Abstract
Cytotoxic anticancer drugs can promote antitumor immune responses. The anticancer activity of 5-fluorouracil (5FU) relies on the restoration of T-cell immunity following the elimination of myeloid-derived suppressor cells (MDSCs). We have recently discovered that the 5FU-driven activation of the NLRP3 inflammasome in MDSCs promotes tumor angiogenesis by eliciting TH17 responses that compromise anticancer immunity. This underscores the need to combine 5-FU with NLRP3 inhibitors to prevent tumor progression.
Keywords: IL1, NLRP3, fluorouracil, inflammasome, myeloid derived suppressor cells
Danger signals produced during tumor cell death as triggered by some chemotherapeutic agents, such as anthracyclines and oxaliplatin, are able to induce CD8+ T-cell antitumor immune responses.1-3 Some cytotoxic drugs also promote anticancer immunity by targeting immunosuppressive cells. For instance, cyclophosphamide targets regulatory T cells, thereby restoring anticancer immune responses that contribute to tumor eradication.4
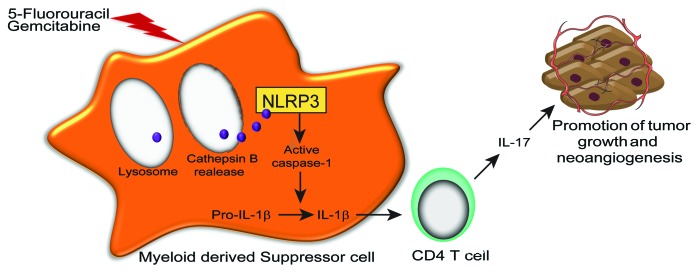
We have investigated the immunological properties of an analog of uracil, 5-fluorouracil (5FU), which operates as an antimetabolite by inhibiting thymidylate synthase. Our findings reveal that 5FU, one of the main drugs employed in the therapy of digestive tract cancers, exerts contrasting effects on anticancer immune responses. We had previously observed that 5FU selectively deplete myeloid-derived suppressor cells (MDSCs), a population of immature myeloid cells that accumulate along with tumor progression and suppress T-cell activation in mice and humans.5 In line with this notion, the 5FU-mediated depletion of MDSCs increased interferon γ (IFNγ) production by tumor-specific CD8+ T cells.5 More recently, we have noted that 5FU, while eliminating immunosuppressive MDSCs, also induces the activation of the NLR family, pyrin domain containing 3 (NLRP3) inflammasome in dying MDSCs, leading to the secretion of interleukin (IL)-1β, elicitation of TH17 cells, IL-17 production and tumor growth following increased angiogenesis.6 These ambivalent effects of 5FU on cancer immunity should therefore be carefully considered for the design of successful 5FU-based anticancer regimens (Fig. 1).
Figure 1. Effect of 5-fluorouracil and gemcitabine on myeloid-derived suppressor cells. IL-1β, interleukin-1β; IL-17, interleukin-17; NLRP3, NLR family, pyrin domain containing 3.
Many endogenous and exogenous activators of NLRP3 have been characterized. Most of these activators share the capacity to promote the accumulation of intracellular radical oxygen species (ROS) or potassium efflux.7 This does not hold true for 5FU, which promotes neither ROS accumulation nor potassium efflux in MDSCs, but triggers NLRP3 activation upon lysosome permeabilization and the consequent release of cathepsin B. Cathepsin B drives NLRP3 activation by binding to its leucin-rich repeat (LRR) domain. Since cathepsin B fails to cleave NLRP3, we speculate that this interaction modifies the spatial conformation of NLRP3 to drive its activation. Our observation reveals an atypical mode of caspase-1 activation that occurs 12 h after the administration of 5FU. This constitutes a longer delay than those observed with classical NLRP3 activation stimuli, and perhaps results from the requirement for 5FU to be metabolized and integrated into DNA to actively induce cell death. 5FU appears to drive caspase-1 activation via lysosome destabilization. This lysosomal rupture stems from the 5FU-driven activation of BAX,8 a pro-apoptotic factor that promotes both mitochondrial and lysosomal permeabilization. In our MDSC model, the inactivation of BAX blunted lysosome permeabilization and caspase-1 activation as triggered by 5FU, linking this specific mechanism of 5FU cytotoxicity to its capacity to trigger caspase-1 activation. The mechanisms underpinning the ability of 5FU to selectively target MDSC remains unclear. We had previously observed that MDSCs express low levels of thymidylate synthase,5 and the expression levels of this enzyme appeared to inversely correlate with caspase-1 activation. Altogether, these data suggest that the selective effect of 5FU on MDSCs might be due to an enzymatic deficiency.
Chronic inflammation and pro-inflammatory cytokines are now recognized as important factors in carcinogenesis and tumor progression. Chronic inflammation resulting from the activation of the IL-1β/IL-1R pathway has indeed been considered as a tumor-promoting condition, arguing in favor of IL-1β inhibition as strategy for tumor prevention.9 In addition, IL-1β has been demonstrated to negatively regulate anticancer immune responses through its capacity to induce the expansion of MDSCs, and to directly impact tumor cell-mediated NF-κB activation, hence promoting progression and resistance to chemotherapy. Finally, IL-1β elicits and stimulated the expansion of TH17 cells, which may promote tumor growth by activating signal transducer and activator of transcription 3 (STAT3) in tumor cells, and by exerting pro-angiogenic functions via the secretion of vascular endothelial growth factor A (VEGFA).10 In contrast, we had previously shown that some anticancer chemotherapeutic agents such as anthracyclines and oxaliplatin elicit tumor-specific immune responses that rely IL-1β signaling for the priming of anticancer CD8+ T cells,2 suggesting that acute inflammation triggered by chemotherapy is beneficial. How to reconcile these seemingly contradictory findings? Of note, 5FU does not induce immunogenic tumor cell death and thereby does not activate CD8+ T cells through the release of high amounts of IL-1β by dendritic cells.5 In this regard, 5FU-treated MDSCs were found to release only low amounts of IL-1β, contrarily to dendritic cells responding to Toll-like receptor 4 (TLR4) ligands such as high mobility group box 1 (HMGB1). Thus, we propose that only high IL-1β concentrations drive the activation of CD8+ T cells, while low concentrations of this cytokine do not affect CD8+ T cells but enhance IL-17 production by CD4+ T cells.6 These data may help to explain the ambiguous role of IL-1β in anticancer immunity. In addition, our study supports the use of inhibitors of IL-1β or the NLRP3 inflammasome to enhance the therapeutic potential of 5FU.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23139
References
- 1.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 2.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 3.Apetoh L, Mignot G, Panaretakis T, Kroemer G, Zitvogel L. Immunogenicity of anthracyclines: moving towards more personalized medicine. Trends Mol Med. 2008;14:141–51. doi: 10.1016/j.molmed.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 6.Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. . Nat Med. 2012 doi: 10.1038/nm.2999. In press. [DOI] [PubMed] [Google Scholar]
- 7.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–5. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Youle RJ. Predominant requirement of Bax for apoptosis in HCT116 cells is determined by Mcl-1's inhibitory effect on Bak. Oncogene. 2012;31:3177–89. doi: 10.1038/onc.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–29. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–64. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]