Abstract
Dendritic cells (DCs) are a heterogeneous group of functionally specialized antigen-presenting cells. We recently characterized the human tissue cross-presenting DCs and aligned the human and mouse DC subsets. Our findings will facilitate the translation of murine DC studies to the human setting and aid the design of DC-based vaccine strategies for infection and cancer immunotherapy.
Keywords: dendritic cell, subset, cross-presentation, transcriptomic analysis, XCR1
Dendritic cells (DCs) are responsible for the induction of immune responses to pathogens, tumors and vaccines as well as for the maintenance of self-tolerance.1 DCs were first described in the mouse spleen and elegant studies in murine models have demonstrated that these cells are heterogeneous and functionally specialized.1 In mice, splenic and lymphoid tissue (LT) DCs were the historical center of research interest but recent work has focused on their migratory counterparts in non-lymphoid tissues (NLTs) such as the skin, gut and lung.2 Two distinct DC lineages have been identified: LT-DCs encompass CD8+ and CD11b+ DC subsets while NLT-DCs include CD103+ and CD11b+ DC subsets.2,3 CD8+ DCs and CD103+ DCs are specialized in processing exogenous antigen for presentation on MHC class I molecules to activate cytotoxic CD8+ T-cell responses, a process known as cross-presentation. In some systems, CD11b+ DCs are superior at MHC class II mediated antigen presentation to activate CD4+ T cells.2,3
Human DC studies have focused on in vitro CD34+ stem cell or monocyte-derived DCs4 and more recently on primary blood CD1c+ DCs and CD141+ DCs.5 The physiological relevance and the in vivo counterpart of human monocyte-derived DCs are still unclear. The accessibility of the human skin enabled investigations on cutaneous DCs, containing two subsets, which are defined by the expression of CD1c/CD1a and CD14 in the dermis, in addition to epidermal Langerhans cells (LCs).6 However, the relationships between human blood CD1c+ DCs and CD141+ DCs with skin CD1c/CD1a+ DCs and CD14+ DCs are uncertain. CD1c+ and CLEC9A+ DCs have been demonstrated in human lymph nodes (LNs).7
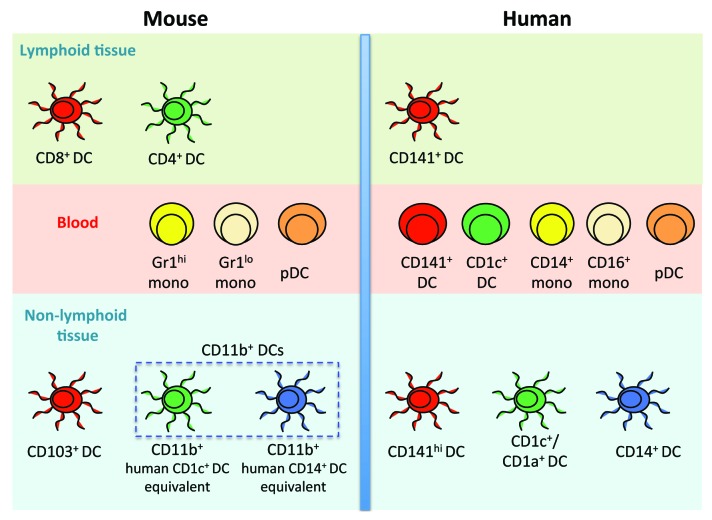
The phenotypic differences, nomenclature issues and 80 million years of independent evolution between mouse and human have prevented an easy translation of results from mouse DC experimentation into knowledge applicable to humans. Dalod and colleagues were the first to attempt steady-state inter-species correlation of DC subsets and suggested by transcriptomic analysis a homologous relationship between human blood CD141+ DCs and CD1c+ DCs with mouse splenic CD8+ DCs and CD11b+ DCs, respectively.8 Recently, functional equivalence between human blood and splenic CD141+ DCs with mouse splenic CD8+ DCs was demonstrated.5 However, the identity of the human tissue cross-presenting DCs, which by virtue of its location constitutes an ideal target for DC-based anticancer therapy, remained elusive. We have recently identified human tissue cross-presenting DCs and aligned the human and mouse NLT-DC networks (Fig. 1).9
Figure 1. Alignment of human and mouse myeloid dendritic-cell and monocyte networks. Functionally homologous subsets and lineages are color-coded across species. Dendritic cells (DCs) have not been identified in mouse blood.
We developed a 12-parameter flow ctyometry strategy to visualize known DC and monocyte subsets in the human blood. By applying the same analysis to cell suspension derived from the human skin, lung and liver we identified a new DC subset, CD141+ DCs, in addition to the known CD1c+ DCs and CD14+ DCs, which were distinct from resident macrophages. In the skin, CD141 expression was promiscuous and was detectable on all DC subsets including CD14+ DCs, as recently described.10 Tissue CD141+ DCs also expressed CD1c and CD1a and hence will be referred to hereafter as CD141hi DCs, to distinguish them from blood CD141+ DCs and other DC subsets co-expressing CD141.10 Blood CD141+ DCs are rare (< 0.1% of CD45+ cells) but tissue CD141hi DCs seem at least 10-fold more abundant. Similar to their circulating counterparts, skin CD141hi DCs express XCR1, TLR3, CLEC9A and CADM1 (NECL2) transcripts and secrete CXCL10 and tumor necrosis factor α (TNFα). In addition, skin CD141hi DCs were found migrating spontaneously in response to XCL1, the ligand for the chemokine receptor XCR1.
We observed that blood CD141+ DCs express the skin homing marker CLA but not the lymph node homing marker CCR7, in contrast to tissue CD141hi DCs which were CLA- but CCR7+. To test if CD141hi DCs migrate to draining LNs, we compared the DC populations in skin-draining LNs with tonsil, which lacks afferent lymphatics. DCs migrating from the periphery were only present in skin-draining LNs and could be identified by comparatively higher expression levels of HLA-DR and lower expression levels of CD11c. CD141hi tissue DCs migrating in the LNs retained the expression of CD1c and CD1a and were distinguishable from resident CD141+ LN DCs, which were CD1c- and CD1a-. Co-stimulatory and DC activation markers, including CD80, CD83 and CD86, were expressed at higher levels by migratory CD141hi DCs as compared with their LN-resident counterparts.
We assessed cross-presentation functions using an in vitro assay, comparing the ability of purified blood and skin DC subsets to cross-present soluble hepatitis B surface (HBs) antigen to an HLA-A*0201 HBs183–91-restricted CD8+ T cell clone. CD141hi DCs were more potent at cross-presentation than all other skin DC subsets including LCs. Cross-presentation by CD141hi DCs was observed even in the absence of any stimuli but was enhanced in the presence of a cocktail containing lipopolysaccharide (LPS), polyinosinic-polycytidylic acid (polyI:C), interferon (IFN)γ, IFNα TNFα, and interleukin-1β (IL-1β). Similar to skin CD141hi DCs, blood CD141+ DCs were superior to CD1c+ DCs and CD14+ monocytes at cross-presentation.
Correlating mouse and human DC subsets is important to justify the continued use of mice as a model for human immunity. Furthermore, important immune functions are often highly conserved across species. Using transcriptomic analysis, we revealed a conserved human cross-presenting CD141+ DC lineage functionally homologous to murine CD8+ LT-DCs and CD103+ NLT-DCs. Interestingly, human CD1c+ DC lineage turned out to be homologous to splenic CD4+ DCs but we did not observe a positive association with mouse CD11b+ NLT-DCs. In contrast, human CD14+ DCs were closely related to human and mouse monocytes and also surprisingly with mouse lung CD11b+ DCs. This suggests that murine tissue CD11b+ DCs are heterogeneous and contain a CD14+ monocyte-like cell equivalent.
Cross-presenting DCs are important for cytotoxic anticancer immune responses. Their presence in the human tissue can be exploited for anticancer DC-based vaccine strategies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23140
References
- 1.Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–9. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 2.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–4. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–45. [PubMed] [Google Scholar]
- 7.Segura E, Valledeau-Guilemond J, Donnadieu M-H, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012;209:653–60. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CC, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, et al. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med. 2012;209:935–45. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]