Abstract
Adipose tissue development is dependent on multiple signaling mechanisms and cell-cell interactions that regulate adipogenesis, angiogenesis and extracellular remodeling. The Notch signaling pathway is an important cell-fate determinant whose role in adipogenesis is not clearly defined. To address this issue, we examined the effect of inhibition of Notch signaling by soluble-Jagged1 in the 3T3-L1 preadipocyte line. In vitro, soluble-Jagged1 expression in 3T3-L1 cells altered cell morphology, increased the rate of cell proliferation and induced an early transcriptional response to differentiation stimuli. However, these cells did not form mature adipocytes due to their inability to exit the cell-cycle in response to serum-starvation and glucocorticoid-induced cell-cycle arrest. In contrast, subcutaneous allografts of soluble-Jagged1 cells formed larger fat pads containing lipid-filled adipocytes with improved neovascularization compared with controls. Since adipogenesis is tightly associated with angiogenesis, we evaluated the influence of soluble-Jagged1 on endothelial cells by culturing them in cell-free conditioned media from preadipocytes. Soluble Jagged1-mediated inhibition of Notch signaling increased levels of secreted cytokines, potentially contributing to the improved cell growth and proliferation observed in these cultures. Our findings demonstrate an initial requirement of Notch signaling inactivation for preadipocyte cell commitment and support the hypothesis that cell-to-cell crosstalk between the preadipocytes and endothelial cells is required for neovascularization and remodeling of the tissue to promote hyperplasia and hypertrophy of differentiating adipocytes.
Keywords: allograft, angiogenesis, Jagged 1, Notch signaling, preadipocyte
Introduction
Adipose tissue development is a complex process involving adipogenesis, angiogenesis and extracellular matrix remodeling.1-4 It is therefore imperative to understand the dynamics of the responsible factors, the cell-cell signaling and cross talk.5 While pro-angiogenic factors like plasminogen activator inhibitor-1 (PAI-1), angiotensin II, phospholipid transfer protein (PLTP), prostaglandin, and cholesterol ester transfer protein (CETP)1,6 secreted from adjacent adipocytes into the blood stream initiate endothelial cell recruitment for expanding adipocytes,7,8 adipose tissue itself functions as an endocrine tissue secreting hormones with cytokine properties like the adipokines, leptin and adiponectin.9-13 These hormones act in a paracrine fashion to support adipose tissue development, and also act on a variety of tissues to regulate the metabolism of the entire organism. Yet, the molecular mechanisms regulating cross talk between adipogenic and endothelial cells within the tissue is not clearly understood.
An important modulator of vascular development and angiogenesis as well as adipogenesis, is the Notch signaling pathway.14-18 Notch proteins are a family of transmembrane receptors whose intercellular signaling determines cell fate in almost all cell types and tissues. Four mammalian Notch receptors (Notch1–Notch4) and 6 Notch ligands (Delta like 1, 2, 4, Jagged 1, 2, F3/contactin) have been identified.19,20 Notch ligand and receptor interaction leads to proteolytic cleavage and release of the Notch intracellular domain (Notch ICD) from the plasma membrane which translocates to the nucleus and regulates transcription of downstream targets.21
Notch signaling is complex and marked by diverse outcomes that reflect different combinations of specific receptor(s)/ligand(s) interaction, the cellular environmental context and developmental outcomes specific to the NotchICD (e.g., stem cell maintenance, progenitor selection, growth organizing boundaries, cell growth or inhibition, differentation).19,22,23 In the context of adipocytes, studies on Notch signaling functionality and target gene expression indicate inhibition of Notch signaling to promote adipocyte differentiation in mesenchymal stem cells (MSCs)24 and preadipocyte cell line 3T3-L1 cells25 in the early stages. The clonally expanded unipotent murine cell line 3T3-L1 is one of the classical and most extensively used cell models for studying adipocyte differentiation for in vitro evaluation in identifying molecular markers, transcription factors and various interactions that are required for preadipocyte differentiation.26 Studies have shown that activation of Notch signaling inhibits adipocyte differentiation from MSCs and 3T3-L1.27-29 While antisense Notch1 prevented 3T3-L1 differentiation, indicating a requirement of Notch signaling,30 Notch was also shown to be dispensable for adipocyte specification and differentiation from either mesenchymal or epithelial progenitors.31 In addition, the non-canonical Notch signaling pathway is also shown to regulate adipogenesis mediated by the ligand Delta-like 1 (Dlk-1/Pref-1).32-34 Given these contrasting reports, the requirement and role of Notch signaling in adipose tissue development and adipocyte differentiation still needs clarification.
In this study we address the effects of inhibition of Notch signaling in adipocytes and the influence on adipocyte differentiation and interaction with endothelial cells (EC). We used the previously described35,36 soluble-Jagged1(sJag1)-mediated suppression of Notch signaling in 3T3-L1 as the model system. In vitro suppression of Notch signaling by sJag1 increased proliferation in 3T3-L1 and EC and triggered the differentiation process in the preadipocytes. In vivo, subcutaneous fat pads formed by 3T3-L1 cells expressing sJag1 in nude mice displayed an increase in vessel formation and production of fat filled adipocytes. Together, these results show that inhibition of Notch signaling by sJag1 promotes both angiogenesis and adipocyte differentiation.
Results
sJag1 expression changes cell morphology
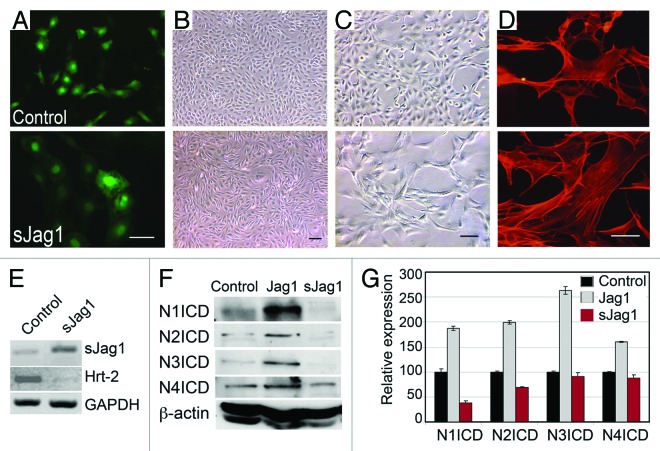
Expression of sJag1 has previously been shown to inhibit endogenous Notch signaling.18,35 Using the same principle, we utilized the 3T3-L1 cell model to define the mechanism of Notch inhibition in adipocyte differentiation. 3T3-L1 cells were transfected with the control vector or sJag1 ligand and a homogenous population of cells expressing sJag1 was selected by sorting for GFP expression (Fig. 1A). Cell passages were checked regularly to confirm GFP expression. sJag1 expressing cells cultured in regular growth media showed distinct phenotypic characteristics. Similar to the behavior of 3T3 fibroblasts expressing a sJag1 construct, stably transfected 3T3-L1 preadipocytes also exhibited a cord-like phenotype in growth media at confluence unlike the monolayer growth of empty vector expressing control cells (Fig. 1B–C). Additionally, similar to the previous report of sJag1 in 3T3 fibroblasts, sJag1 preadipocytes also lift off of the surface of the culture plate indicative of changes in the ability of the cells to adhere to substrate. These cells showed loss of contact inhibition similar to the previous report of sJag1 in 3T3 cells35 and typical fibroblast morphology with large elongated cells, and altered F-actin fiber organization illustrated by phalloidin stain (Fig. 1D).
Figure 1. Expression of soluble form of Jagged1 (sJag1) in 3T3-L1 cells. (A) 3T3-L1 cells were transduced with vector control (control) or sJag1 and sorted for GFP expression. (B and C) Cells under low (B) and higher magnification (C) showing the fibroblastic phenotype in sJag1 cells. (D) Phalloidin staining to show altered F-actin organization in sJag1 expressing cells (bar indicates 50 μm). (E) RT-PCR for sJag1 and Notch target gene Hrt-2 transcripts. (F) Immunoblot for endogenous levels of active Notch receptor activation (NICDs, Notch1, Notch2, Notch3 and Notch4) using whole cell lysates. Full-length Jagged1 overexpressing cells were used as positive control to indicate Notch activation. The graph shows quantification of the immunoblot based on integrated densities (graphed are means ± SD).
sJag1 expression reduces Notch activation in 3T3-L1 cells
RT-PCR for regions specific to the ligands confirmed the expression of the sJag1 at the transcript level and corresponding decrease in Notch target gene Hrt-2, confirming functionality of sJag1 expression (Fig. 1E). Immunoblot for endogenous cleaved Notch intracellular domain (ICD) showed decreased levels in cells expressing sJag1 (Fig. 1F) indicating reduced Notch activation when compared with controls and Jag1 expressing 3T3-L1 cells used as a positive control. sJag1 expression repressed Notch1 and Notch2 activation, while Notch 3 and 4 were less affected as seen in the immunoblot quantifications based on integrated densities (Fig. 1F, graph).
sJag1 expression increases cell proliferation
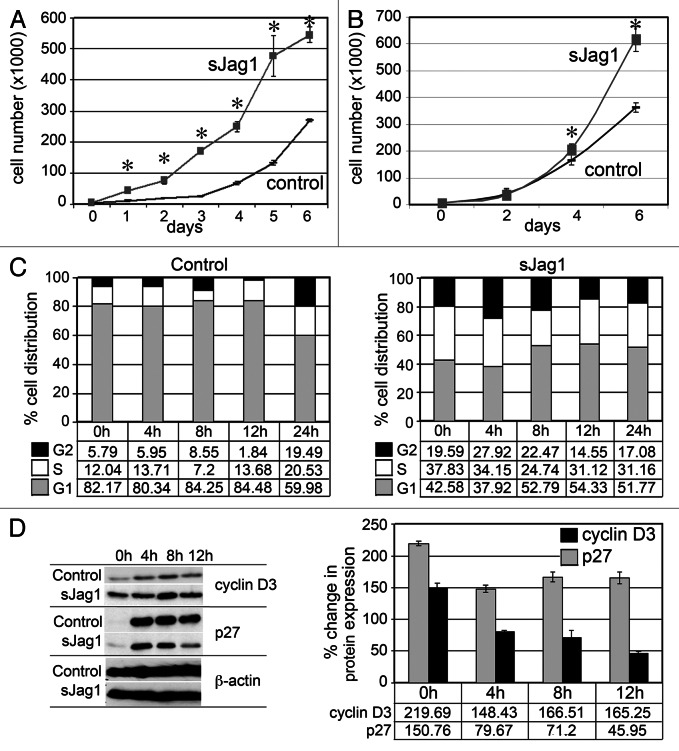
sJag1 expression increased cell growth rate as measured by cell counts. Growth curve analysis showed sJag1-expressing cells had a 2-fold increase in cell numbers compared with control vector transformed cells as early as 2 d (p < 0.01) (Fig. 2A). To determine if the increased growth rate is by virtue of secreted sJag1 acting as a Notch signaling inhibitor, non-transduced 3T3-L1 cells were cultured in cell-free conditioned medium from sJag1 overexpressing cells or controls (Fig. 2B). Growth curve analysis showed a 2-fold increase in cell numbers by 6 d in the sJag1 treated cells over the corresponding control. Although cell proliferation effects due to the conditioned media were not as dramatic as in sJag1 overexpressing cells, it must be noted that the conditioned media is expected to have lower amounts of available (not bound) ligand, and medium was collected after 24–48 h. This result indicates antagonism of the Notch receptor by sJag1 increases proliferation of the 3T3-L1 cells and confirms the earlier findings that the secreted sJag1 form functions as a dominant negative regulator inhibiting Notch signaling that can act in an autocrine and paracrine manner.37
Figure 2. sJag1 expression increases cell proliferation and alters cell cycle. (A) Growth curves of control and sJag1 expressing 3T3-L1 cells cultured in regular growth media (bars indicate means ± SD and * indicates p < 0.05). (B) Growth curve for 3T3-L1 cells cultured in cell-free conditioned media from control or sJag1 expressing 3T3-L1 cells (graphed are means ± SD and * indicates p < 0.05). (C) Cell cycle analysis showing distribution of the cell population in the cell cycle stages G1, S and G2 at different time intervals. (D) Immunoblot for cyclin D3 and p27 at different time intervals; the graph shows quantification of the immunoblots as percent change in expression relative to control at time 0 (graphed are means ± SD).
To further explore cell growth dynamics, we performed cell cycle analysis using flow cytometry on proliferating cells stained with propidium iodide. Cells at ~60% confluence were serum starved (0.2% FBS) for 40 h to synchronize the cells prior to feeding complete media. Cells were collected at 4 h intervals for cell cycle analysis. More than 80% of the control cells were in G1 phase at 0 h, while only ~40% of sJag1 cells were present in G1 phase. This result indicates that serum starvation did not force the sJag1 cells into a resting or quiescence state as in the control line. Five to six percent of control cells were detected at G2 phase, unlike the sJag1 expressing cells with over 20% in the G2 phase (Fig. 2C). Higher numbers of cells were also present in the synthesis (S) phase in the sJag1 cells, indicating that sJag1 cells did not exit cell cycle even after 40 h of serum starvation. Corresponding to the increase in cell proliferation, immunoblot analysis showed increased levels of cyclin D3, a positive regulator of cell cycle progression (Fig. 2D). Quantification of the blots showed that at 0 h, cyclin D3 level was more than 2-fold elevated compared with controls (set at 100%) and subsequently showed 1.5-fold increase throughout the experiment up to 12 h (Fig. 2D, graph). Unlike the controls, serum starvation did not bring down cyclin D3 levels in sJag1 expressing cells. Corresponding to the increased cyclin D3 levels, there was also a decrease in CDK inhibitor p27. Although the initial level at 0 h was 1.5-fold higher than control, serum stimulation dramatically lowered p27 levels in sJag1 cells (Fig. 2D). These results show that sJag1 cells proliferate at a higher frequency than control cells and do not exit cell cycle in response to serum starvation.
sJag1 respond to differentiation signals but are unable to become mature fat cells
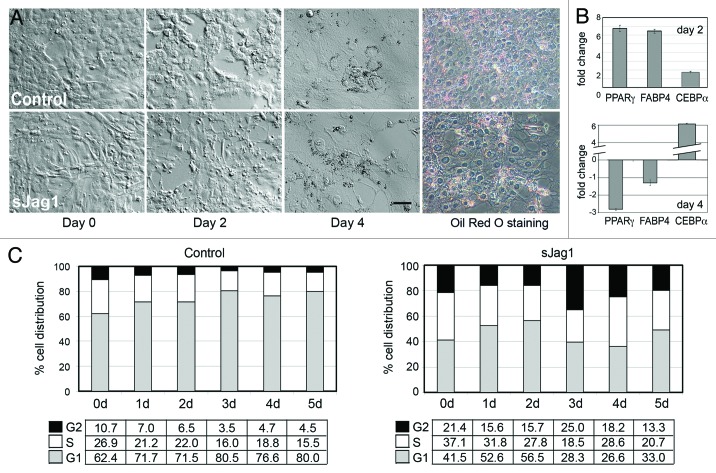
To determine the influence of sJag1 expression on differentiating 3T3-L1 cells, sJag1 and control cells were stimulated with a cocktail containing IBMX, insulin and dexamethasone to induce differentiation. Control cells followed the expected preadipocyte differentiation pattern in which cells stopped proliferating, and changed morphology by becoming large, rounded acquiring lipid droplets (Fig. 3A). On the other hand, sJag1 expressing cells did not show changes in morphology characteristic of differentiation, but instead continued to multiply. After 4 d they became over confluent and began to lift off the surface. However, stimulation with differentiation media did cause the sJag1 cells to initiate the differentiation cascade, based on molecular markers. Two days post induction, quantitative PCR indicated increase in RNA transcript levels of adipogenic markers in sJag1 cells over control cells. Peroxisome proliferator-activated receptor gamma (PPARγ) and Fatty acid binding protein 4 (FABP4) levels were upregulated 6-fold over the corresponding controls (Fig. 3B). However, 4 d post induction, the transcript levels were dramatically downregulated while C/EBP α expression was upregulated in sJag1 expressing cells (Fig. 3B). Cell cycle analysis of cells in the differentiation conditions indicated that sJag1 cells did not exit cell cycle, similar to their behavior in normal growth conditions. On day 2 (48 h in differentiation medium), 56% of sJag1 cells were in G1 phase, while 80% of the control cells were in G1 phase, responding to glucocorticoid induced cell cycle arrest (Fig. 3C). By day 5, continued proliferation of sJag1 cells resulted in over confluence, loss of contact inhibition and cell detachment. These observations indicate that sJag1 cells respond partially to differentiation stimulus, but are unable to exit cell cycle and thus do not form mature adipocytes.
Figure 3. Effect of differentiation on sJag1 expressing cells. (A) Phase contract micrographs of control and sJag1 cells in differentiation conditions. Day 0 represents when cells were stimulated with differentiation media, day 2 represents, two days post stimulation when media was changed to maintenance media, and day 4 represents 4 d post stimulation (bar = 50 μm) and Oil red O staining at 4 d. (B) qPCR for PPARγ, FABP4 and C/EBP α gene transcripts represented as fold change after normalization to internal control and to corresponding control RNA (graphed are means ± SD). (C) Cell cycle analysis showing distribution of cell populations at G1, S and G2 stages for control and sJag1 cells under differentiation conditions.
sJag1 expression induces in vivo fat pad formation
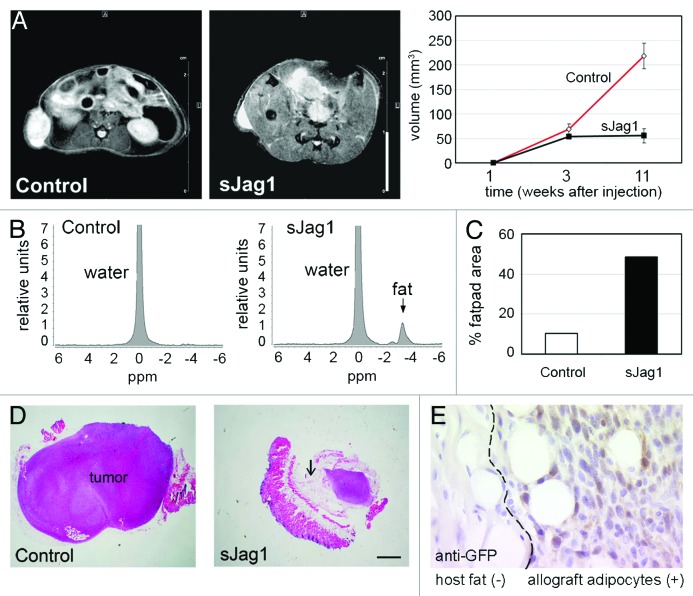
To determine the potential of sJag1 expressing cells to differentiate in vivo, we used the previously described8,38,39 subcutaneous fat pad formation model in nude mice. Injection of 2.5 million proliferating control or sJag1 expressing cells showed fat pad formation within a week. Fat pad formation was visualized by MRI and fat pad volume was determined based on scanned images (Fig. 4A). At 3 weeks, fat pads in control mice measured a volume of 38.16 mm3 while the sJag1 were 30.3 mm3. However, by 11 weeks, the control cells underwent transformation adopting a tumorigenic phenotype, grew larger and measured 218 mm3 while the sJag1 allografts measured 55 mm3 (Fig. 4A, graph). Localized spectroscopy was applied to detect lipid accumulation at 3 and 11 weeks. In the control cells, no peak was detected while in the sJag1 cells, a peak corresponding to the lipid was observed (Fig. 4B). This indicated that the sJag1 cells were accumulating lipid and differentiating into adipocytes. In the control allografts, cells became transformed, and caused excessive growth with histological features of rapidly growing tumors containing necrotic cores (Fig. 4D). Interestingly, although the sJag1 cells were highly proliferative in vitro, sJag1 allografts formed mature adipocytes. The total volume based on MRI (Fig. 4A) therefore indicated the total region comprising the tumor and the fat pad. When the proportion of fat cells in each allograft was calculated, we determined sJag1 expressing allografts had almost half the area consisting of fat cells (48.47%) while in the control allografts, only ~10% of the total area was comprised of fat cells (Fig. 4C). Further, immunostaining of the allographs for GFP clearly shows positive staining of adipocytes and adjacent cells (Fig. 4E), but no staining in the host tissue.
Figure 4. In vivo fat pad formation from 3T3-L1 allographs. (A) Axial images generated by magnetic resonance imaging (MRI) to determine fat pad volume in nude mice injected subcutaneously with control or sJag1 cells in the flanks (bar = 1 cm). The graph shows the fat pad volumes 3 or 11 weeks after injections (n = 6, graphed are means ± SD and * indicates p < 0.05). (B) Localized spectroscopy acquired by placing a 1 mm3 voxel at the center of the fat pad 3 weeks after injection to quantify water and fat content. (C) Graph indicates quantification of fat pad region in each group of mice. (D) Representative micrograph of hematoxylin and eosin stained sections from allografts showing transformation in control and significant fat differentiation in sJag1 allografts (bar = 5 μm). Arrow indicates adipocytes. (E) Immunostaining indicating positive GFP staining in the allograph adipocytes and absence of GFP in the host tissue.
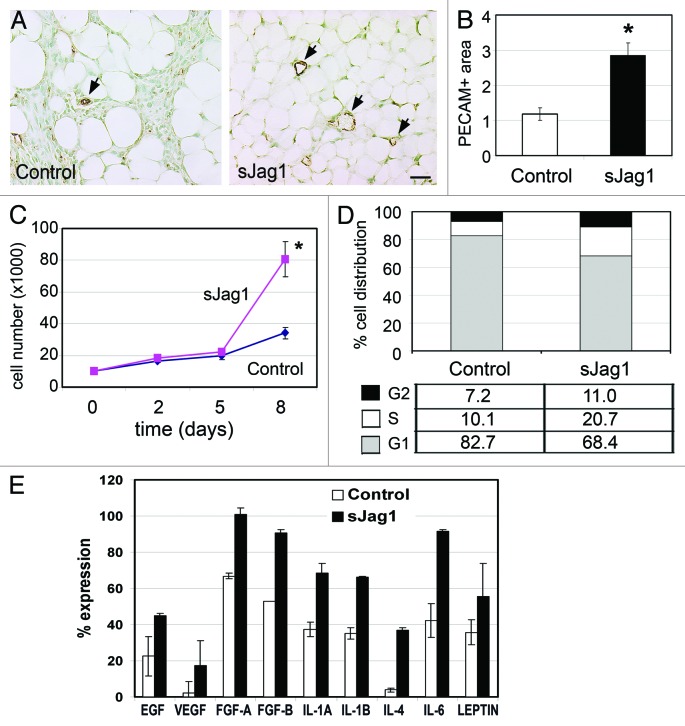
Since angiogenesis often precedes or parallels the formation of adipose tissue8 it is assumed that there is a direct link between adipogenesis and angiogenesis. We therefore used the endothelial specific PECAM staining to visualize vascularization in the fat pads that developed from the allografts (Fig. 5A). Quantification of vascular area normalized to the total fat cell area showed a 2-fold increase in vessel density in the fat pads in allografts from sJag1 cells (Fig. 5B). The vessel area normalized to the total fat pad area was 2.81 in sJag1 fat pads while the controls had 1.18, indicative of increased angiogenesis in the fat pads of sJag1 expressing allografts (Fig. 5B). This result indicates that sJag1 cells were capable of differentiating into adipocytes and form functional fat pads with neovascularization under in vivo conditions.
Figure 5. sJag1 cells promote angiogenesis and endothelial cell proliferation. (A) Representative fat pad sections from subcutaneous injections stained with PECAM to determine vessel density (bar = 50 μm). (B) The graph shows quantification of the vessel area (n = 6 tumors; 20 pictures each; graphed are means ± SD and * indicates p < 0.05). In vitro effect of secreted sJag1 on endothelial cell. (C) Growth curve analysis of HUVEC cells treated with control or sJag1 conditioned media (graphed are means ± SD and * indicates p < 0.05). (D) Cell cycle analysis showing distribution of endothelial cells at G1, S and G2 phase cultured in cell-free conditioned media from control or sJag1 3T3- L1 cells. (E) ELISA based immuno-analysis of pro-angiogenic cytokines secreted from control and sJag1 cells. The graph shows quantification of the immunoblot based on integrated densities (graphed are means ± SD).
sJag1 induces endothelial proliferation
To determine the cause of the observed increase in vessel development within the sJag1 Vs control fat pads, we analyzed the effect of conditioned media from the control and sJag1 cells on endothelial cell proliferation. Endothelial cells treated with sJag1 conditioned media from 3T3-L1 cells showed increased proliferation in vitro. Cell counts on day 8 showed significant 2-fold increase with sJag1 media over control cells (Fig. 5C). Cell cycle analysis of the endothelial cells grown in conditioned media showed differences similar to those found in the sJag1 cells. At 24 h, 68% of the sJag1 conditioned medium-treated endothelial cells were in G1 while the control treated cells had 82% of the population in G1 (Fig. 5D). This change in cell cycle progression indicates that the secreted proteins produced by the sJag1-expressing cells positively influenced endothelial cell growth and proliferation.
To further understand this paracrine signaling, the cytokines secreted by transfected preadipocyte cells were quantified using an ELISA based blot assay. Analysis revealed that there was a general increase in cytokines in conditioned media from sJag1 expressing cells. Quantification based on integrated intensities showed a ~2-fold increase in IL-1A, IL-1B, IL-4, IL-6, EGF, VEGF, FGF-A (FGF1) and FGF-B (FGF2) in sJag1 conditioned media over controls (Fig. 5E). Together, these results suggest that the proliferative effect of sJag1-mediated inhibition of Notch signaling on preadipocytes and endothelial cells may attribute to marked increase in the production of cytokines.
Discussion
The primary goal of this study was to determine the functional role of Notch signaling in adipocyte differentiation. We used the well-established mouse embryonic fibroblast-derived preadipocyte cell line 3T3-L1 to test our hypothesis that suppression of Notch signaling would alter adipogenic differentiation in vitro and in vivo. The role of Notch signaling during adipogenesis is controversial, with data suggesting activities in either promoting or suppressing adipogenesis.28-31,40-42 These in vitro studies support the hypothesis that Notch signaling has a dual role in adipogenesis and that its activity must be tightly controlled. Furthermore, non-canonical Notch signaling through Delta-like 1 (DLK-1) is implicated in regulating adipocyte differentiation.24,34,43,44 While regulation of the Notch signal and its influence on the adipogenic program are still not completely understood, Notch signaling dynamics further increase the complexity of Notch involvement by the multiple ligand-receptor mediated activation and cell type specificity. In this study, we used the previously described35 preferential inhibition of Notch signaling via expression of dominant negative soluble form of the Jagged1 ligand (sJag1) to demonstrate changes in growth and differentiation characteristics in 3T3-L1 cells. The results from this study are not entirely unexpected and are similar to the previous report on sJag1 expression in 3T3 fibroblasts where it was shown that expression of soluble form of Notch ligands Jagged1 (sJag1) and Delta-like1 (sDl1) in NIH3T3 fibroblasts causes cell transformation, increased growth rate and tumors in vivo.18 In smooth muscle cells24 and chondrocytes,25 sJag1 expression has an inhibitory effect on cell proliferation and migration, indicating cell specific effects of sJag1. As in NIH3T3 fibroblast, sJag1 has a similar proliferative effect on 3T3-L1 cells, also a fibroblast derivative. The increased proliferation rate correlated with changes occurring in cell cycle regulation and progression with upregulation of cyclin D3. sJag1 expressing cells do not exit cell cycle even upon serum starvation, and instead are thrust into S-phase, resulting in excessive proliferation. Correspondingly, these cells do not respond to glucocorticoid-induced cell cycle arrest during differentiation, but, respond to insulin by initiating the transcription of the adipogenic regulators PPAR gamma and FABP4, albeit for a short period of time, as the cells continue to proliferate. Interestingly, cyclin D3 is also implicated in promoting adipocyte differentiation,45 which could be a contributing factor stimulating the sJag1 cells to initiate differentiation. Earlier reports have implicated Notch1 in the commitment of 3T3-L1 cells to undergo adipogenesis by controlling the expression of the principal regulators of the adipogenic program. Since impaired Notch1 expression blocks adipocyte commitment and differentiation in 3T3-L1 cells,30 it is possible that sJag1 may not completely inactivate Notch1 signaling; however, it could inhibit either partial or basal level of Notch1 activation or Notch signaling via other Notch receptors. Since Jagged1 is not the only ligand binding to the Notch receptors, we cannot rule out activation of Notch signaling by other ligands through the other Notch receptors (Notch2–Notch4). On the other hand, it possible that sJag1 is not impairing Notch1 activation, but affecting other Notch receptors (Notch2–Notch4), since gamma secretase inhibitor pretreatment promotes adipogenesis and early differentiation in adipose tissue stromal cells.24 sJag1 mediated Notch inactivation elicited a similar response as gamma secretase inhibitor by promoting cell growth, proliferation and adipogenesis and early differentiation in 3T3-L1 cells, both in vivo and in vitro. Together, our results demonstrate that inactivation of Notch signaling is required to initiate the adipocyte differentiation cascade. As continued inactivation inhibits maturation of adipocytes, it is presumable that Notch signaling is required at a later stage during maturation. Our results support the hypothesis that a coordinated and temporal Notch signaling dynamics of initial Jagged1 mediated inactivation and subsequent activation is required for complete adipocyte differentiation.
The subcutaneous injection of preadipocytes in immuno-compromised mice has been used for studying adipogenesis and angiogenesis in adult organisms.38,46 With sJag1 expressing preadipocytes, we have to consider the paracrine effect of secreted sJag1 on surrounding endothelial cells in the event of neovascularization as seen in developing adipose tissue. There is substantial evidence of cross-talk between adipocytes and endothelial cells with bidirectional signaling mediated both by extracellular matrix components and direct cell-cell interaction promoting preadipocyte differentiation.2,8,47,48 The expression of angiopoietin (1–2) and Tie (1–2) receptors and release of critical angiogenic factors by adipose tissue during its development and differentiation7,8 provides considerable proof for implicating its role in neovascularization. Increased vessel area as seen in PECAM staining of the fat pads suggests the positive dynamics of angiogenesis and adipogenesis, although it cannot be confirmed if angiogenesis precedes adipogenesis or vice versa. The pro-angiogenic behavior of the sJag1 ligand in our in vitro and in vivo studies corroborates previous reports of sJag1 promoting fibroblast growth factor receptor (FGFR) dependent signaling and inducing a cord-like phenotype in NIH3T3 fibroblasts, and producing large, dilated vessels in chick chorioallantoic assays.18,49,50 Although adenoviral sJag1 expression in endothelial cells did not affect proliferation and migration,51 conditioned media from sJag1 3T3-L1 cells had a proliferative effect on endothelial cells as demonstrated by the increase in secreted cytokines and endothelial cell growth rate. Adipose tissue-secreted adipokines like leptin and adiponectin signal to the vascular endothelium, stimulating angiogenic response and are therefore believed to be the functional link between adipocytes and the vasculature.52,53 Additionally, subcutaneous 3T3-F442A fat pads express high levels of leptin not attainable in cell culture.54 Two distinct features of the in vivo study are (1) the use of the analogous preadipocyte cell line 3T3-L1 while most of the previous studies have used 3T3-F442A cells to form in vivo subcutaneous fat pads8,38,55,56 and (2) use of lower number of cells (2.5 × 106 instead of 3 × 107 cells/mouse) to avoid cell overdose and limit secreted factors. Nevertheless, the two cell lines are comparable and show similar fat pad dynamics including presence of a necrotic core.8 It is important to note that 3T3-L1 cells underwent transformation in the presence or absence of matrigel, the latter being less aggressive. This raises the question of whether the presence of sJag1 in vivo prevents 3T3-L1 cells from becoming tumorigenic, possibly by favoring adipocyte differentiation. Additionally, matrigel has been shown to influence vessel development, decrease adipocyte maturation and microvessel maturation in 3T3-F442A cells, facilitating transformation to form tumors and dense connective tissue which suppress adipogenesis by physically impairing the development of capillaries.8 In the tissue perspective, the cell-cell interactions and cell-extracellular matrix play a regulatory role in cell differentiation and formation of the mature adipocytes.38 Studies have shown that influence of extracellular matrices on preadipocyte development is dependent on the source of substrate.57,58 Remodeling of the adipose tissue matrix is an essential prerequisite for creating the space for triglyceride deposition. In particular, laminin has been implicated in matrix-enhanced preadipocyte attachment and spreading, indicating a pivotal role in preadipocyte differentiation.58 Remodeling also involves a decrease in the key matrix protein fibronectin brought about by degradation by cathepsin-K, itself an adipokine. Cathepsin-K also degrades other matrix constituents such as collagen types I and II and osteonectin, which is a non-structural extracellular matrix protein modulating cell matrix interactions.59 Additionally, enhanced proliferation leads to impaired adipocyte development since proliferation is inversely related to differentiation. Thus, it becomes necessary to consider these requirements for structural modifications to support adipocyte differentiation along with the cell cycle arrest. In the context of sJag1 overexpressing preadipocytes, there is continuous proliferation in the absence of any matrix changes as seen in vitro. However, in vivo, in the presence of a complex extracellular matrix and other cell-cell interactions (specifically, the endothelial cells), there is considerable remodeling of the matrix impacting the differentiation of preadipocytes, as evidenced by the formation of fat pads. Therefore, it is imperative to consider that in vivo preadipocyte differentiation is influenced by host response as triggered by matrix remodeling and neovascularization.
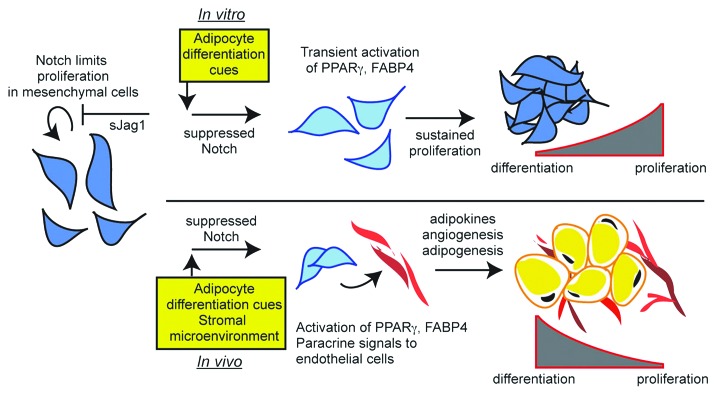
Given these complex interactions affecting in vivo differentiation of preadipocytes, we demonstrate that sJag1 cells secrete pro-angiogenic cytokines that can potentially support recruitment of endothelial cells and thereby neovascularization in the subcutaneous fat pads as represented in the schematic model (Fig. 6). In conclusion, our results confirm that (1) Notch signaling plays a role in preadipocyte cell response to proliferation and differentiation, (2) Inhibition of Notch signaling by soluble Jagged1 induces cell proliferation of preadipocytes and endothelial cells and (3) In the presence of sJag1, there is increased angiogenesis that correlates with the formation of a larger fat pad.
Figure 6. Schematic model describing sJag1 induced Notch signaling modulation of adipogenesis. sJag1 mediated inhibition of Notch signaling promotes cell growth and initiates the adipogenic cascade. Under in vivo conditions, the secreted sJag1 stimulates angiogenesis by inhibiting Notch signaling in the endothelial cells. This cross-talk potentially supports extracellular matrix remodeling and development of the composite adipose tissue.
Materials and Methods
Cell lines and culture
The stable cell lines were generated using the 3T3-L1 mouse preadipocyte cell line from ATCC (Catalog Number CL173) and transduced retrovirally to generate populations expressing the soluble and full length forms of the Notch ligands Jagged1 (Jag1). HUVEC cells (CC-2517) are from Lonza. The empty vector was used as the control. The retroviral constructs were a kind gift from Cathrin Brisken [Ecole Polytechnique Fédérale de Lausanne (EPFL); Swiss Institute for Experimental Cancer Research (ISREC), NCCR Molecular Oncology, Lausanne, Switzerland]. The cells were selected for GFP positive expression by FACS Aria (BD BioScience) sorting. The cells were maintained at low passage numbers to maintain high level of expression. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 5% penicillin/streptomycin. For differentiation, cells were stimulated at 70–80% confluence in the same media supplemented with 0.25 μM dexamethasone, 0.5 mM isobutylmethylxanthine and 166 nM insulin for 2 d. This medium was replaced with DMEM regular medium with 10% FBS and 166 nM insulin thereafter.
Characterization of the stable cell line
Morphology
Semi-confluent cells were fixed with 4% paraformaldehyde, washed with PBS and stained for 30 min with phalloidin to visualize F-actin, washed and mounted for imaging.
Cell cycle analysis
Cells were synchronized by culturing in media containing minimum serum (0.5% FBS) for 24–30 h. At time 0, fresh media with 10% FBS was added and cells collected at time intervals of 0, 3, 6, 9, 12 and 15 h, fixed in ice cold 70% ethanol, stained with propidium iodide (PI) and analyzed by flow cytometry (FACS, BD Bioscience) to determine G1, G2 and S phases. Cell cycle analysis was also done for longer intervals of time for each cell line.
Evaluation of bioactivity of conditioned media
Conditioned cell-free media from transduced stable cell populations (control and sJag1) were collected from confluent plates. The conditioned media was concentrated using vivaspin cell concentrators by centrifugation or used as such at a 1:1 concentration.
Effect on growth of endothelial cells
Primary human umbilical vein endothelial cells (HUVEC) at passage 4 or 5 were seeded at a density of 5,000 cells per well in a 24-well plate and cultured in endothelial basal media (EBM) with 10% FBS. After 12 h, the EBM medium was replaced with 50% fresh EBM medium and 50% cell-free conditioned medium harvested previously from transduced stable lines. The growth of endothelial cell was monitored by cell count for 6 d, and the data expressed as a growth curve.
Evaluation of angiogenic factors
Angiogenic factors in conditioned media were detected using an ELISA based blot assay (Panomics Inc.). Briefly, concentrated cell free conditioned media (1 mg) from the stable cell lines was added to the blot for 2 h, and binding visualized by streptavidin conjugated antibody following the protocol provided with the kit. Expression level was based on the blot intensity as quantified using image quant.
In vivo tumor allografts in immunocompromised mice
All protocols involving mice were evaluated and approved by our Institutional Animal Care and Use Committee, and performed under veterinary supervision. The in vivo model for subcutaneous tumor development in nude mice was performed as described.50 Briefly, 3T3-L1 stable lines were grown to confluence and 24 h prior to injection the medium was changed to DMEM containing 10% FBS. The transfectants were washed with PBS, harvested by trypsin digestion and resuspended in PBS. Male athymic nude mice (nu/nu) from Taconic between 6–8 weeks of age were injected subcutaneously in the right flank with 2 × 106 transfected cells in a total volume of 200 μl. Tissue growth was monitored by palpation, and the onset noted when nodules were palpable. Tissue nodule size was measured with calipers, and volume calculated assuming the shape as ellipsoid. Representative data were obtained from 5 mice/experimental group, and the entire experiment was repeated in three independent trials. Prior to collection, mice were injected subcutaneously with 200 μl of 25 mg/ml BrdU solution at 15 h and 1 h before collection. The mice were euthanized and the tissue allografts overlying skin collected 3–5 weeks after injection, depending on growth. Allografts were individually weighed, half was snap frozen for RNA and protein, and the rest fixed in 4% paraformaldehyde for histological studies.
Quantification of vessel area in allograft sections
Non-counterstained Platelet endothelial cell adhesion molecule-1 (PECAM) stained sections (five tumors per condition) were quantified for vessel area. Ten pictures of comparable regions of each tumor were taken and quantified in a blinded fashion. In Photoshop 7.0, the vessels were outlined in a transparent layer and filled in with black. The outlined vessel image was opened in Scion Image, converted to binary, threshold set to constant, and area of black pixels measured. Shown is average percentage of vessel area per allograft, and results were analyzed by Student’s t test to determine statistical significance compared with the control group.
Quantitative reverse transcriptase-PCR (qRT-PCR)
Total RNA was collected using TRI Reagent (Sigma) following the manufacturer’s protocol. RNA was reverse transcribed using oligodT in the presence of AMV reverse transcriptase to make cDNA. Successful cDNA production was verified using primers against GAPDH or β-actin. Quantitative PCR was performed using Bio-Rad (Bio Rad) cybergreen supermix and amplification on the Bio-Rad Icycler and quantified based on Ct values as described earlier.60 Primers used for PCR reactions are as follows:
sJag1 Forward primer 5′-TGCCTCTGTGAGACCAACTG-3′
Reverse primer 5′-TGGGCAACACTCACACTCAA-3′
Hrt2 Forward primer 5′-CCAGAAAAAGACGGAGAGGA-3′
Reverse primer 5′-GCGCGTCAAAGTAACCTTTC-3′
GAPDH Forward primer 5′-GCCTCAAGATCATCAGCAAT-3′
Reverse primer 5′-GGACTGTGGTCATGAGTCCT-3′
PPARγ1+2: Forward primer 5′-TCATCTCAGAGGGCCAAGGA-3′
Reverse primer 5′-CACCAAAGGGCTTCCGC-3′
FABP4: Forward primer 5′-TGGAAGCTTGTCTCCAGTGA-3′
Reverse primer 5′-AATCCCCATTTACGCTGATG-3′
Cyclophilin: Forward primer 5′-CTCGAATAAGTTTGACTTGTGTTT-3′
Reverse primer 5′-CTAGGCATGGGAGGGAACA-3′
Immunoblot analysis
Total cell lysate was prepared using cell lysis buffer consisting of 150 mM NaCl, 50 mM Tris pH = 8, 1% Triton X-100, and proteinase inhibitors. Samples were analyzed by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted as indicated. Immunoblots were visualized using chemiluminescence (Amersham). Cytokine levels in cell free conditioned medium was quantified using an ELISA based dot-blot assay (Panomics Inc.). Expression level was quantified using image quant software. The intensity of the predetermined positive controls was set at 100.
MRI and 1H spectroscopy
Axial and coronal images through the allograft area were acquired from isoflurane (1%, 0.4 l/min) anesthetized mice in a 7.0T Bruker PharmaScan magnet. A rapid acquisition method with enhanced relaxation (RARE) was used (TR 2500 ms, TE 10.64 ms, FOV 3.5 cm, matrix size 256 × 256, slice thickness 1 mm, total scan time 5 min 30 sec). The allograft dimensions were measured at the largest width, length, and height. For localized spectroscopy PRESS (TR 2000 ms, TE 21.4 ms) was used. A voxel of 1 mm3 was placed into the center of the tumor. Placement of the voxel had no influence on the spectrum. One spectrum is the sum of 800 fields with a total scan time of 26 min 40 sec.
Acknowledgments
The authors gratefully acknowledge Dr. Cathrin Brisken for the viral constructs, Marilena Preda and Dr. Ilka Pinz for mouse imaging support, Kathleen Carrier and Dr. Volkhard Lindner for histology support, and Jane Mitchell for assistance with flow cytometry. This research was supported by NIH grant R01HL070865 (to L.L), and the Mouse Transgenic and In Vivo Imaging Core (supported by NIH grant P30GM103392, R. E. Friesel, PI, Phase II COBRE in Vascular Biology). This work was also supported by the FACS and ES Cell Core (supported by NIH grant P20GM103465, D. Wojchowski, PI) and the Histopathology Core supported by NIH grants P20GM103465 and P30GM103392. B. Turner was supported by a Founders Affiliate American Heart Association summer student research fellowship.
Glossary
Abbreviations:
- C/EBP
CCAAT enhancer binding protein
- EC
endothelial cell
- EGF
epidermal growth factor
- FABP
fatty acid binding protein
- FGF
fibroblast growth factor
- IL
Interleukin
- NICD
Notch intracellular domain
- PECAM
platelet endothelial cell adhesion molecule 1
- PPAR
peroxisome proliferator-activated receptor
- sDl1
soluble Delta-like 1
- sJag1
soluble Jagged1
- VEGF
vascular endothelial growth factor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/19186
References
- 1.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–93. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 2.Aoki S, Toda S, Sakemi T, Sugihara H. Coculture of endothelial cells and mature adipocytes actively promotes immature preadipocyte development in vitro. Cell Struct Funct. 2003;28:55–60. doi: 10.1247/csf.28.55. [DOI] [PubMed] [Google Scholar]
- 3.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 4.Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, et al. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–37. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 5.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–34. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 6.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146:4545–54. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 7.Dallabrida SM, Zurakowski D, Shih SC, Smith LE, Folkman J, Moulton KS, et al. Adipose tissue growth and regression are regulated by angiopoietin-1. Biochem Biophys Res Commun. 2003;311:563–71. doi: 10.1016/j.bbrc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18:983–5. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 9.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 10.Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–57. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010;95:2476–85. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–9. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–6. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–53. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100:1556–68. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 16.Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–83. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesh DA, Park KS, Harrington A, Miceli-Libby L, Yoon JK, Liaw L. Cardiovascular and hematopoietic defects associated with Notch1 activation in embryonic Tie2-expressing populations. Circ Res. 2008;103:423–31. doi: 10.1161/CIRCRESAHA.108.177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urs S, Roudabush A, O’Neill CF, Pinz I, Prudovsky I, Kacer D, et al. Soluble forms of the Notch ligands Delta1 and Jagged1 promote in vivo tumorigenicity in NIH3T3 fibroblasts with distinct phenotypes. Am J Pathol. 2008;173:865–78. doi: 10.2353/ajpath.2008.080006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 20.Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–9. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 21.Trifonova R, Small D, Kacer D, Kovalenko D, Kolev V, Mandinova A, et al. The non-transmembrane form of Delta1, but not of Jagged1, induces normal migratory behavior accompanied by fibroblast growth factor receptor 1-dependent transformation. J Biol Chem. 2004;279:13285–8. doi: 10.1074/jbc.C300564200. [DOI] [PubMed] [Google Scholar]
- 22.Bray S, Bernard F. Notch targets and their regulation. Curr Top Dev Biol. 2010;92:253–75. doi: 10.1016/S0070-2153(10)92008-5. [DOI] [PubMed] [Google Scholar]
- 23.Krejcí A, Bernard F, Housden BE, Collins S, Bray SJ. Direct response to Notch activation: signaling crosstalk and incoherent logic. Sci Signal. 2009;2:ra1. doi: 10.1126/scisignal.2000140. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Yang X, Wu Y, Jing W, Cai X, Tang W, et al. gamma-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-gamma. Cell Prolif. 2010;43:147–56. doi: 10.1111/j.1365-2184.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SM, Jeong YH, Kim HM, Park HY, Yoon D, Kim DH, et al. Presenilin enhancer-2 (PSENEN), a component of the gamma-secretase complex, is involved in adipocyte differentiation. Domest Anim Endocrinol. 2009;37:170–80. doi: 10.1016/j.domaniend.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood) 2010;235:1185–93. doi: 10.1258/ebm.2010.010063. [DOI] [PubMed] [Google Scholar]
- 27.Ugarte F, Ryser M, Thieme S, Fierro FA, Navratiel K, Bornhäuser M, et al. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37:867–75, e1. doi: 10.1016/j.exphem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Ross DA, Hannenhalli S, Tobias JW, Cooch N, Shiekhattar R, Kadesch T. Functional analysis of Hes-1 in preadipocytes. Mol Endocrinol. 2006;20:698–705. doi: 10.1210/me.2005-0325. [DOI] [PubMed] [Google Scholar]
- 29.Ross DA, Rao PK, Kadesch T. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol Cell Biol. 2004;24:3505–13. doi: 10.1128/MCB.24.8.3505-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcés C, Ruiz-Hidalgo MJ, Font de Mora J, Park C, Miele L, Goldstein J, et al. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J Biol Chem. 1997;272:29729–34. doi: 10.1074/jbc.272.47.29729. [DOI] [PubMed] [Google Scholar]
- 31.Nichols AM, Pan Y, Herreman A, Hadland BK, De Strooper B, Kopan R, et al. Notch pathway is dispensable for adipocyte specification. Genesis. 2004;40:40–4. doi: 10.1002/gene.20061. [DOI] [PubMed] [Google Scholar]
- 32.Nueda ML, Baladrón V, Sánchez-Solana B, Ballesteros MA, Laborda J. The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J Mol Biol. 2007;367:1281–93. doi: 10.1016/j.jmb.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 33.Nueda ML, García-Ramírez JJ, Laborda J, Baladrón V. dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 cells. J Mol Biol. 2008;379:428–42. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 34.Sul HS. Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate. Mol Endocrinol. 2009;23:1717–25. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Small D, Kovalenko D, Kacer D, Liaw L, Landriscina M, Di Serio C, et al. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J Biol Chem. 2001;276:32022–30. doi: 10.1074/jbc.M100933200. [DOI] [PubMed] [Google Scholar]
- 36.Nikopoulos GN, Duarte M, Kubu CJ, Bellum S, Friesel R, Maciag T, et al. Soluble Jagged1 attenuates lateral inhibition, allowing for the clonal expansion of neural crest stem cells. Stem Cells. 2007;25:3133–42. doi: 10.1634/stemcells.2007-0327. [DOI] [PubMed] [Google Scholar]
- 37.Small D, Kovalenko D, Kacer D, Liaw L, Landriscina M, Di Serio C, et al. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J Biol Chem. 2001;276:32022–30. doi: 10.1074/jbc.M100933200. [DOI] [PubMed] [Google Scholar]
- 38.Fischbach C, Spruss T, Weiser B, Neubauer M, Becker C, Hacker M, et al. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54–64. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 39.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross DA, Kadesch T. Consequences of Notch-mediated induction of Jagged1. Exp Cell Res. 2004;296:173–82. doi: 10.1016/j.yexcr.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–9. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- 42.Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278:46107–16. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 43.Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–52. doi: 10.1359/jbmr.040118. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez P, Higueras MA, González-Rajal A, Alfranca A, Fierro-Ferńndez M, García-Ferńndez RA, et al. The non-canonical NOTCH ligand DLK1 exhibits a novel vascular role as a strong inhibitor of angiogenesis. Cardiovasc Res. 2011 doi: 10.1093/cvr/cvr296. In press. [DOI] [PubMed] [Google Scholar]
- 45.Sarruf DA, Iankova I, Abella A, Assou S, Miard S, Fajas L. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 2005;25:9985–95. doi: 10.1128/MCB.25.22.9985-9995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, et al. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varzaneh FE, Shillabeer G, Wong KL, Lau DC. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism. 1994;43:906–12. doi: 10.1016/0026-0495(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 48.Lai N, Jayaraman A, Lee K. Enhanced proliferation of human umbilical vein endothelial cells and differentiation of 3T3-L1 adipocytes in coculture. Tissue Eng Part A. 2009;15:1053–61. doi: 10.1089/ten.tea.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Small D, Kovalenko D, Soldi R, Mandinova A, Kolev V, Trifonova R, et al. Notch activation suppresses fibroblast growth factor-dependent cellular transformation. J Biol Chem. 2003;278:16405–13. doi: 10.1074/jbc.M300464200. [DOI] [PubMed] [Google Scholar]
- 50.Wong MK, Prudovsky I, Vary C, Booth C, Liaw L, Mousa S, et al. A non-transmembrane form of Jagged-1 regulates the formation of matrix-dependent chord-like structures. Biochem Biophys Res Commun. 2000;268:853–9. doi: 10.1006/bbrc.2000.2173. [DOI] [PubMed] [Google Scholar]
- 51.Caolo V, Schulten HM, Zhuang ZW, Murakami M, Wagenaar A, Verbruggen S, et al. Soluble Jagged-1 inhibits neointima formation by attenuating Notch-Herp2 signaling. Arterioscler Thromb Vasc Biol. 2011;31:1059–65. doi: 10.1161/ATVBAHA.110.217935. [DOI] [PubMed] [Google Scholar]
- 52.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 53.Huang PH, Chen JS, Tsai HY, Chen YH, Lin FY, Leu HB, et al. Globular adiponectin improves high glucose-suppressed endothelial progenitor cell function through endothelial nitric oxide synthase dependent mechanisms. J Mol Cell Cardiol. 2011;51:109–19. doi: 10.1016/j.yjmcc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci U S A. 1997;94:4300–5. doi: 10.1073/pnas.94.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979;101:169–71. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- 56.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci U S A. 1997;94:4300–5. doi: 10.1073/pnas.94.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hausman GJ, Richardson RL. Newly recruited and pre-existing preadipocytes in cultures of porcine stromal-vascular cells: morphology, expression of extracellular matrix components, and lipid accretion. J Anim Sci. 1998;76:48–60. doi: 10.2527/1998.76148x. [DOI] [PubMed] [Google Scholar]
- 58.Hausman GJ, Wright JT, Richardson RL. The influence of extracellular matrix substrata on preadipocyte development in serum-free cultures of stromal-vascular cells. J Anim Sci. 1996;74:2117–28. doi: 10.2527/1996.7492117x. [DOI] [PubMed] [Google Scholar]
- 59.Achike FI, To NH, Wang H, Kwan CY. Obesity, metabolic syndrome, adipocytes and vascular function: A holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38:1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- 60.Urs S, Venkatesh D, Tang Y, Henderson T, Yang X, Friesel RE, et al. Sprouty1 is a critical regulatory switch of mesenchymal stem cell lineage allocation. FASEB J. 2010;24:3264–73. doi: 10.1096/fj.10-155127. [DOI] [PMC free article] [PubMed] [Google Scholar]