Abstract
Radiotherapy sensitizes unresponsive tumors to the antineoplastic activity of antibodies that target the inhibitory receptor CTLA-4 on T cells. One molecular mechanism accounting for this therapeutic synergy is the induction of NKG2D ligands on irradiated tumor cells. The fact that NKG2D receptors must be engaged for the elicitation of CD8+ T-cell antitumor responses has important clinical implications.
Keywords: abscopal effect, CTLA-4, ipilimumab, NKG2D, RAE-1, radiotherapy, synergy, tumor rejection
Antibodies against cytotoxic T lymphocyte-associated protein 4 (CTLA-4, also known as CD152) were the first therapeutics targeting a “checkpoint” receptor on T cells to be approved by the FDA for use in metastatic melanoma patients. While responses to anti-CTLA-4 antibodies are durable, they develop in a minority of patients,1 prompting studies to investigate the determinants of such responses and to identify agents that may synergize with this therapeutic approach.
In mice bearing poorly immunogenic tumors that do not respond to anti-CTLA-4 antibodies given as a standalone intervention, the administration of anti-CTLA-4 antibodies together with local radiotherapy induces antitumor T-cell responses that inhibit both irradiated lesions and metastases that lay outside the radiation field (abscopal effect).2,3 The clinical relevance of these data is supported by the recent observation of abscopal effects in a melanoma patient subjected to radiotherapy while progressing in the course of anti-CTLA-4 treatment.4 While ionizing radiation was known to exert multiple effects that promote tumor immunogenicity,5 the mechanisms accounting for its synergistic interaction with anti-CTLA-4 antibodies were not fully understood.
We have recently explored these aspects focusing on the effector phase of antitumor immune responses.6 For this purpose, we used intravital microscopy and a well-characterized mouse model of poorly immunogenic breast cancer,2 which allows for the comparison of T-cell behavior within tumors that respond, or not, to anti-CTLA-4 antibodies. We studied the endogenous repertoire of activated CD8+ tumor-infiltrating lymphocytes (TILs) by exploiting their expression of green fluorescent protein (GFP) in Cxcr6gfp/+ mice.7 While antigen specificity could not be determined, antibodies blocking the binding of MHC class I molecules to CD8 reduced the interaction of TILs with tumor cells, consistent with a polyclonal TIL population enriched in tumor-specific CD8+ T cells. The polyclonal nature of endogenous TILs is likely to mimic the clinical reality of cancer patients undergoing anti-CTLA-4 antibody-based therapy. Hence, our model appeared to be better than others based on reporter antigens and T-cell receptor (TCR)-transgenic T cells, which mostly reflect the behavior of a single T-cell clone that usually bears a high-affinity TCR.
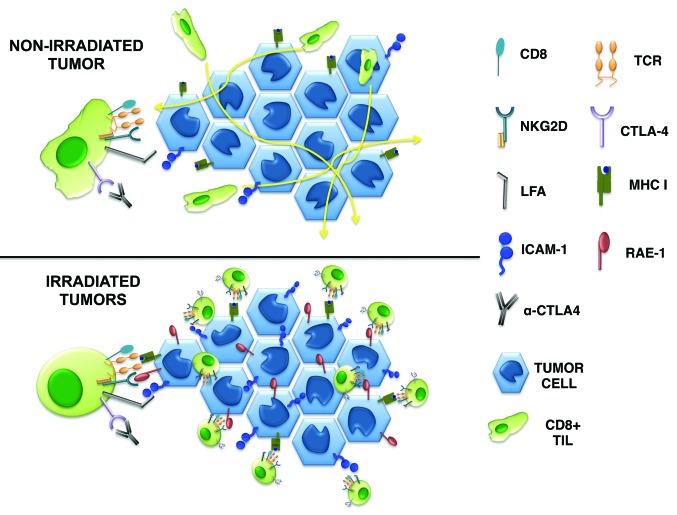
TILs in mice receiving anti-CTLA-4 antibodies alone moved significantly faster than those in untreated mice, and were unable to form stable interactions with tumor cells. While radiotherapy per se also slightly increased TILs motility, the combination of anti-CTLA-4 antibodies and irradiation surprisingly produced the opposite effect: The motility of TILs was markedly reduced and TILs engaged in stable interactions with neoplastic cells, which are required for tumor-cell killing by T cells.8 Retinoic acid early inducible-1 (RAE-1) expressed on the surface of irradiated tumor cells appears to be implicated in this phenomenon, as both the stable interaction of TILs with tumor cells and the therapeutic effect of the combinatorial regimen were blocked by antibodies targeting the RAE-1 receptor NKG2D.6 Our data suggest that the stability of interactions between CD8+ effector T lymphocytes and neoplastic cells is regulated by signals transduced by the TCR, NKG2D, and CTLA-4 (Fig. 1). While it remains to be determined if the strength of the TCR-mediated signals dictates the requirement for NKG2D engagement, the ligation of CTLA-4 increases the signal threshold that is needed for immunological synapses between CD8+ T cells and their targets to form. This function of CTLA-4 has previously been demonstrated in CD4+ T cells,9 but the fact that CTLA-4 also regulates tumor rejection by CD8+ T cells was unanticipated.
Figure 1. Expression of the NKG2D ligand RAE-1 on malignant cells, as induced by radiotherapy, is required for the rejection of poorly immunogenic tumors by effector CD8+ T cells responding to anti-CTLA-4 antibodies. In mice receiving anti-CTLA-4 antibodies, T cells move rapidly through the tumor and do not stably interact with neoplastic cells. Conversely, upon irradiation malignant cells upregulate RAE-1, in turn promoting their NKG2D-dependent interaction with T cells and the formation of an immunological synapse that eventually leads to tumor-cell killing.
The suppression of T-cell responses by CTLA-4 involves multiple cell-autonomous and non-autonomous mechanisms, acting at the level of naïve T-cell priming as well as on regulatory and effector CD4+ T-cell functions.10 While the success of anti-CTLA-4 antibodies is likely to depend on a multipronged effect, the relative contribution of each of these immunosuppressive mechanisms in a given tumor model (or a given patient) may differ. Poorly immunogenic tumors, such as the one used in our study, are well known for their unresponsiveness to anti-CTLA-4 antibody-based monotherapy. Irradiation can convert a tumor into a vaccine in situ, hence generating T-cell responses that, upon priming in the context of CTLA-4 blockade, reject tumors also at non-irradiated metastatic sites.2-4 Our data suggest that the T cell-mediated elimination of cancer cells that survived irradiation by mounting a stress response, which are marked by the expression of NKG2D ligands, contributes to therapeutic success in response to anti-CTLA-4 antibodies.
The increased motility of activated CD8+ T cells in response to CTLA-4-targeting antibodies was confirmed in vitro. Anti-CTLA-4 antibodies were also able to reverse the stop signal induced by soluble anti-CD3 antibodies in the absence, but not in the presence, of RAE-1.6 Altogether, these results support a partial agonistic function of anti-CTLA-4 antibodies that is regulated by the molecular context in which effector T cells interact with their targets.
The critical role played by NKG2D ligands in determining the response of poorly immunogenic tumors to anti-CTLA-4 antibodies has important clinical implications. The upregulation of NKG2D ligands on the surface of tumor cells by some chemotherapy drugs or by radiotherapy may indeed provide a biomarker that predicts the success of combinatorial regimens involving anti-CTLA-4 antibodies. In addition, NKG2D ligand expression in normal tissues such as the colon may help to explain autoimmune toxicities that are commonly associated with treatment, and influence the patterns of toxicity seen with combination therapies. Irradiation induces the expression of NKG2D ligands in humans and mice, but shedding of soluble forms of these molecules adds an additional level of complexity in humans. Thus, the role of NKG2D ligands in the therapeutic responses to anti-CTLA-4 antibodies can only be addressed in a clinical setting. Currently ongoing clinical trials involving radiotherapy plus anti-CTLA-4 antibodies will establish the true therapeutic value of this approach.
Glossary
Abbreviations:
- CTLA-4
cytotoxic T lymphocyte-associated protein 4
- GFP
green fluorescent protein
- RAE-1
retinoic acid early inducible-1
- TCR
T-cell receptor
- TIL
tumor-infiltrating lymphocyte
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23127
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 3.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–26. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breart B, Lemaître F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–7. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 10.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–65. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]