Abstract
Obesity is associated with a low-grade, chronic inflammation that promotes the development of a variety of diseases, most notably type 2 diabetes. A number of cell types of the innate and adaptive immune systems have been implicated in this process. Recent studies have focused on the role of natural killer T (NKT) cells, a subset of T lymphocytes that react with lipids, in the development of obesity-associated diseases. These studies have shown that invariant NKT (iNKT) cells, a population of NKT cells expressing a semi-invariant T cell receptor, become rapidly activated in response to lipid excess, and that these cells influence the capacity of other leukocytes to produce cytokines during the progression of obesity. The role of NKT cells in obesity-associated inflammation and insulin resistance has been investigated using NKT cell-deficient animals, adoptive transfer of NKT cells and an iNKT cell agonist. While divergent results have been obtained, it is now clear that NKT cells can modulate the inflammatory milieu in obesity, suggesting that these cells could be targeted for therapeutic intervention in obesity-associated diseases.
Keywords: CD1d, diabetes, immunotherapy, inflammation, insulin resistance, lipid, metabolic disease, natural killer T cells, obesity
Introduction
Approximately 30% of the adult population in the United States is obese, and this obesity epidemic has also spread to children. Obesity is associated with susceptibility to a variety of diseases, including type 2 diabetes, non-alcoholic fatty liver disease, cardiovascular disease, airway disease and cancer, resulting in reduced life expectancy. A common denominator that links these maladies to obesity is inflammation (for a review, see refs. 1–5). Inflammation represents the response of the host to pathogenic insults or tissue injury. Obesity is associated with a chronic low-grade inflammation, now referred to as “metainflammation,” in metabolically active tissues such as adipose, liver and muscle, as well as pancreas and brain. While the signals that initiate these inflammatory processes remain unclear, emerging evidence suggests that nutrients and the modifications they cause in adipose tissue during situations of nutritional overload can activate immune sensors. Activation of immune sensors such as the Toll-like receptors (TLRs) in obese tissues results in the induction of inflammatory kinases and transcription factors, followed by production of pro-inflammatory cytokines. In turn, these cytokines may interfere with insulin signaling pathways in a variety of cell types, resulting in insulin resistance, which might ultimately progress to type 2 diabetes.
A variety of cell types of the innate and adaptive immune systems have been implicated in regulating the inflammatory process during obesity (for a review, see refs. 1–5). Much research has focused on macrophages, which can adopt a pro-inflammatory M1 or an anti-inflammatory M2 phenotype and, thus, contribute either positively or negatively to the inflammatory process during obesity. Other innate cell types such as neutrophils and mast cells have been implicated in promoting inflammation and insulin resistance during obesity, whereas eosinophils and myeloid-derived suppressor cells have been suggested to play a suppressive role. Cells of the adaptive immune response are also involved, with B cells, CD8+ T lymphocytes and T helper type 1 (Th1)-polarized CD4+ T cells playing a pathogenic role, and CD4+CD25+ regulatory T cells playing a suppressive role. Recent studies have focused on another regulatory T cell subset, natural killer T (NKT) cells, in the development of obesity-associated inflammation and diseases.
Natural Killer T Cells
NKT cells are a group of T lymphocytes that react with lipid antigens bound with the antigen-presenting molecule CD1d, which is expressed by hematopoietic cells, such as macrophages, dendritic cells and B cells, as well as by other cell types such as hepatocytes (for a review, see refs. 6–11). NKT cells also express several surface markers such as NK1.1 that are characteristic of the natural killer (NK) cell lineage, which belongs to the innate arm of the immune system. Two subpopulations of NKT cells have been identified that differ in their lipid antigen-specificity and functions.12 Type 1 or invariant NKT (iNKT) cells express T cell receptors (TCRs) with an invariant α chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans), whereas Type 2 or variant NKT (vNKT) cells express more diverse TCRs. Hence, both of these cell types are absent in CD1d-deficient mice, whereas Jα18-deficient mice only lack iNKT cells. iNKT cells are also distinguished from vNKT cells by their reactivity with α-galactosylceramide (α-GalCer), a glycosphingolipid derived from bacteria associated with a marine sponge.13 Consequently, iNKT cells can be identified most reliably by staining with α-GalCer-loaded recombinant CD1d molecules (i.e., CD1d/α-GalCer-tetramers or -dimers).12 iNKT cells also react with lipid antigens derived from several pathogenic bacteria (e.g., Borrelia burgdorferi, Helicobacter pylori and Streptoccoccus spp) and with endogenous lipids such as isoglobotrihexosylceramide (iGb3) and β-glucosylceramide (β-GluCer).11 The antigen-specificity of vNKT cells is less clear, although many of these cells can react with the β-linked glycolipid sulfatide. NKT cells can contribute to a variety of diseases, including infections, cancer, autoimmunity, transplant rejection, airway disease and other inflammatory conditions. Although most of these studies have focused on iNKT cells, vNKT cells can contribute to some of these maladies as well, often exhibiting opposing effects to iNKT cells.14 Because of their reactivity with lipids, their capacity to produce a variety of both pro- and anti-inflammatory cytokines, and their abundance in metabolically active organs, recent studies have investigated the role of NKT cells in the development of obesity-related inflammation and insulin resistance.
Functional Status of NKT Cells in Metabolically Active Organs During Obesity
NKT cells are abundant in the liver15 and white adipose tissue,16 two organs that play a critical role in the development of metainflammation. Several earlier studies, mostly employing surrogate markers for NKT cells, reported that hepatic NKT cells in mice on a high-fat diet (HFD), or in leptin-deficient ob/ob mice on a normal chow diet, gradually decline in numbers.17-20 This finding was subsequently confirmed for iNKT cells, using α-GalCer/CD1d-tetramers to specifically identify this NKT cell subset in liver of wild-type C57BL/6 mice fed with a HFD, and in ob/ob or leptin receptor-deficient db/db mice fed with a normal chow diet.21,22 Similar results were obtained for adipose tissue,22 although only a partial loss of iNKT cells was observed in some studies.21 Furthermore, it was found that within several days on a HFD, iNKT cells showed evidence of activation,21,23 as demonstrated by early but transient expansion of this cell population, and increased expression of the activation marker ICOS (inducible costimulatory molecule) on these cells, concomitant with a reduction in the expression of the NK cell marker NK1.1. These alterations in iNKT cells, particularly in the white adipose tissue, occurred before significant increases in macrophages and CD8+ T cells, two cell types that play a pathogenic role in the development of obesity-associated inflammation and insulin resistance, were observed.21 These findings therefore suggested that iNKT cells are one of the first cell types that become activated in response to nutrient lipid excess.
Several research groups analyzed the functional properties of iNKT cells in obesity.21,22,24,25 Although iNKT cells in adipose tissue were able to produce a variety of pro- and anti-inflammatory cytokines, divergent results were obtained regarding the profile of cytokines produced by iNKT cells during the progression of obesity. Some research groups reported that iNKT cells from adipose tissue, as compared with iNKT cells from spleen and liver, exhibit a Th2-biased cytokine profile.22,24 Another group of investigators further reported that the cytokine profile of iNKT cells becomes progressively biased toward Th2 cytokine production in obese mice.25 However, another study reported that iNKT cells gradually acquire increased capacity to secrete both pro- and anti-inflammatory cytokines during the progression of obesity.21 In turn, these alterations in iNKT cells, regardless of their nature, correlated with the increased pro-inflammatory environment in liver and white adipose tissue.
Collectively, these findings suggested that iNKT cells are one of the first cell types that become activated in response to lipid excess, and that their chronic stimulation gradually modifies their functional properties, which contributes to the overall cytokine environment in metabolically active organs.
Although it is clear that iNKT cells become activated in response to lipid excess, mechanisms involved remain unknown. Based on studies that have investigated the response of iNKT cells to various microorganisms that lack cognate iNKT cell antigens (for a review, see refs. 10 and 11), we favor the idea that nutrient lipids can activate immune sensors on antigen-presenting cells, resulting in cytokine secretion and alterations in the endogenous lipid pool that is available for binding with CD1d molecules (Fig. 1). Additional studies will be needed to investigate this and other possible mechanisms by which NKT cells become activated during situations of nutrient excess.
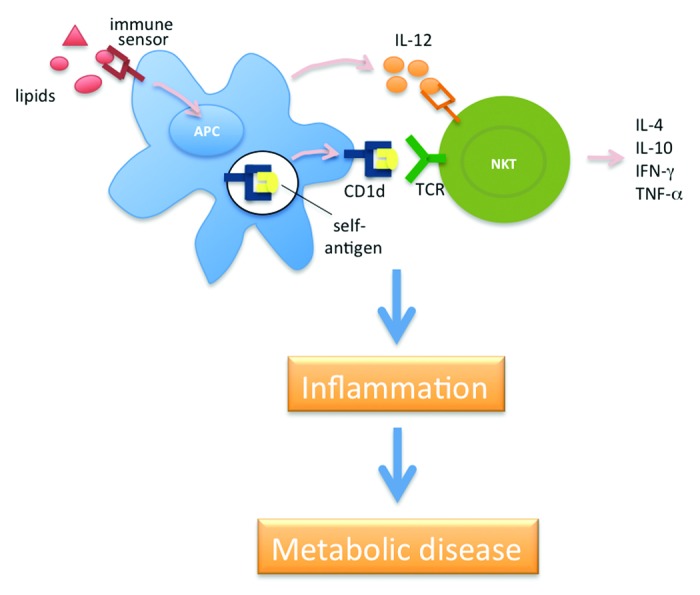
Figure 1. Proposed role of NKT cells in obesity-associated inflammation and metabolic diseases. In this model, nutrient lipids activate immune sensors expressed on antigen-presenting cells (APCs), inducing these cells to produce cytokines such as IL-12, and to induce the synthesis of NKT cell antigens. The pro-inflammatory cytokines subsequently synergize with the CD1d-bound lipid antigens to activate NKT cells. In turn, the NKT cells produce cytokines such as IL-4, IL-10, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) and contribute to the overall inflammatory milieu, which influences the development of insulin resistance and other metabolic maladies.
Role of NKT Cells in Metainflammation and Obesity-Associated Insulin Resistance
Earlier studies using adoptive transfer of NK1.1-expressing T cells suggested a suppressive role of NKT cells in the generation of obesity-associated insulin resistance (Table 1).20,26 However, a subsequent study argued for a pathogenic role of NKT cells in this process, using β2-microglobulin-deficient mice,27 which lack not only NKT cells but also other T cell subsets such as CD8+ T cells that play a pathogenic role in obesity-associated inflammation. To investigate the role of NKT cells in the development of obesity-associated diseases more directly, several research groups employed models of selective NKT cell-deficiency. This included CD1d-deficient mice, which lack both iNKT and vNKT cells, and Jα18-deficient mice, which only lack iNKT cells. These studies were performed with both diet-induced and genetic models of obesity. While most studies showed that NKT cell-deficiency only has minor effects on the development of obesity, food uptake, energy expenditure and blood lipid levels,21,24,28-31 one group of investigators found that NKT cell-deficient animals gained more weight than their wild-type controls when fed either a high-fat or low-fat diet.22 Widely divergent results have been obtained for the effects of NKT cell-deficiency on the development of obesity-associated fatty liver and insulin resistance (Table 1). Some of these studies found a pathogenic role of NKT cells,21,30 whereas others found no significant role24,28,29,31 or evidence for disease amelioration.22,23 One study further suggested that NKT cells played a protective role in the development of insulin resistance in lean animals.24 In most of these studies, similar results were obtained for CD1d- and Jα18-deficient mice, pointing toward iNKT cells as the main contributors. However, in one study, reduced insulin resistance was observed in CD1d-deficient but not Jα18-deficient mice, as compared with wild-type animals,30 suggesting that vNKT cells might contribute to obesity-associated disease as well.
Table 1. Effect of NKT cells on insulin resistance reported in different studies.
| Study* | Mice† | Diet‡ | Manipulation§ | Effect on insulin resistance|| |
|---|---|---|---|---|
| Elinav et al.20 |
ob/ob |
chow |
Transfer of B6 NK1.1+CD3+ cells, then 12 d rest |
amelioration |
| Margalit et al.32 |
ob/ob |
chow |
β-GluCer, chronic (daily) |
amelioration |
| Ma et al.26 |
B6 |
HFD |
Transfer of B6 NK1.1+TCR+ cells, then 4 d rest |
amelioration |
| Ohmura et al.27 |
β2m KO |
HFD |
- |
amelioration |
| |
B6 |
HFD |
α-GalCer, single, then 8 d rest |
exacerbation |
| |
ob/ob |
chow |
α-GalCer, single, then 8 d rest |
no effect |
| Mantell et al.28 |
CD1d KO |
HFD |
- |
no effect |
| Kotas et al.29 |
CD1d KO |
HFD |
- |
mild exacerbation |
| |
Jα18 KO |
HFD |
- |
no effect |
| |
CD1d KO |
chow |
- |
no effect |
| Satoh et al.30 |
CD1d KO |
HFD |
- |
amelioration |
| |
Jα18 KO |
HFD |
- |
no effect |
| |
CD1d KO |
HFD |
Transfer of Jα18 KO HMNC, then 14 week HFD |
exacerbation |
| |
CD1d KO |
LFD |
- |
no effect |
| |
Jα18 KO |
LFD |
- |
no effect |
| |
B6 |
HFD |
α-GalCer, chronic, then 1 wk rest |
exacerbation |
| Wu et al.21 |
CD1d KO |
HFD |
- |
amelioration |
| |
Jα18 KO |
HFD |
- |
amelioration |
| |
db/db;CD1d KO |
chow |
- |
amelioration |
| |
db/db;Jα18 KO |
chow |
- |
amelioration |
| |
B6 |
HFD |
α-GalCer, chronic, then 2 week rest |
exacerbation |
| Ji et al.31 |
CD1d KO |
HFD |
- |
no effect |
| |
B6 |
HFD |
α-GalCer, day 0 and 2, then 2 d rest |
amelioration |
| Ji et al.23 |
CD1d KO |
HFD, 4 d |
- |
exacerbation |
| Schipper et al.24 |
CD1d KO |
HFD |
- |
mild exacerbation |
| |
Jα18 KO |
HFD |
- |
mild exacerbation |
| |
CD1d KO |
LFD |
- |
exacerbation |
| |
Jα18 KO |
LFD |
- |
exacerbation |
| |
B6 |
LFD |
NK1.1+ cell depletion |
exacerbation |
| |
B6 |
LFD |
α-GalCer, single, then 3 d rest |
no effect |
| Lynch et al.22 |
CD1d KO |
HFD |
- |
exacerbation |
| |
Jα18 KO |
HFD |
- |
exacerbation |
| |
CD1d KO |
LFD |
- |
exacerbation |
| |
Jα18 KO |
LFD |
- |
exacerbation |
| |
Jα18 KO |
HFD |
transfer of B6 hepatic iNKT cells, then 4 d rest |
amelioration |
| B6 | HFD | α-GalCer, single, then 4 d rest | amelioration |
Individual studies are listed in chronological order of publication date. †All mice employed were on a C57BL/6 (B6) background. ‡Although diets are listed here as high-fat diet (HFD) and low-fat diet (LFD), these differed in their fat content and source, as well as other nutritional components. §For details about the different manipulations, including dosing and timing of treatments, please see the original studies. ||The effects listed are as compared with control mice, which are either wild-type, db/db, untreated or treated with PBS, vehicle or isotype antibody. Diverse measurements of insulin resistance or glucose intolerance have been employed in these studies and only the main outcomes, as reported by the authors of the original studies, are listed. Abbreviations: -, none; α-GalCer, α-galactosylceramide; β-GluCer, β-glucosylceramide; β2m, β2-microglobulin; HMNC, hepatic mononuclear cells; KO, knockout; TCR, T cell receptor.
To complement these studies with a gain-of-function approach, several groups treated mice with the iNKT cell-specific agonist α-GalCer during the development of diet-induced obesity (Table 1). Again, divergent results were obtained, with studies reporting disease exacerbation,21,27,30 no effect24,27 or disease amelioration.22,31 Yet another study reported that β-GluCer ameliorated insulin-resistance in ob/ob mice, possibly by antagonizing iNKT cell functions.32
When investigated, mechanistic studies revealed that the effects of NKT cells on obesity-associated disease correlated with alterations in macrophage accumulation, macrophage polarization and levels of pro-inflammatory cytokines in liver and white adipose tissue, and in the size of adipocytes and levels of the adipokines leptin and adiponectin in white adipose tissue.21-24,27,30,31
Although precise reasons remain unclear, a variety of factors might explain these divergent effects of NKT cells and their subsets on obesity-associated inflammation and disease. Possible contributing factors include differences in the genetic backgrounds of the animals employed, diet compositions, feeding durations, experimental procedures such as the protocol for α-GalCer treatment, as well as the endogenous microbiota that are present in the animal facilities where the mice were housed. With respect to the latter possibility, it is interesting to note that NKT cells can influence microbial colonization in the gut33 and, conversely, that the normal microbiota can impact NKT cell numbers and functions.34-36 In this context, it has been suggested that NKT cells can contribute to the protective effects of probiotics on obesity-associated inflammation and glucose intolerance.26
Concluding Remarks
The studies discussed here have provided strong evidence that NKT cells are one of the first cell types that become activated in response to nutritional lipid excess. These cells can contribute to the low-grade inflammation that is associated with obesity and influence the development of metabolic diseases (Fig. 1; Table 1). Outstanding questions in this field include the mechanisms that activate NKT cells during lipid excess and the effects of the endogenous microbiota on the functional status of NKT cells that impacts their role in the development of metainflammation and metabolic diseases. Collectively, these studies place NKT cells within the complex network that links nutrient excess to inflammation in obesity. These findings also suggest NKT cells as potential therapeutic targets for obesity-associated disorders. NKT cells could be targeted by lipid antigens, TCR antagonists, blocking or activating CD1d antibodies or NKT cell-depleting antibodies.
Acknowledgments
We thank Drs Sebastian Joyce (Vanderbilt University School of Medicine) and Kazuya Iwabuchi (Hokkaido University) for helpful discussions. Work in the authors’ laboratory was supported by grants from the National Institutes of Health and the American Diabetes Association.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/22296
References
- 1.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–55. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 5.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 7.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 8.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 10.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–64. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Van Kaer L, Parekh VV, Wu L. iNKT cells as sensors and managers of inflammation. Trends Immunol. 2012 doi: 10.1016/j.it.2012.08.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 13.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 14.Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218:246–50. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24:364–9. doi: 10.1016/S1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 16.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893–901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–41. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–5. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Jhaveri R, Huang J, Qi Y, Diehl AM. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest. 2007;87:927–37. doi: 10.1038/labinvest.3700603. [DOI] [PubMed] [Google Scholar]
- 20.Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, et al. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121–8. doi: 10.1002/path.1950. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA. 2012;109:E1143–52. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–87. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Y, Sun S, Xia S, Yang L, Li X, Qi L. Short term high fat diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem. 2012;287:24378–86. doi: 10.1074/jbc.M112.371807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–54. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki Y, Iwabuchi K, Iwata D, Miyazaki A, Kon Y, Niino M, et al. Effect of high fat diet on NKT cell function and NKT cell-mediated regulation of Th1 responses. Scand J Immunol. 2008;67:230–7. doi: 10.1111/j.1365-3083.2007.02062.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–30. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–9. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 28.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O’Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS One. 2011;6:e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotas ME, Lee HY, Gillum MP, Annicelli C, Guigni BA, Shulman GI, et al. Impact of CD1d deficiency on metabolism. PLoS One. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One. 2012;7:e30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, et al. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–71. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margalit M, Shalev Z, Pappo O, Sklair-Levy M, Alper R, Gomori M, et al. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther. 2006;319:105–10. doi: 10.1124/jpet.106.104950. [DOI] [PubMed] [Google Scholar]
- 33.Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–50. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei B, Wingender G, Fujiwara D, Chen DY, McPherson M, Brewer S, et al. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J Immunol. 2010;184:1218–26. doi: 10.4049/jimmunol.0902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143:418–28. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


