Abstract
High expression levels of cyclooxygenase 2 expression and infiltration by regulatory T cells (Tregs) are often associated with tumor progression. We have recently reported a prostaglandin E2 (PGE2)-dependent recruitment of Tregs to the tumor, suggesting that targeting specific PGE2 receptors may constitute a valuable approach to ablate the immuno-editing that occurs along with disease progression.
Keywords: EP2, EP4, immunosuppression, immunotherapy, mammary tumor, regulatory T cell
Harnessing the immune system, either by potentiating immune responses or by inhibiting cancer cell-elicited immunosuppressive mechanisms (immuno-editing), to understand and counteract tumor progression is currently at the forefront of cancer research. A subset of CD4+ T cells known as regulatory T cells (Tregs) is instrumental in the maintenance of normal peripheral tolerance and in the control of immune responses to pathogens. Tregs mediate immunosuppressive functions by directly inhibiting T cells, killing them or suppressing clonal expansion. Notably, Tregs can dampen many of the host defenses utilized against cancer, making Treg recruitment by developing tumors a critical step in the evasion of antitumor immune responses. Both pre-clinical and clinical studies have associated the progression of various neoplasms to the high levels of circulating and/or intratumoral Tregs. For instance, in human breast cancer patients, the percentage of Tregs at the tumor site is positively correlated with disease progression to normal tissue to ductal carcinoma in situ (DCIS), and from DCIS to invasive carcinoma.1 Despite the correlation between Treg accumulation and worsened disease outcome, the mechanisms by which Tregs promote tumor progression remain unclear. Of note, the levels of cyclooxygenase 2 (COX2) and of its main product prostaglandin E2 (PGE2) have also been associated to poor outcome in many tumor models and clinical studies.2 Although reports have correlated the upregulation of COX2 with increased levels of Tregs in breast cancer, no mechanistic data on this observation was available.
While attempting to elucidate the role of COX2/PGE2 in breast carcinoma progression, we observed that—compared with poorly aggressive mammary TM40D tumor cells—TM40D cells overexpressing COX2 (TM40D-COX2) exhibit an increased rate of bone metastasis, which is comparable to that of a highly-metastatic mammary cancer cell line (TM40D-MB), an effect that can be ablated by the stable depletion of COX2 with short-hairpin RNAs (shRNAs).3 As these cells did not differ relative to in vitro and in vivo proliferation rates, the effects of COX2 on metastatic potential must reflect proliferation-independent phenomena. Additionally, the overexpression of COX2 in TM40D tumor cells altered the immunological profile of tumors, shifting it from one characterized by high levels of intratumoral CD4+ T helper cells to one featuring intense infiltration by CD4+ FOXP3+ Tregs. Others have shown that PGE2 induces the accumulation of myeloid-derived suppressor cells (MDSCs) and that specific receptor antagonists can block this process.4 Moreover, 4T1 mammary carcinoma cells inoculated into PGE2 receptor 2 (EP2)-deficient mice grew less efficiently and accumulated lower numbers of MDSCs than similar cells injected into wild-type mice. Although we could not reveal differences in the number of monocytic and granulocytic MDSCs in response to varying levels of COX2 expression/PGE2 production, we cannot rule out that this may influence the activation state of intratumoral MDSCs.
Conversely, our study specifically addressed the ability of mammary tumors developing from cells that express different levels of COX2 to recruit Tregs from the periphery. Purified Tregs that express the PGE2 receptors EP2 and EP4 preferentially migrated in response to factors released by TM40D-COX2 and TM40D-MB cells, an effect that was attenuated using by anti-PGE2 antibodies. Though we suggest one mechanism involving an increased infiltration of the primary tumor by Tregs, others have shown that this phenomenon can be due to the local differentiation of FOXP3+ Tregs from naïve T cells, occurring independent of transforming growth factor β (TGFβ) and interleukin-10 (IL-10).5 Of note, the PGE2-induced development of Tregs from naïve CD4+ cells requires EP receptors.6 Specifically, FOXP3 expression in response to PGE2 was significantly reduced in the absence of EP4 and entirely ablated in the absence of EP2. Although it has previously been shown that PGE2 alone can directly induce FOXP3 expression, we believe that multiple mechanisms can manipulate the immune system to promote an immunosuppressive environment (Fig. 1).
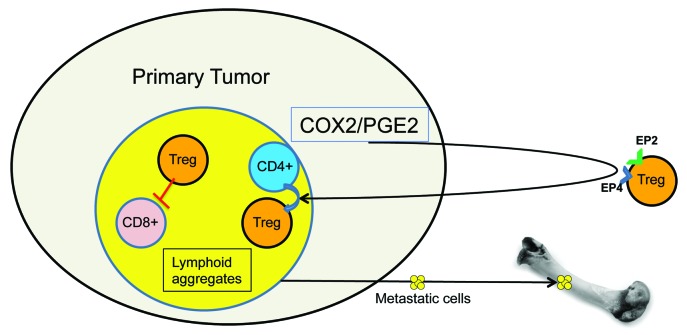
Figure 1. Role of cyclooxygenase 2 and prostaglandin E2 in tumor progression. The overexpression of cyclooxygenase 2 (COX2) and the consequent increased production of prostaglandin E2 (PGE2) promote the recruitment of regulatory T cells (Tregs) from the circulation and/or their local differentiation. Immunosuppressive microenvironments are characterized by elevated levels of Treg-induced CD8+ T-cell apoptosis in lymphoid aggregates, or lymphoid-rich regions of the tumor, and eventually favor metastatic dissemination.
In our attempt to better elucidate the mechanism of PGE2-driven immunosuppression, we observed that COX2-overexpressing tumors contained a higher frequency of apoptotic CD8+ T cells than their wild-type counterparts. These cells are known to be required for the inhibition of tumor progression and metastasis.7,8 Of note, we observed the accumulation of CD8+/cleaved caspase-3+ cells in specific lymphoid-rich areas of the tumor. Similar observations have been reported by others, including Menetrier-Caux et al., who have recently described the effects of Tregs within lymphoid aggregates in breast tumors.9
Targeting COX2 as the therapeutic approach to breast cancer has been the focus of both clinical and laboratory investigations. A broad inhibition of COX2 may result in undesirable cardiovascular and gastrointestinal side effects that are due, at least in part, to reduced levels not only of PGE2, but also of prostaglandin D2, F2R, I2 (the cardioprotective prostacyclin) and thromboxane A2. Specifically targeting one of the many downstream effectors of COX2 would curb potential side effects for both breast cancer patients and individuals treated with COX2 inhibitors for other indications. Along similar lines, the treatment with anti-EP agents following lumpectomy may result in 2-fold benefits. First, it may prevent the establishment of tumor cells to secondary sites by inhibiting the recruitment of immunosuppressive Tregs, therefore allowing the immune system to clear residual cancer cells. Second, it may decrease osteolytic bone lesions secondary to metastatic dissemination by suppressing the effects of PGE2 on osteoblasts, and hence ultimately inhibiting osteoclast function.10 A large amount of data indicates that strategies for the therapeutic targeting of COX2 and Tregs against breast cancer should focus on antagonists that are specific for EP2 and EP4.
Glossary
Abbreviations:
- COX2
cyclooxygenase 2
- DCIS
ductal carcinoma in situ
- MDSC
myeloid derived suppressor cell
- PE2
prostaglandin E2
- Treg
regulatory T cell
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23129
References
- 1.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 2.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 2012;7:e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 5.Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277–88. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 7.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 8.Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120:2030–9. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ménétrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–8. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Schem C, Shi YH, Medina D, Zhang M. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:389–400. doi: 10.1007/s10585-007-9117-3. [DOI] [PubMed] [Google Scholar]