-expressing cells become lineage-restricted to skeletal muscle,35 suggesting that early Pax7-expressing cells are multi-potent, but become committed to the myogenic lineage as embryonic development proceeds. However, not all of brown fat cells are derived from precursors expressing myogenic markers. In adult life, brown fat cells located in the non-classic sites, such as WAT and skeletal muscle, are derived from the myf5 negative progenitors.36 These non-classic brown adipocytes have been given different names, such as ‘brite cells’37 and ‘beige cells,’38 designations that reflect their recruitable and inducible nature. We have recently identified and prospectively isolated a subpopulation of adipogenic progenitors (Sca-1+/CD45-/Mac1-; referred to as Sca-1+ progenitor cells, ScaPCs) residing in murine brown fat, white fat and skeletal muscle. Using the myf5 lineage tracing reporter mice, we and others have demonstrated that ScaPCs derived from skeletal muscle and subcutaneous WAT develop from cells that have never expressed myf5.39 Nevertheless, it is likely an oversimplification to divide into only myf5 positive and myf5 negative lineages, because progenitors derived from different tissues possess unique molecular expression signatures and adipogenic capacities, further supporting the notion that brown fat depots located in different anatomical locations arise from distinct developmental origins (Fig. 1). Another non-classic brown fat depot is the perivascular fat. Perivascular adipose tissue around the thoracic region has been found to express gene profiles highly similar to the interscapular BAT,40 but the developmental origin of this BAT depot remains to be determined. Recently, growing evidence has indicated that increased ‘browning’ in WAT can counteract diet-induced obesity, suggesting that inducible brown adipocytes may be potential targets for developing anti-obesity therapies. We review the recent evidence on browning in WAT in detail in the next section
Figure 1. Regulation of brown adipocyte development. Brown adipocytes located in different anatomical locations of the body arise from different developmental origins. While the Pax7+/En1+/Myf5+ dermomyotome progenitor gives rise to interscapular brown fat, a distinct myf5− tissue resident progenitor serves as the common precursor for white adipocytes and systemic brown adipocytes. With the stimulation of appropriate developmental cues, these progenitors become committed to the adipocyte lineage. The Sca-1+ progenitor cells isolated from interscapular brown fat serve as constitutively committed brown fat precursors, and Sca-1+ progenitor cells from skeletal muscle and subcutaneous white fat are highly inducible to become mature brown adipocytes. These precursors possess unique molecular signatures that allow designation of the distinction of cellular origin. Agents that can promote brown adipocyte differentiation include norepinephrine, insulin/IGF1, thiazolidinedione (TZD), cyclooxygenase 2 (COX2), orexin, BMP7 and others. At the molecular level, a number of transcriptional/post-transcriptional regulators have been shown to specify or enhance brown fat phenotype, such as PRDM16, PGC1α, FOXC2, C/EBPβ, Plac8 and miR-193b-365. The brown adipocytes in white fat may come from de novo differentiation and/or transdifferentiation. Dashed lines in this figure indicate links that are only partially established.
Cell fate determination in pluripotent stem/progenitor cells is controlled by the integration of cell intrinsic factors with extrinsic cues supplied by the surrounding microenvironment, known as the stem cell niche.41 Prototypical stem cell niches include the stem cells, stromal support cells, soluble factors, extracellular matrix, blood vessels and neuronal inputs. The identification and characterization of niches within tissues and how niches support specific stem cell function remain key topics in understanding tissue development and homeostasis. Several developmental signaling molecules implicated in the evolution of mesodermal tissue have been shown to impact early stages of fat development (Fig. 1). These include members of the transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) family, the fibroblast growth factor (FGF) family, the wingless (Wnt) family, the hedgehog family, and others. The exact effects of these factors depend on the concentration, stage of differentiation, cell-cell interactions, and nature of the extracellular matrix. While TGF-β inhibits adipocyte differentiation in vitro,42 TGF-β expression in fat is paradoxically increased with obesity in rodents and humans.43,44 We have demonstrated that in contrast to the roles of BMP2 and BMP4 in development of white fat, BMP7 serves as a potent inductive signal for brown adipogenesis.45 Some members of the FGF family, such as FGF 1, 10, 16, 19 and 21, have been implicated in adipose development. In particular, FGF 16, 19 and 21 are specifically involved in brown fat formation.46-48 Wnt signaling is known to suppress adipogenic and favor myogenic or osteogenic differentiation in MSCs.49 Finally, while the anti-adipogenic role of the hedgehog pathway has been established,50 a recent Drosophila genome-wide screen identified hedgehog as a determinant of brown vs. white cell fate.51
Molecular Control of Brown Fat Development
Despite the distinct functions of brown and white fat, these two cell types share a similar transcriptional cascade for adipocyte differentiation. This is a process involving adipogenic transcription factors PPARγ (peroxisome proliferator-activated receptor-γ) and CCAAT/enhancer-binding proteins (C/EBPs; for reviews see refs. 52–54). Importantly, a number of nuclear factors that specify or enhance brown fat phenotype have been indentified. Before the initiation of the adipogenic program, the preadipocytes need to be released from suppression and become committed to terminal differentiation. Among the known inhibitors of preadipocyte-adipocyte transition, proteins of the retinoblastoma (Rb) family and necdin, a growth repressor functionally resembling Rb, selectively inhibit brown preadipocyte differentiation.55-58 Consistent with these findings, Calo et al. recently demonstrated that deletion of both Rb and tumor suppressor p53 in primitive mesenchymal cells shifts the tumor spectrum away from osteosarcoma to the brown fat tumor hibernoma.59
After release from suppression, the adipose precursors initiate a transcriptional cascade to turn on lipid synthesis and other adipocyte specific programs. A number of transcription factors and co-regulators appear to play important roles in specifying brown fat cell fate or modulation of the expression of thermogenic genes, especially UCP1. Nuclear co-repressor RIP140 directs histone and DNA methylation to silence UCP1 expression and suppress mitochondrial biogenesis in white adipocytes.60,61 Nuclear receptor liver X receptor (LXR) can suppress UCP1 gene expression by binding to the LXRE element of the UCP1 promoter and recruiting co-repressor RIP140.62 Thus, LXR KO mice display increased energy expenditure and UCP1 expression.63 The zinc-finger containing protein PRDM16, which is expressed at higher levels in brown compared with white adipocytes,64 has been shown to drive differentiation of white preadipocytes or myoblasts into functional brown adipocytes. This effect depends on the interaction of PRDM16 with nuclear coactivator PGC-1α and transcription factors PPARγ and C/EBPβ, while binding of PRDM16 to CtBP-1 and CtBP-2 suppresses expression of white fat-selective genes.65,66 In addition, the forkhead family transcription factor forkhead box C2 (FOXC2) can induce expression of the R1 subunit of cAMP-dependent protein kinase A (PKA), thereby sensitizing cells to β-adrenergic stimulation and promoting brown adipogenesis.67 Recently, Plac8, a 12.5 kDa protein containing a cysteine-rich domain, has been found to play a critical role in promoting brown adipogenesis via induction of C/EBPβ expression.68
Recently, microRNAs (miRNAs) have emerged as important regulators of diverse biological processes and pathologies, including cell fate decision. The role of miRNAs in brown adipogenesis has just begun to emerge. Certain myogenic miRNAs have been shown to be enriched in BAT compared with WAT.69 Sun et al. have recently shown that the miR-193b-365 cluster is required for brown fat differentiation from the myf5+ progenitors, and that miR-193b-365 expression was regulated via the PRDM16-PPARγ transcriptional cascade70; however, whether this miRNA cluster could regulate the formation of the inducible brown adipocytes arising from the myf5− lineage is unknown.
‘Browning’ in White Adipose Tissue
Recent years have brought a greater appreciation for potential beneficial effects of acquiring brown fat cells in non-classic BAT locations, such as WAT and skeletal muscle.71 Obesity-resistant strains of mice contain higher amounts of brown adipocytes dispersed in their WAT and muscle,72-75 and physiological stimuli, such as cold exposure and sympathetic activation, are also known to induce brown adipogenesis in white depots.76 Over the past two years, several studies reported that mouse models with increased UCP1-positive brown adipocytes in WAT are protected from high-fat diet-induced obesity. Transgenic expression of PRDM16 in fat tissue using the aP2 promoter induced the formation of brown adipocytes in subcutaneous but not epididymal fat, and the transgenic mice exhibited increased energy expenditure, limited weight gain, and improved glucose tolerance in response to a high-fat diet.77 Similarly, transgenic mice with overexpression of FOXC2 in adipose tissue induced the emergence of brown fat cells in WAT. This interconversion of white to brown adipose was recently shown to be reliant on the C/EBPα signaling pathway, which acts with co-repressors to reduce the expression of certain visceral WAT genes in order to promote BAT genes.78
Hormone-sensitive lipase (HSL) null mice have increased UCP1 expression and enhanced mitochondrial activity in WAT,79 while mice with adipose triglyceride lipase (ATGL, also known as desnutrin) ablation display a conversion of BAT to a WAT phenotype,80 suggesting that these two lipases may have opposite effects in adipose cell fate decision. A lipid droplet protein, perilipin, was recently demonstrated to induce a brown-like phenotype in WAT upon its overexpression, thereby reducing lipid-droplet size.81 However, mice deficient in another lipid droplet protein, fsp27, also display brown fat properties in WAT.82 Interestingly, mice living in an enriched environment with increased social interactions, novel objects and physical activity also displayed a brown-phenotype occurring in WAT, which was reproduced upon hypothalamic overexpression of brain-derived neurotrophic factor (BDNF).83 BDNF has also been recently linked to ventromedial hypothalamus control of energy expenditure, through sympathetic activation of BAT.84 As detailed below, the central nervous system appears to play an important role in the induction of brown fat cells within WAT.
Pharmacological agents that regulate different biological pathways have also been demonstrated to induce browning in WAT. For example, synthetic PPARγ agonists, such as the thiazolidinedione (TZD) family members, are also able to induce a brown phenotype in WAT.85 At the cellular level, Petrovic et al. have demonstrated that TZDs can induce brown adipogenesis in adipose precursors isolated from white fat.86 Furthermore, cyclooxygenase (COX)2, a rate-limiting enzyme in prostaglandin (PG) synthesis, can promote de novo BAT in WAT and increased energy expenditure, and also exerts anti-obesity effects in high-fat fed mice.87 COX2 induces UCP1 expression specific to inguinal WAT, as expression is not induced in classic interscapular BAT.88 Interestingly, co-injection of brown adipogenic factor BMP7 and β3-adrenergic receptor agonist CL316,243 to mice maintained on a high-fat diet resulted in significant increases in the expression of brown fat marker genes UCP1 and CIDEA in both WAT and interscapular BAT. These data suggest that BMP7 may act in concert with other brown adipogenic agents to promote the formation of energy-dissipating brown adipocytes from tissue-resident brown fat cells.
The exact cellular and molecular mechanisms contributing to the browning phenomenon in WAT have not been fully elucidated. Two potential mechanisms have been proposed and each is supported by experimental evidence (Fig. 1). First, the presence of brown fat cells in WAT may come from de novo differentiation. Indeed, as described in the sections above, several brown fat inducers, such as TZD, BMP7 and COX2, can induce de novo brown fat differentiation from tissue resident progenitors. Second, the brown fat cells in WAT may come from the existing white adipocytes, a process called transdifferentiation. Recent evidence for this includes observation of an ‘intermediate’ cell type in cold-exposed WAT, which has ‘paucilocular’ lipid droplets and mitochondria characteristic of both WAT and BAT.89 These two mechanisms are not exclusive to each other; in fact, it is likely that both mechanisms may co-exist in the body and different stimuli may preferentially activate one pathway over the other. Importantly, several findings also indicate that induction of brown adipocytes in WAT is directly reliant on the sympathetic nervous system. Therefore, it appears that complex signaling and neuronal inputs converge to either induce or maintain a BAT phenotype, providing potential in-roads for converting WAT to BAT.
New Physiological Functions of Brown Fat
In addition to thermogenesis, recent studies have demonstrated that BAT is involved in triglyceride clearance and glucose disposal, serves as a source of adipokines, and posseses distinct inflammatory function compared with WAT. For example, Bartelt et al. recently demonstrated a new function of BAT in triglyceride clearance and glucose disposal, a process which involves whole-particle uptake of triglyceride-rich lipoproteins through activation of the cell-surface fatty acid translocase CD36.90-92
White adipose tissue secretes many cytokines, hormones, and other factors which are collectively termed ‘adipokines,’ leading to the classification of adipose tissue as an endocrine organ. While it is expected that BAT secretes many of the same factors which it also expresses (adiponectin, for instance), there are also several factors which may be uniquely secreted by BAT alone, which we are calling BATokines, for BAT-derived adipokines. Several BATokines have already been demonstrated in the literature, including FGF21, which is induced upon cold and adrenergic stimulation.93-95
Furthermore, BAT secretes other factors such as IL6 and neurotrophic factors such as BDNF and NGF,96-99 which may play unique roles in BAT vs. WAT. For example, BAT is more highly innervated by the sympathetic nervous system and contains a more richly developed vasculature. Therefore, the paracrine and autocrine environment of BAT may have evolved to respond uniquely to various adipokines, given the metabolic functions of BAT.
White adipose tissue readily becomes infiltrated with immune cells and macrophages upon high-fat feeding and obesity, believed to be the trigger of the inflammation observed in obese adipose tissue. BAT, by contrast, does not appear to accumulate pro-inflammatory macrophages with a high-fat diet, though this may depend on mouse strain or diet conditions.40 The authors of this study postulate that the high metabolic rate of mitochondria-rich BAT allows tissue to readily utilize free fatty acids through β-oxidation, whereas the overload of free fatty acids (lipotoxicity) in WAT may be the precipitating event leading to the influx of immune cells, including pro-inflammatory macrophages.
Interestingly, another group has similarly demonstrated that macrophages in BAT do not develop the same chemokine and cytokine expression profile as those in WAT.100 Together, these studies indicate that the microenvironment provided by different adipose depots likely influence whether or not the tissue recruits immune cells such as macrophages, and whether these tissues become inflamed. They further suggest that the microenvironment of BAT may be protective against the pro-inflammatory state which may lead to insulin resistance, and interventions which convert white depots to BAT may be protective against this effect.
Mechanisms of Increased Metabolic Activity in BAT
The traditional view of BAT is that its metabolic activity (both β-oxidation and thermogenesis via UCP1) is regulated via input from the sympathetic nervous system (SNS). SNS input to BAT results in the release of the catecholamine neurotransmitter norepinephrine (NE), which binds to adrenergic receptors in BAT. BAT expresses G-protein coupled adrenergic receptors (classified as α1–α3 or β1–β3), and while NE is believed to be the main catecholamine from sympathetic nerves which acts on these receptors, there is emerging evidence that epinephrine may also affect PGC1 α and UCP1 expression in BAT.101 Activation of the β3-adrenergic receptor stimulates cAMP production and PKA activation. It is believed that the β3-adrenergic receptor isoform is the main isoform in mouse BAT, while β1 may be the predominant isoform in human BAT. Indeed, a recent study found that non-shivering thermogenesis (activated in the absence of shivering thermogenesis only after several weeks in the cold) in β3-adrenergic receptor knockout mice is still activated, and the knockout mice are able to survive 7 weeks or more. This period was characterized by upregulation of UCP1 and the β1 adrenergic receptor in BAT, indicating that these KO mice may be a good model for human BAT thermogenesis where β1 appears to be the main responsible isoform.102
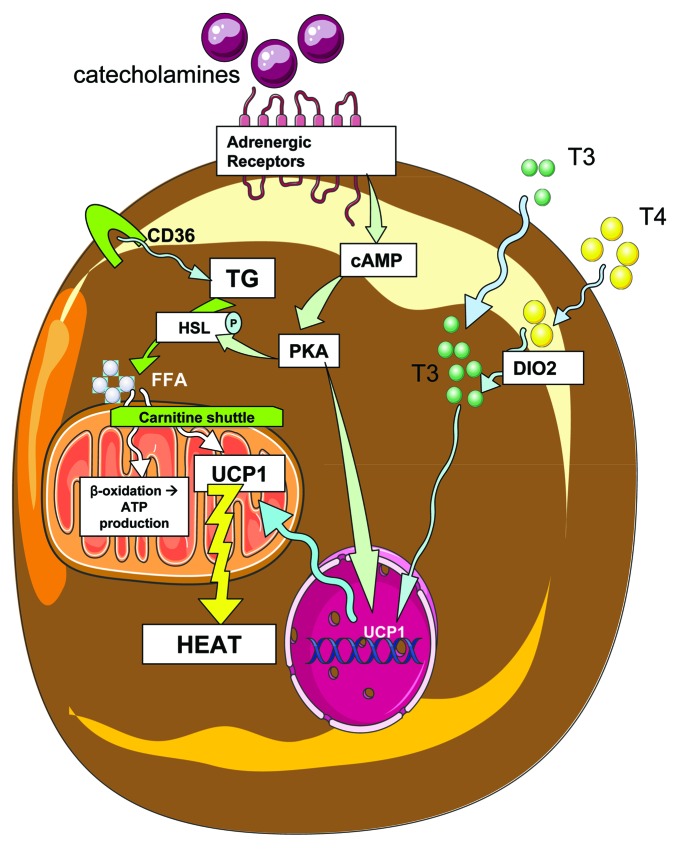
Downstream of cAMP and PKA, HSL is phosphorylated and activated, as well as perilipin A.103 Normally, perilipin A protects the lipid droplet, but after phosphorylation and activation it induces fatty acid cleavage. HSL also promotes fatty acid release from triglycerides. The newly available fatty acids are brought to the mitochondria by the carnitine shuttle, where they can activate UCP1 as well as be metabolized through β-oxidation (pathways summarized in Fig. 2).
Figure 2. Brown adipose activity in response to sympathetic input and thyroid hormone. Catecholamines bind to adrenergic receptors on the surface of brown adipocytes, initiating signaling cascades that include cAMP and protein kinase A (PKA), which then phosphorylates and activates the enzyme hormone sensitive lipase (HSL), which then cleaves triglycerides (TG) into free fatty acids (FFA). Triglycerides enter the cell by uptake of triglyceride rich lipoproteins via CD36 transport, and fatty acids then enter the mitochondria through the carnitine shuttle. Mitochondrial fatty acids may be oxidized via β-oxidation, or serve to activate UCP1 thermogenesis. Additionally, thyroid hormones T3 and T4 enter the cell, and T4 is further converted to T3 by type 2 deiodinase (DIO2). T3 is then able to affect mitochondrial activity and nuclear transcription of genes that affect energenesis, including UCP1. PKA also affects nuclear transcription of UCP1, a protein which acts in the mitochondria to uncouple oxidative phosphorylation from ATP production, resulting in heat generation.
For BAT thermogenic function, there is a synergy between sympathetic inputs and thyroid hormone action (reviewed in ref. 104). Thyroid hormones (as both the T3 and T4 forms, which are more or less active respectively) are transported into brown adipocytes, where T4 is further converted to T3 by type 2 deiodinase (DIO2), followed by T3-driven expression of genes such as UCP1.105 The thyroid hormone is essential for adaptive thermogenesis, as demonstrated by hypothyroid mammals which succumb quickly to reduced environmental temperature. However, a recent study clarified that this metabolic response of hypothyroid animals is temperature-specific and does not occur at ambient room temperatures.106 Relevant to BAT thyroid hormone signaling, the β isoform of the thyroid receptor has recently been demonstrated to mediate T3 regulation of UCP1.107 Other pathways are also implicated in the increased expression of UCP1, such as the PKA and protein kinase G (PKG) pathways, and the p38-CREB pathway.108-110
Recently, several novel factors have been implicated in the regulation of these energetic processes in BAT. Sympathetic input to BAT can be removed through surgical or chemical denervation of the tissue. A recent study demonstrated that unilateral BAT denervation not only reduced UCP1 expression, but also reduced activity of AMPK, the cellular energy sensor which is activated in BAT upon β-adrenergic receptor stimulation.111
The regulation of fuel supply in BAT may also be a point of metabolic control. As mentioned above, BAT is involved in triglyceride clearance, through whole-particle uptake of triglyceride-rich lipoproteins, and Vergnes et al. have shown that FABP3 (the heart-type fatty acid binding protein) is required for BAT fatty acid oxidation and cold tolerance, despite FABP4 (or ap2) being the most abundant FABP in BAT.112 Mitochondrial activity and energy expenditure in BAT are therefore complex processes with multiple points of potential regulation, from sympathetic/catecholamine stimulation of adrenergic receptors, to UCP1 and other genes involved in thermogenesis, to mitochondrial activity and fuel utilization.
Central Nervous System (CNS) Control of BAT Function
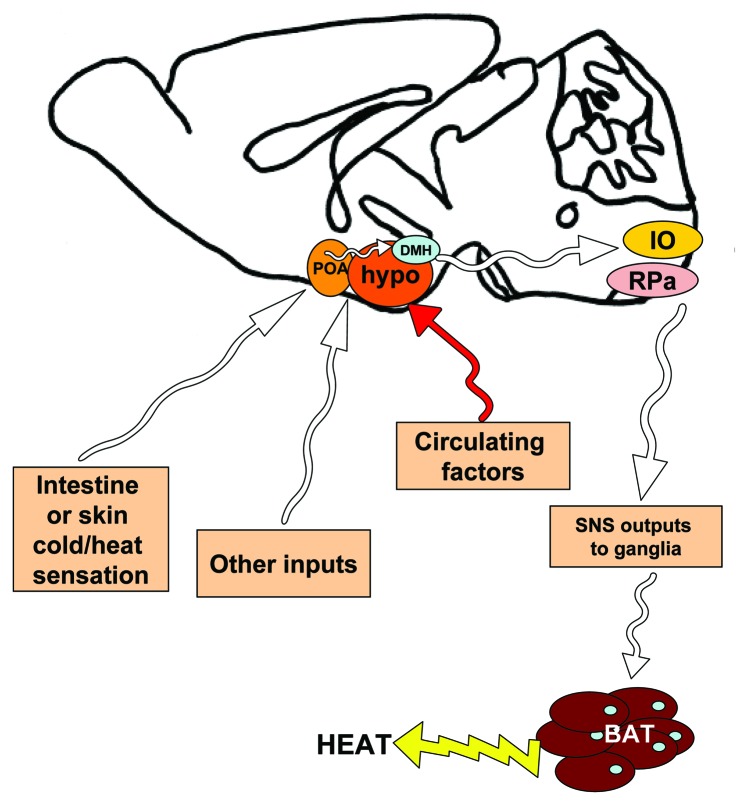
The brain remains the most important organ for control and coordination of systemic energy balance. Several brain regions, including the hypothalamus, are involved in the central regulation of appetite and energy expenditure, including the activation of sympathetic output to adipose depots. For example, when cold is sensed by the pre-optic area (POA) of the brain, signals are relayed to the dorsomedial hypothalamus (DMH), then the GABAergic neurons of the rostroventral medulla in the brainstem, followed by sympathetic outflow to the tissue113-116 (summarized in Fig. 3). Several hypothalamic signaling pathways have been identified which can increase sympathetic output to adipose tissue, including leptin receptors in the ventromedial hypothalamus (VMH) and POA, as shown through retroviral tract tracing from BAT to the hypothalamic leptin-receptor neurons.117
Figure 3. Simplified schematic of neural pathways in the mouse brain which affect sympathetic outflow to brown adipose tissue. Using rodent models, neuroscientists have begun to identify which neural pathways are involved in signaling temperature status (such as cold) to the brain, followed by sympathetic stimulation of brown adipose tissue in order to initiate thermogenesis. Some of these findings are summarized in this mid-sagittal view of a mouse brain, but for a complete review see Morrison et al. Cold temperature is sensed by the pre-optic area (POA), rostral to the hypothalamus (hypo). The POA sends signals to the hypothalamus, including the dorsomedial hypothalamus (DMH). Other hypothalamic nuclei are also involved in relaying various signals related to energy status, in response to various neural inputs and circulating factors. Neural outputs from the hypothalamus reach the inferior olive and GABAergic centers in the raphe pallidus (RPa) in the medulla of the brain stem. From here, sympathetic outputs are activated and send afferents to the sympathetic ganglia, followed by the brown adipose tissue, where catecholamine neurotransmitters are released from sympathetic nerve terminals, to act on adrenergic receptors there. White arrows represent neural pathways.
In response to diet or cold, hypothalamic pathways like those described above lead to sympathetic activation of BAT, as well as increased sympathetic input to WAT depots, which in turn respond by upregulating brown-adipocyte genes such as UCP1. The exact CNS pathways involved in this communication with adipose depots to control peripheral energy expenditure continue to be elucidated, but much of the neural connections and neurotransmitters have already been identified. Recently several candidate signaling molecules have been identified with a CNS role in sympathetic outflow to adipose. For instance, the sirtuin deacetylase SIRT1 in pro-opiomelanocortin (POMC) neurons appears to be responsible for ‘browning’ of perigonadal WAT in response to cold or diet.118 Similarly, neurons in the paraventricular hypothalamus (PVH) appear to also inhibit sympathetic outflow to adipose tissue. Upon activation of PVH neurons by NMDA (an agonist for the NMDA glutamate receptor), no effect on BAT thermogenesis was observed, and in fact NMDA treatment to the PVH reversed BAT activation that had been stimulated by cold exposure. In addition to this, DMH neuropeptide Y (NPY) appears to be an inhibitory signal for adipose expression of UCP1, as its knockdown produces an increase in UCP1 expression in inguinal and BAT depots.119 However, previous studies have demonstrated that the DMH is involved in activation of BAT thermogenesis.120
The same group of researchers has also recently identified orexigenic projections from hypothalamus to raphe pallidus, a region of serotonergic neurons in the medulla that plays an important role in sympathetic activation of thermogenesis.121,122 Indeed, previous studies have shown that orexin infusions to the lateral ventricle affect sympathetic outflow and BAT thermogenesis.123-125 Interestingly, Sellayah et al. recently showed that the neuropeptide orexin, which is known to stimulate feeding and arousal in the hypothalamus, is involved in BAT differentiation and function. This was exhibited in orexin knockout mice which display impaired thermogenesis and smaller BAT depots, due to decreased ability of progenitor cells to differentiate. Additionally, in vitro studies showed that orexin was able to have a direct effect on BAT differentiation by inducing expression of PRDM16 and PGC1α.126 It is currently not clear if orexin’s effects are solely mediated by CNS pathways and sympathetic nervous system activity,127 or whether orexin is reaching the brown adipocytes (either via sympathetic neuron secretion or through the circulation) to act on BAT directly. Indeed, brown adipocytes have recently been shown to express orexin receptors.128 However, orexin is a neurotransmitter and its expression has not yet been shown in sympathetic nerve terminals in BAT, it is not expressed in brown adipocytes, and its levels in the circulation are low, leaving open the question regarding the physiological pathways by which orexin regulates BAT.
Taken together, nearly every region of the hypothalamus has been implicated in some manner in the regulation of sympathetic outflow to adipose tissues and the resulting regulation of thermogenesis and energy expenditure; however a complete understanding of the circuitry involved remains to be fully described.
Lessons from Human Brown Fat
Recently, several studies concurrently demonstrated that human BAT is present and active in adult humans. These findings reignited research on human BAT, which had essentially been ignored and believed to be inactive beyond the infant stage when it is important for maintenance of body temperature in the setting of high surface-to-volume ratio with no capacity for shivering. These studies have further clarified conditions in humans which correlate with increased (cold-exposure) or decreased (obesity) BAT activity, as measured by imaging modalities such as 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) combined with X-ray CT (CT). BAT activity also declines with age, from 50% activity in subjects in their 20s, down to 10% for subjects in their 50s and 60s.129 In accordance with this, it was also found that BAT is more prevalent in children than adults, and BAT activity increases into adolescence when it may play a specific metabolic role.130
Additionally, more recent studies have confirmed that circulating catecholamines such as epinephrine and norepinephrine also activate BAT.131 While it is enticing to consider adrenergic stimulation as a means to increase BAT mass and activity in humans, thereby increasing energy expenditure and decreasing body weight, adrenergic stimulation through various pharmaceutical means results in non-specific activation in other tissues and undesirable, or even dangerous, side effects (reviewed in ref. 132). Interestingly, a recent study showed that blockade of β-adrenergic receptors in fact does not inhibit cold-induced thermogenesis in humans, an effect thought to be due to the differing roles of β-adrenergic isoforms in BAT vs. skeletal muscle, another thermogenic tissue.133 This finding was surprising and important, taking into account that the three β-adrenergic receptors (β1–β3) are considered the most important for thermogenic effects, illustrated by the obesity observed in mice with total depletion of the β-adrenergic receptors.134
Instead of therapeutic approaches which may induce sympathetic drive to BAT in order to increase its activity, another approach is to induce tissue-resident progenitor cells in human BAT to differentiate, thereby increasing the total mass of human BAT. A recent study has provided promising evidence for this direction, demonstrating that progenitor cells derived from PET-CT positive BAT are able to differentiate to brown adipocytes in vitro, in contrast to PET-CT negative subcutaneous white tissue progenitors which did not develop into brown adipocytes.135
Increasing BAT mass and activity not only provide increased energy expenditure to potentially combat obesity, but BAT also serves to improve lipid and glucose homeostasis (reviewed in ref. 132). Cold may not be the only parameter to activate BAT in adult humans, as BAT also has the capacity to act as a glucose sink. Comparing cold to insulin stimulation, Orava et al. measured BAT perfusion as well as glucose uptake, and observed that insulin leads to increased glucose uptake independent of perfusion, while cold leads to increased thermogenesis correlated with perfusion rates,136 thereby indicating two distinct mechanisms of BAT stimulation. Other signals beyond cold and adrenergic stimulation include leucine deficiency, which leads to decreased abdominal fat mass and has now been associated with increased BAT UCP1 expression and thermogenesis-related energy expenditure due to increased sympathetic innervation.137,138
It remains to be determined whether rodent BAT studies are translatable to understanding the biology of human BAT. Thus far, human BAT has been found to display a unique distribution, but an overall similar morphology and gene expression profile (including high UCP1 and type 2 deiodinase) as the mouse. However, a recent comparison of BAT vs. WAT in humans showed that many genes enriched in human BAT differ from those found in murine interscapular BAT.139 Localization of human BAT is another difficulty, and PET-CT is the most common method for this in humans, where many small BAT depots are interspersed with WAT. However, recent data indicate that PET-CT is able to localize only dense regions of BAT, and BAT can be found in PET-CT negative fat biopsies. Also, given the mechanism of PET-CT (i.e., essentially a measurement of tissue glucose uptake) and the fact that BAT also utilizes fatty acids as fuel, not all BAT activity may be detected using this method. Overall, recent research regarding human BAT has provided the promise that this metabolically active tissue may be coaxed to increase in size or activity in order to boost energy expenditure and combat obesity. However, given the difficulty in obtaining BAT samples from human adults, most future research into the function of BAT may come from rodent models.140
The Promise of Brown Adipose Tissue
As presented in this review, research into the development, function and control of BAT has now reached an exciting pace following the re-discovery of BAT in adult humans. Given the global pandemic of obesity and the projection for epidemic rates of co-morbidities like diabetes, as well as the limited success rate with various interventions to treat and prevent obesity, knowledge about BAT and its promise as a potential therapeutic agent are exciting areas for biological and translational research. BAT activity may be increased in order to elevate whole-body energy expenditure, either through sympathetic or other activation of UCP1 and mitochondria pathways, increased differentiation from progenitor cells, or stimulation of a brown phenotype in WAT depots. Interestingly, transplantation of as little as 0.1–0.4 g of BAT into the visceral cavity of recipient mice is able to prevent weight gain and improve glucose homeostasis in diet-induced obese mice.141 Given its huge capacity for energy expenditure, newly identified effects on fatty acid and glucose metabolism, as well as potential resistance from infiltrating pro-inflammatory macrophages, increasing the amount and function of brown fat may not only combat obesity, but may also prevent type 2 diabetes and other metabolic disorders. Therefore, future research regarding BAT function will further our understanding of its unique physiology as well as its therapeutic promise.
Acknowledgments
We thank T.J. Schulz, A.M. Cypess, and H. Zhang for a critical reading of the manuscript, and E. Caniano and M. Hirshman for administrative assistance. Cellular components in Figures 1–3 were based on templates provided by Servier (www.servier.com/servier-medical-art). This work was supported in part by NIH grants 5R01 DK077097 (Y.-H.T.), and the Joslin Diabetes Center’s Diabetes Research Center (P30 DK036836 from the NIDDK), a research grant from the Eli Lilly Research Foundation and by funding from the Harvard Stem Cell Institute (to Y.-H.T.). K.L.T. was supported by NIH T32 DK007260–33 and NIH F32 DK091996. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH.
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest or financial disclosures.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/18951
References
- 1.Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- 2.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J Obes (Lond) 2010;34(Suppl 1):S7–16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 4.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–72. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci. 2010;1212:E20–36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 6.Richard D, Picard F. Brown fat biology and thermogenesis. Front Biosci. 2011;16:1233–60. doi: 10.2741/3786. [DOI] [PubMed] [Google Scholar]
- 7.Richard D, Carpentier AC, Dore G, Ouellet V, Picard F. Determinants of brown adipocyte development and thermogenesis. Int J Obes (Lond) 2010;34(Suppl 2):S59–66. doi: 10.1038/ijo.2010.241. [DOI] [PubMed] [Google Scholar]
- 8.Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes Rev. 2009;10:265–8. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 10.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–82. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond) 1983;64:19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]
- 12.Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond) 1986;71:291–7. doi: 10.1042/cs0710291. [DOI] [PubMed] [Google Scholar]
- 13.Nedergaard J, Bengtsson T, Cannon B. Unexpected Evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–52. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 14.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 16.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 18.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–20. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 19.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–8. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 20.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–6. [PubMed] [Google Scholar]
- 21.Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11:248–52. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl 1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu Rev Nutr. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr. 2005;25:469–97. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- 25.Williams CT, Goropashnaya AV, Buck CL, Fedorov VB, Kohl F, Lee TN, et al. Hibernating above the permafrost: effects of ambient temperature and season on expression of metabolic genes in liver and brown adipose tissue of arctic ground squirrels. J Exp Biol. 2011;214:1300–6. doi: 10.1242/jeb.052159. [DOI] [PubMed] [Google Scholar]
- 26.Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–4. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–9. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Oelkrug R, Heldmaier G, Meyer CW. Torpor patterns, arousal rates, and temporal organization of torpor entry in wildtype and UCP1-ablated mice. J Comp Physiol B. 2011;181:137–45. doi: 10.1007/s00360-010-0503-9. [DOI] [PubMed] [Google Scholar]
- 30.Kitao N, Hashimoto M. Increased thermogenic capacity of brown adipose tissue under low temperature and its contribution to arousal from hibernation in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 2011; [DOI] [PubMed]
- 31.Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. 2011;12:167–88. doi: 10.1111/j.1467-789X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 32.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–6. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–76. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 35.Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–36. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA. 2011;108:143–8. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic PPAR{gamma} activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, ucp1-containing adipocytes molecularly distinct from classical brown adipocytes. J Biol Chem. 2010;285:7153–64. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–62. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–37. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 42.Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem. 2003;278:9609–19. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- 43.Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, et al. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000;49:1374–80. doi: 10.2337/diabetes.49.8.1374. [DOI] [PubMed] [Google Scholar]
- 44.Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3:37–48. [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–4. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konishi M, Mikami T, Yamasaki M, Miyake A, Itoh N. Fibroblast growth factor-16 is a growth factor for embryonic brown adipocytes. J Biol Chem. 2000;275:12119–22. doi: 10.1074/jbc.275.16.12119. [DOI] [PubMed] [Google Scholar]
- 47.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–7. doi: 10.1210/en.143.5.1741. [DOI] [PubMed] [Google Scholar]
- 48.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–83. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 50.Cousin W, Fontaine C, Dani C, Peraldi P. Hedgehog and adipogenesis: fat and fiction. Biochimie. 2007;89:1447–53. doi: 10.1016/j.biochi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–60. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 54.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function–of mice and men. Genes Dev. 2009;23:788–97. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA, et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci USA. 2004;101:4112–7. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scimè A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, et al. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–95. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Tseng YH, Butte AJ, Kokkotou E, Yechoor VK, Taniguchi CM, Kriauciunas KM, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7:601–11. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 58.Cypess AM, Zhang H, Schulz TJ, Huang TL, Espinoza DO, Kristiansen K, et al. Insulin/IGF-I regulation of necdin and brown adipocyte differentiation via CREB- and FoxO1-associated pathways. Endocrinology. 2011;152:3680–9. doi: 10.1210/en.2011-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–4. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiskinis E, Hallberg M, Christian M, Olofsson M, Dilworth SM, White R, et al. RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. EMBO J. 2007;26:4831–40. doi: 10.1038/sj.emboj.7601908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, et al. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–36. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Zhang Y, Yehuda-Shnaidman E, Medvedev AV, Kumar N, Daniel KW, et al. Liver X receptor alpha is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Mol Cell Biol. 2008;28:2187–200. doi: 10.1128/MCB.01479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korach-André M, Archer A, Barros RP, Parini P, Gustafsson JA. Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity. Proc Natl Acad Sci USA. 2011;108:403–8. doi: 10.1073/pnas.1017884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008;22:1397–409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009; [DOI] [PMC free article] [PubMed]
- 67.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–73. doi: 10.1016/S0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 68.Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, et al. Plac8 Is an inducer of C/EBPbeta required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab. 2011;14:658–70. doi: 10.1016/j.cmet.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Walden TB, Timmons JA, Keller P, Nedergaard J, Cannon B. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J Cell Physiol. 2009;218:444–9. doi: 10.1002/jcp.21621. [DOI] [PubMed] [Google Scholar]
- 70.Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, et al. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol. 2011;13:958–65. doi: 10.1038/ncb2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kozak LP. The genetics of brown adipocyte induction in white fat depots. Front Endocrin 2011; Vol 2, article 64, e-published October 31, [DOI] [PMC free article] [PubMed]
- 72.Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:143–9. doi: 10.1097/MED.0b013e328337a81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–20. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci USA. 2007;104:2366–71. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–6. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, et al. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–28. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strom K, Hansson O, Lucas S, Nevsten P, Fernandez C, Klint C, et al. Attainment of brown adipocyte features in white adipocytes of hormone-sensitive lipase null mice. PLoS One 2008; 3: e1793. [DOI] [PMC free article] [PubMed]
- 80.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–48. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sawada T, Miyoshi H, Shimada K, Suzuki A, Okamatsu-Ogura Y, Perfield JW, et al. Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS ONE. 2010;5:e14006. doi: 10.1371/journal.pone.0014006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, et al. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS ONE. 2008;3:e2890. doi: 10.1371/journal.pone.0002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–38. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C, Bomberg E, Billington CJ, Levine AS, Kotz CM. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010;1336:66–77. doi: 10.1016/j.brainres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.del Mar Gonzalez-Barroso M, Pecqueur C, Gelly C, Sanchis D, Alves-Guerra MC, Bouillaud F, et al. Transcriptional activation of the human ucp1 gene in a rodent cell line. Synergism of retinoids, isoproterenol, and thiazolidinedione is mediated by a multipartite response element. J Biol Chem. 2000;275:31722–32. doi: 10.1074/jbc.M001678200. [DOI] [PubMed] [Google Scholar]
- 86.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–61. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 88.Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS ONE. 2010;5:e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–53. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 90.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 91.Williams KJ, Fisher EA. Globular warming: how fat gets to the furnace. Nat Med. 2011;17:157–9. doi: 10.1038/nm0211-157. [DOI] [PubMed] [Google Scholar]
- 92.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011;13:238–40. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 93.Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17:736–40. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–90. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–12. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nisoli E, Tonello C, Carruba MO. Nerve growth factor, beta3-adrenoceptor and uncoupling protein 1 expression in rat brown fat during postnatal development. Neurosci Lett. 1998;246:5–8. doi: 10.1016/S0304-3940(98)00220-1. [DOI] [PubMed] [Google Scholar]
- 97.Nisoli E, Tonello C, Benarese M, Liberini P, Carruba MO. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503. doi: 10.1210/en.137.2.495. [DOI] [PubMed] [Google Scholar]
- 98.Néchad M, Ruka E, Thibault J. Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp Biochem Physiol Comp Physiol. 1994;107:381–8. doi: 10.1016/0300-9629(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 99.Sornelli F, Fiore M, Chaldakov GN, Aloe L. Adipose tissue-derived nerve growth factor and brain-derived neurotrophic factor: results from experimental stress and diabetes. Gen Physiol Biophys. 2009;28:179–83. [PubMed] [Google Scholar]
- 100.Ortega MT, Xie L, Mora S, Chapes SK. Evaluation of macrophage plasticity in brown and white adipose tissue. Cell Immunol. 2011;271:124–33. doi: 10.1016/j.cellimm.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharara-Chami RI, Joachim M, Mulcahey M, Ebert S, Majzoub JA. Effect of epinephrine deficiency on cold tolerance and on brown adipose tissue. Mol Cell Endocrinol. 2010;328:34–9. doi: 10.1016/j.mce.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 102.Mattsson CL, Csikasz RI, Chernogubova E, Yamamoto DL, Hogberg HT, Amri EZ, et al. beta1-Adrenergic receptors increase UCP1 in human MADS brown adipocytes and rescue cold-acclimated beta3-adrenergic receptor-knockout mice via nonshivering thermogenesis. Am J Physiol Endocrinol Metab. 2011;301:E1108–18. doi: 10.1152/ajpendo.00085.2011. [DOI] [PubMed] [Google Scholar]
- 103.Chaudhry A, Granneman JG. Differential regulation of functional responses by beta-adrenergic receptor subtypes in brown adipocytes. Am J Physiol. 1999;277:R147–53. doi: 10.1152/ajpregu.1999.277.1.R147. [DOI] [PubMed] [Google Scholar]
- 104.Silva JE. Physiological importance and control of non-shivering facultative thermogenesis. Front Biosci (Schol Ed) 2011; 3:352-71.: 352 - 371. [DOI] [PubMed] [Google Scholar]
- 105.Martinez dM, Scanlan TS, Obregon MJ. The T3 receptor beta1 isoform regulates UCP1 and D2 deiodinase in rat brown adipocytes. Endocrinology. 2010;151:5074–83. doi: 10.1210/en.2010-0533. [DOI] [PubMed] [Google Scholar]
- 106.Ueta CB, Olivares EL, Bianco AC. Responsiveness to thyroid hormone and to ambient temperature underlies differences between brown adipose tissue and skeletal muscle thermogenesis in a mouse model of diet-induced obesity. Endocrinology. 2011;152:3571–81. doi: 10.1210/en.2011-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ribeiro MO, Bianco SD, Kaneshige M, Schultz JJ, Cheng SY, Bianco AC, et al. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-beta isoform specific and required for adaptive thermogenesis. Endocrinology. 2010;151:432–40. doi: 10.1210/en.2009-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pedersen SB, Bruun JM, Kristensen K, Richelsen B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem Biophys Res Commun. 2001;288:191–7. doi: 10.1006/bbrc.2001.5763. [DOI] [PubMed] [Google Scholar]
- 109.Haas B, Mayer P, Jennissen K, Scholz D, Diaz MB, Bloch W, et al. Protein kinase g controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal. 2009;2:ra78. doi: 10.1126/scisignal.2000511. [DOI] [PubMed] [Google Scholar]
- 110.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24:3057–67. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pulinilkunnil T, He H, Kong D, Asakura K, Peroni OD, Lee A, et al. Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J Biol Chem. 2011;286:8798–809. doi: 10.1074/jbc.M111.218719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vergnes L, Chin R, Young SG, Reue K. Heart-type fatty acid-binding protein is essential for efficient brown adipose tissue fatty acid oxidation and cold tolerance. J Biol Chem. 2011;286:380–90. doi: 10.1074/jbc.M110.184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R831–43. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–97. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–49. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–84. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–83. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–5. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- 121.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–55. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Madden CJ, Morrison SF. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R776–83. doi: 10.1152/ajpregu.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yasuda T, Masaki T, Kakuma T, Hara M, Nawata T, Katsuragi I, et al. Dual regulatory effects of orexins on sympathetic nerve activity innervating brown adipose tissue in rats. Endocrinology. 2005;146:2744–8. doi: 10.1210/en.2004-1226. [DOI] [PubMed] [Google Scholar]
- 124.Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, et al. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol. 2010;588:4117–29. doi: 10.1113/jphysiol.2010.195099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Verty AN, Allen AM, Oldfield BJ. The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinology. 2010;151:4236–46. doi: 10.1210/en.2009-1235. [DOI] [PubMed] [Google Scholar]
- 126.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–90. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 127.Seale P. Orexin turns up the heat on obesity. Cell Metab. 2011;14:441–2. doi: 10.1016/j.cmet.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 128.Digby JE, Chen J, Tang JY, Lehnert H, Matthews RN, Randeva HS. Orexin receptor expression in human adipose tissue: effects of orexin-A and orexin-B. J Endocrinol. 2006;191:129–36. doi: 10.1677/joe.1.06886. [DOI] [PubMed] [Google Scholar]
- 129.Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011;19:1755–60. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 130.Drubach LA, Palmer EL, III, Connolly LP, Baker A, Zurakowski D, Cypess AM. Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J Pediatr. 2011;159:939–44. doi: 10.1016/j.jpeds.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 131.Wang Q, Zhang M, Ning G, Gu W, Su T, Xu M, et al. Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS ONE. 2011;6:e21006. doi: 10.1371/journal.pone.0021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Whittle AJ, Lopez M, Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends Mol Med. 2011;17:405–11. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 133.Wijers SL, Schrauwen P, van Baak MA, Saris WH, Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab. 2011;96:E598–605. doi: 10.1210/jc.2010-1957. [DOI] [PubMed] [Google Scholar]
- 134.Jimenez M, Leger B, Canola K, Lehr L, Arboit P, Seydoux J, et al. Beta(1)/beta(2)/beta(3)-adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett. 2002;530:37–40. doi: 10.1016/S0014-5793(02)03387-2. [DOI] [PubMed] [Google Scholar]
- 135.Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011;152:3597–602. doi: 10.1210/en.2011-1349. [DOI] [PubMed] [Google Scholar]
- 136.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–9. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 137.Cheng Y, Zhang Q, Meng Q, Xia T, Huang Z, Wang C, et al. Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Mol Endocrinol. 2011;25:1624–35. doi: 10.1210/me.2011-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59:17–25. doi: 10.2337/db09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Svensson PA, Jernas M, Sjoholm K, Hoffmann JM, Nilsson BE, Hansson M, et al. Gene expression in human brown adipose tissue. Int J Mol Med. 2011;27:227–32. doi: 10.3892/ijmm.2010.566. [DOI] [PubMed] [Google Scholar]
- 140.Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes (Lond) 2010;34(Suppl 1):S23–7. doi: 10.1038/ijo.2010.179. [DOI] [PubMed] [Google Scholar]
- 141.Stanford KI, Middelbeek RJ-W, An D, Townsend KL, Hitchcox KM, Jung DY, et al. Transplantation of Brown Adipose Tissue Exerts Beneficial Effects on Glucose Homeostasis. Diabetes 2011; Abstract presented at annual meeting of the American Diabetes Association, San Diego CA, 60: A24 - A25. [Google Scholar]