Abstract
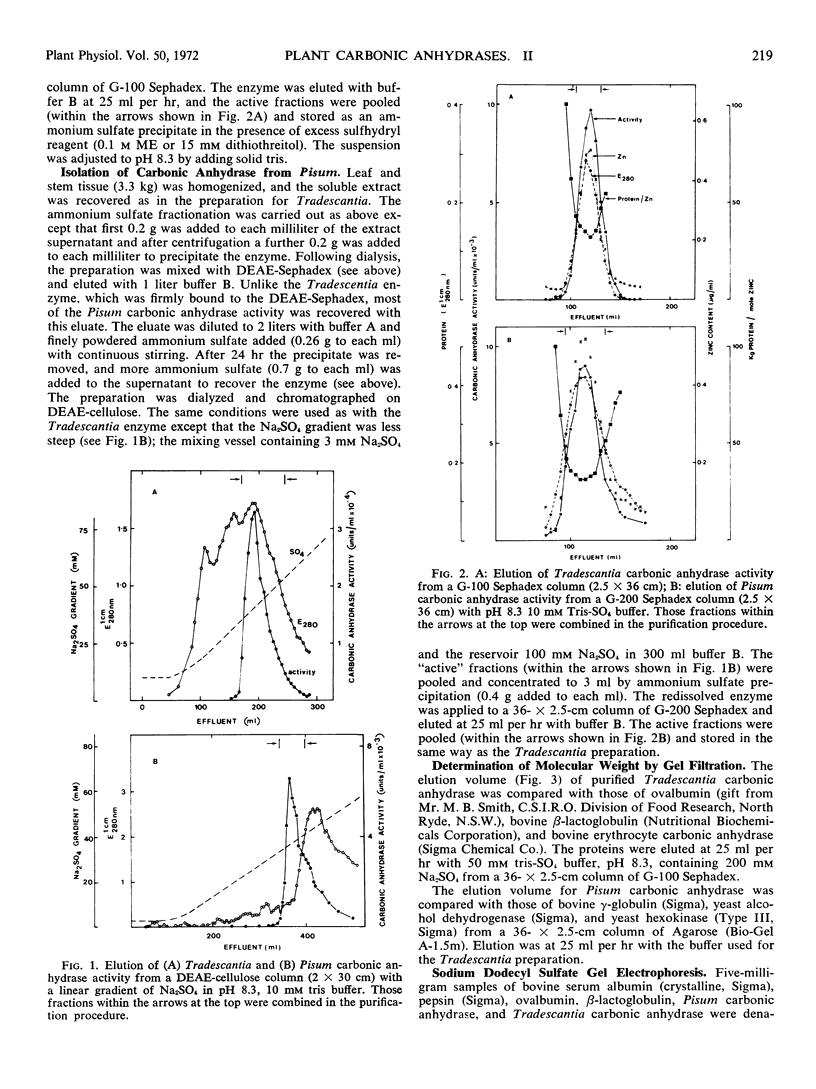
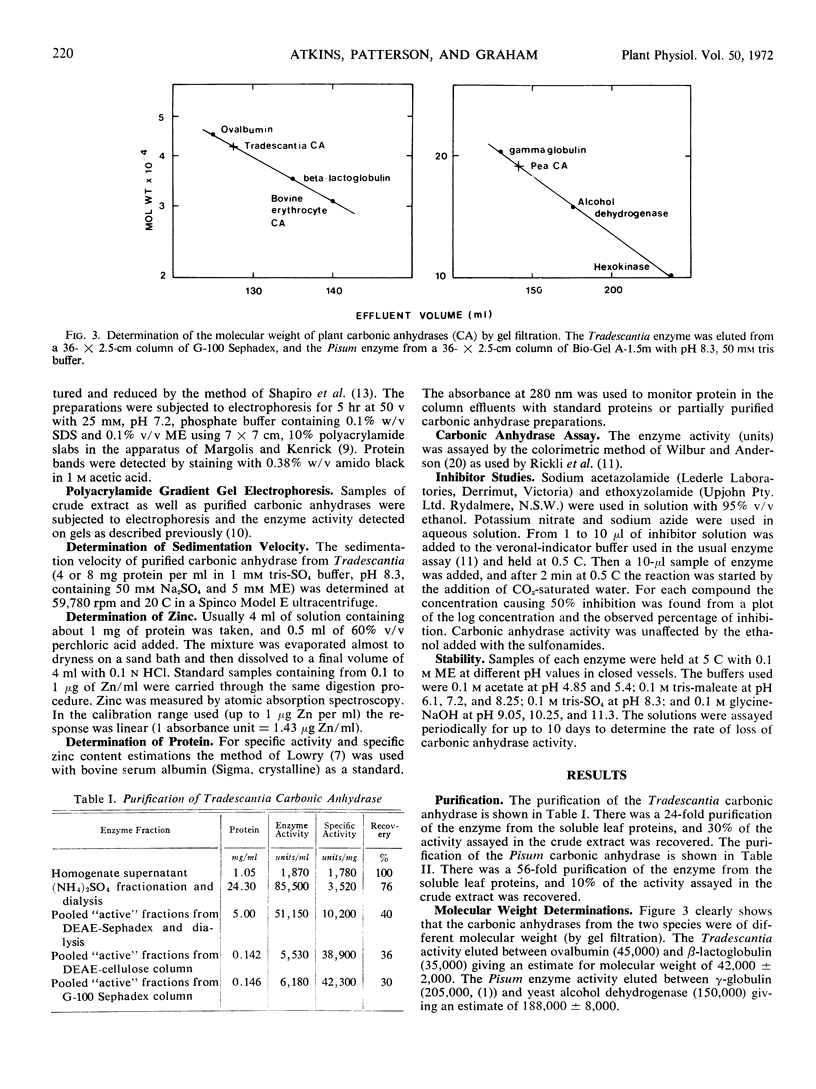
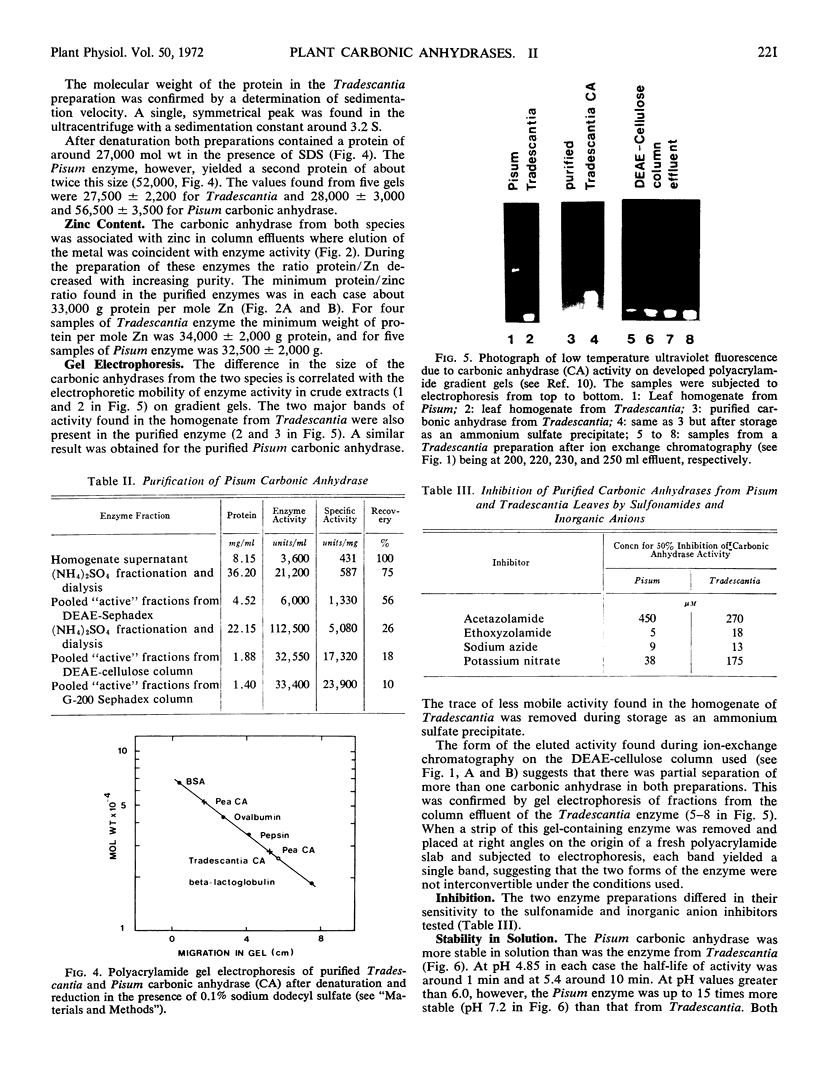
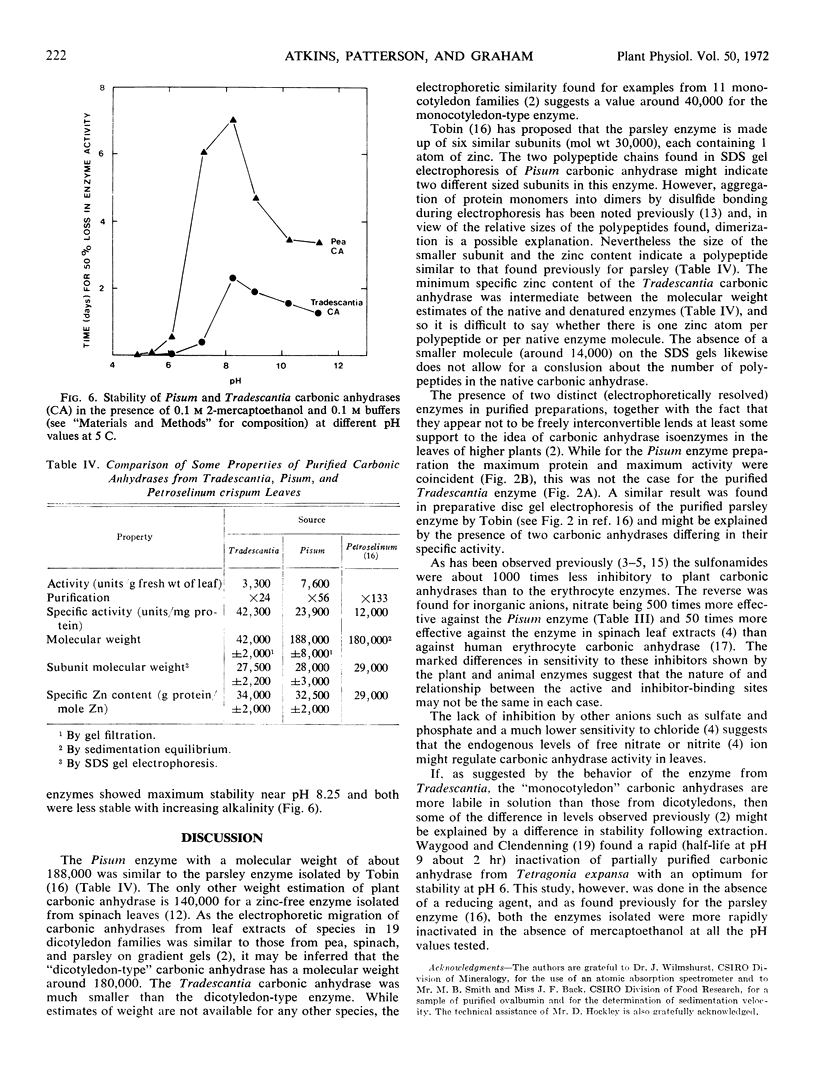
Carbonic anhydrase (EC.4.2.1.1) was purified from leaves of the dicotyledon Pisum sativum L. (56-fold) and from leaves of the monocotyledon Tradescantia albiflora Kunth. (24-fold). The molecular weight of the Pisum enzyme was estimated to be 188,000 ± 8,000 with subunit sizes of 28,000 ± 3,000 and 56,600 ± 3,500. It contained 1 mole zinc per 32,500 ± 2,000 g protein. The molecular weight of the Tradescantia enzyme was estimated to be 42,000 ± 2,000 with a subunit size of 27,500 ± 2,200. It contained 1 mole zinc per 34,000 ± 2,000 g protein. The two enzyme preparations were different in specific activity, stability in solution, and sensitivity to sulfonamides and inorganic anions. Gel electrophoresis separated each purified preparation into two active enzyme bands.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C. A., Patterson B. D., Graham D. Plant Carbonic Anhydrases: I. Distribution of Types among Species. Plant Physiol. 1972 Aug;50(2):214–217. doi: 10.1104/pp.50.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELLNER S. K. ZINC-FREE PLANT CARBONIC ANHYDRASE; LACK OF INHIBITION BY SULFONAMIDES. Biochim Biophys Acta. 1963 Sep 3;77:155–156. doi: 10.1016/0006-3002(63)90483-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Patterson B. D., Atkins C. A., Graham D., Wills R. B. Carbonic anhydrase: a new method of detection on polyacrylamide gels using low-temperature fluorescence. Anal Biochem. 1971 Dec;44(2):388–391. doi: 10.1016/0003-2697(71)90224-7. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- SIBLY P. M., WOOD J. G. The nature of carbonic anhydrase from plant sources. Aust J Sci Res B. 1951 Nov;4(4):500–510. doi: 10.1071/bi9510500. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Tobin A. J. Carbonic anhydrase from parsley leaves. J Biol Chem. 1970 May 25;245(10):2656–2666. [PubMed] [Google Scholar]
- Verpoorte J. A., Mehta S., Edsall J. T. Esterase activities of human carbonic anhydrases B and C. J Biol Chem. 1967 Sep 25;242(18):4221–4229. [PubMed] [Google Scholar]




