Abstract
In bone marrow, the differentiation of osteoblasts and adipocytes is reciprocally regulated. This inverse regulation occurs mainly through complex signaling crosstalk between transcriptional factors such as peroxisome proliferator-activated receptor-γ (PPARγ) and runt-related transcription factor 2 (Runx2). This commentary addresses the role of myeloid elf-1 like factor (MEF) and distal-less homeobox 5 (Dlx5) in the lineage commitment of bone marrow mesenchymal stem cells into adipocytes and osteoblasts, respectively. MEF suppresses osteoblastogenesis by preventing Runx2 from binding to the promoters of target genes and enhancing adipogenesis via transactivation of PPARγ expression. Conversely, Dlx5 enhances osteoblastogenesis through upregulation of the expression of Runx2 and osteoblast marker genes while suppressing adipogenesis through the downregulation of PPARγ expression by sequestering the cAMP response element binding protein and CCAAT/enhancer-binding protein α. Studies designed to examine the effects of physiological and pathologic signals on the expression of MEF and Dlx5 will provide further insight to the function of these transcription factors in vivo.
Keywords: adipocyte, osteoblast, myeloid elf-1-like factor, distal-less homeobox 5, bone marrow mesenchymal stem cells
In bone marrow, osteoblasts and adipocytes arise from a common progenitor: bone marrow mesenchymal stem cells (bMSCs). Even though bMSCs are considered multipotent, clonal analysis of bMSC populations has revealed that only 20–30% of total bMSCs are multipotent1 and the remainder of the population is a mixture of bi-potential and uni-potential MSCs.2 Two of the uni-potent populations of bMSCs are cells that are committed to either osteoblastic or adipocytic lineages.3
In several in vivo studies, a decrease in bone mass has been shown to be associated with an increase in the adipocyte cell population in the bone marrow, suggesting an inverse relationship between adipocyte differentiation and osteoblast differentiation.4 Extracellular signals that promote commitment of bMSCs to the osteoblast lineage actively suppress mechanisms that induce adipocyte lineage commitment and vice versa. There exist various regulation mechanisms involved in this lineage commitment. At transcriptional regulation level, transcription factors including myeloid elf-1 like factor (MEF),5,6 distal-less homeobox 5 (Dlx5),5-8 Wnt/β-catenin,9 meshless homeobox 2,10 secreted frizzled-related protein 111 and delta-like1/preadipocyte factor 112 are now known to reciprocally regulate bone marrow adipogenesis and osteogenesis mainly via modulation of complex crosstalk pathway between master switch genes, peroxisome proliferator-activated receptor-γ (PPARγ) and runt-related transcription factor 2 (Runx2). PPARγ, a ligand-activated transcription factor, is a master regulator of adipocyte differentiation. PPARγ knockout mice failed to generate adipose tissue, even when fed a high fat diet.13 PPARγ forms heterodimers with retinoid X receptors and regulates the transcription of various adipogenic genes. Runx2 is a master transcription factor of osteoblast differentiation and bone mineralization.14 Targeted disruption of Runx2 induced a complete lack of bone formation due to maturational arrest of osteoblasts.15 Interaction between PPARγ and Runx2 regulates the reciprocal differentiation pathways that control commitment of bMSCs to osteoblast and adipocyte lineages. PPARγ negatively regulates stromal cell plasticity by suppressing Runx2 and osteoblast-like biosynthetic activity, while promoting adipocyte differentiation.16 Heterozygous PPARγ-deficient mice exhibited a high bone mass with increased osteoblastogenesis and decreased marrow fat,17 while osteoblast-targeted overexpression of PPARγ decreased bone mass in male mice and accelerated ovariectomy-induced bone loss in female mice.18 Regulations of osteoblast/adipocyte lineage commitment at the post-transcriptional level such as microRNA (miRNA) control have been recently explored. miRNAs, are endogenous non-coding RNAs, 22–25 nucleotides in length, which bind to complementary sequences on target mRNA transcripts and post-transcriptionally regulate various biological processes.19 miRNAs can stimulate or suppress adipocyte differentiation and also regulate the lineage commitment of bMSC.20 Few miRNA targets have been experimentally verified in adipocytes. But recently it has been demonstrated that both miR-27 and miR-519d suppress adipogenesis by targeting PPAR family members21,22 whereas miR-204/211 inhibits osteogenesis and promotes adipogenesis of mesenchymal progenitor cells and bMSCs by targeting Runx2.23
In the next two sections, we will focus on the regulation of marrow adiposity at transcriptional level and especially on two transcription factors, MEF and Dlx5, which are recently demonstrated to inversely regulate osteoblast/adipocyte differentiation by acting upstream of Runx2 and PPARγ.11,12
Myeloid Elf-1 Like Factor (MEF)
MEF was originally isolated from a human megakaryocytic leukemia cell line and belongs to the Elf-1/E74 group of E-20 six (Ets) family transcription factors.24 All Ets family members encode unique transcriptional regulators that have a highly conserved DNA binding domain. Ets family transcription factors have been implicated in various biological processes, including hematopoiesis, innate immunity and extracellular matrix mineralization. Kim et al. previously demonstrated that MEF expression is highest during early differentiation of MC3T3-E1 osteoblasts and that its expression level is decreased by bone morphogenetic protein-2 (BMP2), one of the strongest inducers of osteoblast differentiation.6 MEF was shown to form a complex with Runx2 and prevent it from binding to the cis-acting element OSE2 in the osteocalcin promoter, which in turn, suppressed BMP2-induced osteocalcin expression in MC3T3-E1 osteoblasts. Osteoblast-specific MEF transgenic mice (Col1a1-MEF TG mice) exhibited decreased bone mass and higher marrow fat accumulation in long bones than their wild-type counterparts.25
Recently, we demonstrated that MEF is a positive regulator of adipocyte differentiation.5 bMSCs derived from Col1a1-MEF TG mice showed a higher adipogenic differentiation potential than those from wild-type mice. Adipogenic stimuli increased MEF expression, and knockdown of MEF suppressed adipogenic differentiation of 3T3-L1 pre-adipocytes. Forced expression of MEF in MC3T3-E1 osteoblasts induced them to secrete higher levels of 15d-PGJ2, a strong endogenous PPARγ ligand, thereby creating a microenvironment in the marrow that favors adipogenesis. Furthermore, MEF overexpression upregulated the expression of adipogenic marker genes, including PPARγ and CCAAT/enhancer-binding protein α (C/EBPα), and increased lipid droplet accumulation in MC3T3-E1 osteoblasts and 3T3-L1 pre-adipocytes. PPARγ was shown to be a target gene of MEF, which directly binds to the PPARγ promoter and enhances its transcription.
Distal-Less Homeobox 5 (Dlx5)
Bone formation is orchestrated by multiple homeodomain (HD) proteins, which are sub-grouped based on their sequences and their homeobox motifs. Dlx5 is a member of the Distal-less HD protein family and has been implicated in the commitment of mesenchymal progenitors to the osteoblast lineage through the upregulation of Runx2 and Osterix expression.7 Dlx5-deficient mice showed defects in bone formation,26 and expression of Dlx5 is rapidly induced by BMP2.27 Dlx5 also upregulates the expression of alkaline phosphatase and osteocalcin through direct binding to their respective gene promoters.28 We recently demonstrated that Dlx5 exerts an anti-adipogenic effect through downregulation of PPARγ expression and that adipogenic stimuli rapidly downregulate the expression levels of Dlx5.8 Overexpression of Dlx5 suppressed the adipogenic differentiation of bMSCs and 3T3-L1 pre-adipocytes while knockdown of Dlx5 promoted adipogenic differentiation. Overexpression of PPARγ or treatment with rosiglitazone, a PPARγ ligand, rescued adipogenic differentiation of 3T3-L1 cells overexpressing Dlx5, indicating that downregulation of PPARγ expression was a major anti-adipogenic mechanism. Dlx5 suppresses PPARγ expression by sequestering the cAMP response element binding protein (CREB) and C/EBPα, transcriptional activators of PPARγ, rather than by direct binding to the PPARγ promoter.
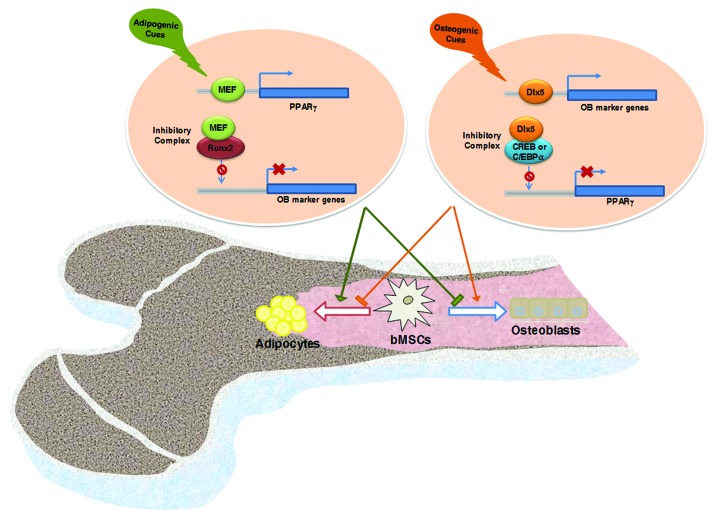
These reports indicate that in the bone marrow, MEF and Dlx5 play a role as lineage determinants for the differentiation of adipocytes and osteoblasts, respectively. As described above, extracellular osteogenic signals, such as those stemming from BMP2-activated signaling, increased the expression of Dlx5, an osteogenic transcription factor, but decreased the expression of MEF, an adipogenic transcription factor. Conversely, extracellular adipogenic signals increased the expression of MEF but suppressed the expression of Dlx5. These findings further support the concept that specific extracellular cues dictate the commitment and subsequent differentiation of bMSCs toward one lineage via suppression of the expression or functions of transcription factors in one lineage and induction of transcription factors in the other lineage. Another common theme of these transcription factor studies was that MEF and Dlx5 act as transcriptional activators of PPARγ and Runx2, respectively, by directly binding to their promoter regions, whereas they negatively regulate the function of Runx2 and the expression of PPARγ, respectively, through protein-protein interactions. Figure 1 summarizes how Dlx5, whose expression is increased by osteogenic signals, or MEF, whose expression is increased by adipogenic signaling, regulate Runx2 and PPARγ expression and function and subsequently induce osteogenic and adipogenic differentiation of bMSCs.
Figure 1. Marrow fat accumulation is closely related to bone formation. Osteoblasts and adipocytes are derived from common multipotential mesenchymal stem cells. Extracellular signals that promote the differentiation of bMSCs into adipocytes induce MEF expression. MEF then enhances adipogenesis via transactivation of PPARγ expression and suppresses osteoblastogenesis by preventing Runx2 from binding to the promoter of target genes such as osteocalcin. Extracellular signals that promote the differentiation of bMSCs into osteoblasts induce Dlx5 expression. Dlx5 then enhances osteoblastogenesis through the upregulation of Runx2 and osteoblast marker gene expression, and suppresses adipogenesis through the downregulation of PPARγ expression by sequestering CREB and C/EBPα.
Classically, bone marrow fat has been considered to be primarily a filler for the void vacated by bone and was believed to be metabolically inert. However, in light of several studies demonstrating the dynamic features of bone marrow fat, a new function for marrow adipocytes as modulators of adjacent cell activity has been suggested. Endosteal adipocytes may suppress osteoblast proliferation and differentiation by releasing adipokines, such as leptin and adiponectin, and/or by making direct contact in the bone marrow.29,30,31 Furthermore, it has been demonstrated that uni-potent bMSCs of the adipocyte lineage also negatively regulate osteogenic differentiation of neighboring bMSCs via the secretion of frizzled-related protein 1, an inhibitor of the canonical Wnt pathway.32 In contrast to reports showing an anti-osteogenic role for bone marrow adipocytes, another report has demonstrated a potentially positive role for adipocytes in bone formation. When new bone formation was triggered in skeletal muscle by BMP2 injection, accumulation of brown-like adipocytes was observed transiently, followed by heterotopic bone formation, suggesting that brown-like adipocytes may stimulate bone formation.33 Considering that the primary role of brown fat is thermogenesis, brown-like adipocytes may provide energy to the neighboring bone-forming microenvironment. Recently, it was demonstrated that bone marrow fat expresses genetic markers of brown adipocytes, including Prdm16 (PR domain containing 16) and Ppargc1a (peroxisome proliferative activated receptor, gamma, coactivator 1 α), at levels characteristic of brown adipose tissue and that the expression levels of brown adipocyte marker genes decrease in the bones of aged and diabetic mice.34 Considering that aging and diabetes are representative risk factors for decreased bone formation and increased bone fat,4 the question arises as to whether aging and diabetes induce these changes in the characteristics of bone marrow adipocytes. Whether the transcription factors for white adipogenesis would also positively regulate the brown adipogenesis is yet to be known. That is, it is uncertain how MEF or Dlx5 effect on the lineage commitment of bMSCs into brown adipocytes. Further research is warranted to determine the exact nature of bone marrow fat and marrow adiposity. Investigations into the physiological significance of bone marrow infiltration with adipocytes, particularly with respect to growth/aging, and the exact roles of locally secreted hormones and adipokines will permit a better understanding of the physiological and pathologic roles of bone marrow fat.
In conclusion, we present here recent evidence demonstrating that the transcription factors MEF and Dlx5 inversely regulate osteoblast and adipocyte differentiation upstream of Runx2 and PPARγ in bMSCs. The physiological and molecular changes that occur in association with aging, diet restriction or high fat diets, diabetes, disuse and/or sympathetic nervous system activation are known to increase bone marrow fat deposition. Further research regarding the effects of these risk factors on the expression of MEF and Dlx5 is necessary. In addition, further investigation into the nature of adipocytes, brown-like or white, whose levels are increased in bone marrow by aging, obesity, diabetes and disuse, would provide insight into the function of bone fat under these osteopenic conditions.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2009-0068779, 2011-0016548).
Glossary
Abbreviations:
- BMP2
bone morphogenetic protein-2
- bMSCs
bone marrow mesenchymal stem cells
- C/EBPα
CCAAT/enhancer-binding protein α
- CREB
cAMP response element binding protein
- Dlx5
distal-less homeobox 5
- HD
homeodomain
- MEF
myeloid elf-1 like factor
- miRNA
microRNA
- PPARγ
peroxisome proliferator-activated receptor-γ
- Runx2
runt-related transcription factor 2
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/22019
References
- 1.Larsen KH, Frederiksen CM, Burns JS, Abdallah BM, Kassem M. Identifying a molecular phenotype for bone marrow stromal cells with in vivo bone-forming capacity. J Bone Miner Res. 2010;25:796–808. doi: 10.1359/jbmr.091018. [DOI] [PubMed] [Google Scholar]
- 2.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–98. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 3.Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43:32–9. doi: 10.1016/j.bone.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–24. doi: 10.1615/CritRevEukarGeneExpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek K, Cho JY, Hwang HR, Kwon A, Lee HL, Park HJ, et al. Myeloid Elf-1-like factor stimulates adipogenic differentiation through the induction of peroxisome proliferator-activated receptor γ expression in bone marrow. J Cell Physiol. 2012;227:3603–12. doi: 10.1002/jcp.24064. [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Kim BG, Lee SJ, Lee HK, Lee SH, Ryoo HM, et al. The suppressive effect of myeloid Elf-1-like factor (MEF) in osteogenic differentiation. J Cell Physiol. 2007;211:253–60. doi: 10.1002/jcp.20933. [DOI] [PubMed] [Google Scholar]
- 7.Holleville N, Matéos S, Bontoux M, Bollerot K, Monsoro-Burq AH. Dlx5 drives Runx2 expression and osteogenic differentiation in developing cranial suture mesenchyme. Dev Biol. 2007;304:860–74. doi: 10.1016/j.ydbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Lee HL, Woo KM, Ryoo HM, Baek JH. Distal-less homeobox 5 inhibits adipogenic differentiation through the down-regulation of peroxisome proliferator-activated receptor γ expression. J Cell Physiol. 2012;228:87–98. doi: 10.1002/jcp.24106. [DOI] [PubMed] [Google Scholar]
- 9.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 10.Ichida F, Nishimura R, Hata K, Matsubara T, Ikeda F, Hisada K, et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004;279:34015–22. doi: 10.1074/jbc.M403621200. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone. 2012;50:540–5. doi: 10.1016/j.bone.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–52. doi: 10.1359/jbmr.040118. [DOI] [PubMed] [Google Scholar]
- 13.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005;102:6207–12. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/S0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 15.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. doi: 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 16.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–71. doi: 10.1002/(SICI)1097-4644(19990901)74:3<357::AID-JCB5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho SW, Yang JY, Her SJ, Choi HJ, Jung JY, Sun HJ, et al. Osteoblast-targeted overexpression of PPARγ inhibited bone mass gain in male mice and accelerated ovariectomy-induced bone loss in female mice. J Bone Miner Res. 2011;26:1939–52. doi: 10.1002/jbmr.366. [DOI] [PubMed] [Google Scholar]
- 19.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 20.McGregor RA, Choi MS. microRNAs in the regulation of adipogenesis and obesity. Curr Mol Med. 2011;11:304–16. doi: 10.2174/156652411795677990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Q, Gao ZG, Alarcon RM, Ye JP, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009;276:2348–58. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, et al. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun. 2009;390:247–51. doi: 10.1016/j.bbrc.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–64. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki Y, Sun X, Uchida H, Zhang J, Nimer S. MEF, a novel transcription factor with an Elf-1 like DNA binding domain but distinct transcriptional activating properties. Oncogene. 1996;13:1721–9. [PubMed] [Google Scholar]
- 25.Seul KJ, Cho HS, Heo SH, Baek WY, Kim JE, Park EK, et al. Osteoblast-specific expression of MEF induces osteopenia through downregulation of osteoblastogenesis and upregulation of osteoclastogenesis. J Bone Miner Res. 2011;26:341–50. doi: 10.1002/jbmr.208. [DOI] [PubMed] [Google Scholar]
- 26.Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan MQ, Tare RS, Lee SH, Mandeville M, Morasso MI, Javed A, et al. BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network. J Biol Chem. 2006;281:40515–26. doi: 10.1074/jbc.M604508200. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Lee MH, Wozney JM, Cho JY, Ryoo HM. Bone morphogenetic protein-2-induced alkaline phosphatase expression is stimulated by Dlx5 and repressed by Msx2. J Biol Chem. 2004;279:50773–80. doi: 10.1074/jbc.M404145200. [DOI] [PubMed] [Google Scholar]
- 29.Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26:485–9. doi: 10.1016/S8756-3282(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 30.Benayahu D, Zipori D, Wientroub S. Marrow adipocytes regulate growth and differentiation of osteoblasts. Biochem Biophys Res Commun. 1993;197:1245–52. doi: 10.1006/bbrc.1993.2611. [DOI] [PubMed] [Google Scholar]
- 31.Pun S, Dearden RL, Ratkus AM, Liang H, Wronski TJ. Decreased bone anabolic effect of basic fibroblast growth factor at fatty marrow sites in ovariectomized rats. Bone. 2001;28:220–6. doi: 10.1016/S8756-3282(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 32.Taipaleenmäki H, Abdallah BM, AlDahmash A, Säämänen AM, Kassem M. Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations. Exp Cell Res. 2011;317:745–56. doi: 10.1016/j.yexcr.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–32. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–52. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]