Abstract
The immune system recognizes pathogens and other danger by means of pattern recognition receptors. Recently, we have demonstrated that the orphan Toll-like receptor 13 (TLR13) senses a defined sequence of the bacterial rRNA and that bacteria use specific mechanisms to evade macrolide lincosamide streptogramin (MLS) antibiotics detection via TLR13.
Keywords: antibiotic resistance, bacteria, erythromycin, immune evasion, pattern recognition, rRNA, TLR13
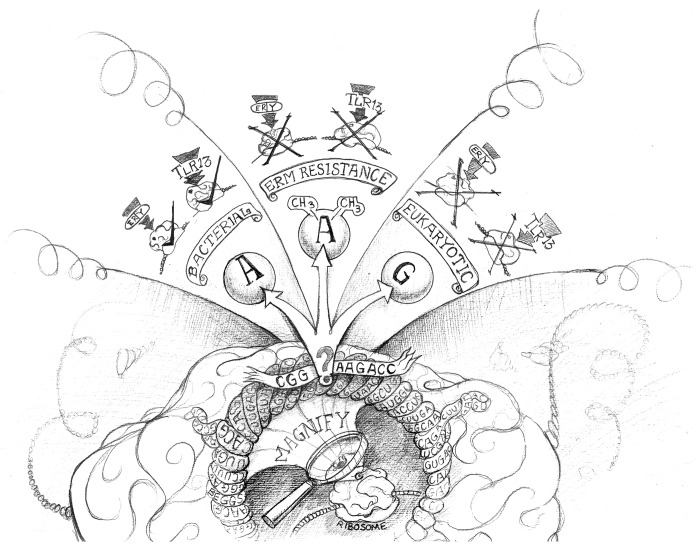
Pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) sense specific products of viral, bacterial, fungal or parasitic origin.1 This sensing initiates innate immune responses that are interconnected, by various means, with adaptive immunity. Analyzing the host recognition of Gram-positive bacteria, we observed that Staphylococcus aureus (SA) activates immune cells such as macrophages and conventional dendritic cells (DCs) even in the absence of TLR2, TLR3, TLR4, TLR7 and TLR9.2 This said, our data indicated that this detection depended on the adaptor molecule MYD88 while excluding the implication of interleukin (IL)-1-type MYD88-dependent pathways, overall suggesting that a TLR was involved. Since TLR10, TLR1 and TLR6 depend upon TLR2 function and SA lacks flagella (which are sensed by TLR5), TLR8, TLR11, TLR12 and TLR13 remained as candidate SA sensors. Since SA sensing was abrogated upon pharmacological blockade of endosomal acidification and in UNC93B1-mutant cells, we suspected an involvement of an endosomal TLR. Comparing the responses of DC subsets from multiple TLR knockout mice with DC-subset specific TLR expression profiles (previously generated by proteomics) narrowed down as candidate molecule the TLR13.3 TLR13 sensed SA as revealed by gain and loss of function analyse. To identify the TLR13 ligand, we enzymatically treated SA preparations and found that the TLR13 stimulatory activity was abrogated by the single-strand RNA (ssRNA) specific RNase. Bacterial RNA fractionation identified the 23S rRNA (rRNA) as the TLR13 ligand, excluding other bacterial RNA such as mRNA and tRNA as well as eukaryotic RNAs. Notably, TLR13-mediated sensing of rRNA from antibiotic-resistant clinical SA isolates was largely abrogated when bacteria had been grown in erythromycin. Erythromycin binds to a conserved domain in the peptidyl transferase loop V positioned in the exit tunnel of the 23S rRNA, thus blocking bacterial protein synthesis.4 Erythromycin is a bacteria-derived antibiotic produced by Saccharopolyspora erythraea (formerly known as Streptomyces erythraeus). Saccharopolyspora gained resistance to self-produced erythromycin by the expression of erythromycin resistance methylases (erms), often encoded on mobile genetic elements such as transposons or plasmids. Erms catalyze methylation of the highly conserved adenosine 2058/2085 (A2058 Escherichia coli nomenclature; A2085 SA nomenclature) of 23S rRNA, contained within the major binding site for erythromycin, thus preventing erythromycin binding and enabling bacteria to grow in the presence of it. Transfection of erythromycin-susceptible bacteria with erm-expression plasmids thus not only led to antibiotic resistance but also abrogated bacteria detection via TLR13. Erythromycin selectively binds to bacterial 23S rRNA, but not to eukaryotic 28S rRNA, because eukaryotic ribosomes, like those of MLS resistant bacteria, principally carry a guanosine (G) at the site corresponding to A2058/2085, which also prevents binding (Fig. 1). Using synthetic oligoribonucleotides (ORNs) representing 23S rRNA segments encompassing A2058/2085 we identified the sequence motif “CGGAAAGACC” as the minimal stimulatory segment of 23S rRNA and TLR13 ligand (Fig. 1). This sequence is highly conserved in prokaryotes and is identical in numerous Gram-positive and Gram-negative bacteria. In accordance with the erm-mediated inhibition of TLR13 recognition of bacterial rRNA, the methylation or substitution of the adenosine corresponding to A2058/2085 on stimulatory ORNs completely abrogated TLR13 activation. Thus, the same mechanisms (methylation or mutation of A2058/2085) that prevent binding of erythromycin to 23S rRNA (causing resistance to macrolides, lincosamides and streptogramin, collectively known as MLS antibiotics) also abrogate detection of SA by TLR13 (Fig. 1). We propose that erms in ancient times evolved in erythromycin-producing bacteria to gain supremacy over bacterial competitors. Subsequently, other bacterial species such as SA acquired erm-plasmids from Saccharopolyspora, probably facilitated by the location of erms-encoding sequences in mobile genetic elements such as plasmids, and hence gained an ability to grow in the close vicinity to erythromycin-producing bacteria. In the animals, a panel of PRRs to detect the presence of pathogens, including TLR13, evolved. TLR13 is a specific sensor of bacteria because it detects a sequence-specific, at least 10-mer, highly conserved segment of the bacterial 23S rRNA, which differs from eukaryotic “self” 28S rRNA, at different sites including “A2058/2085” (Fig. 1). Among nucleic-acid sensing TLRs, TLR13 display the highest sequence specificity identified so far, while TLR3 senses dsRNA based on the structure but not on the sequence.5 TLR7 and TLR8 detect ssRNA containing poly(U), poly (GU) or poly (AU) stretches, whereas TLR9 preferentially binds unmethylated CpG DNA generally but principally binds to the sugar backbone of DNA molecules.6-8 Based on the low sequence specificity of TLR3, TLR7, TLR8 and TLR9, it is not surprising that these TLRs have all been implicated in autoimmune diseases.9 Bacterial 23S rRNA, however, is a confined non-self pathogen-associated molecular pattern, as it might be the most conserved, abundant and stable RNA within a bacterial cell and the “CGGAAAGACC” sequence is most likely detectable by TLR13 without the need for specific processing. For a long time, the erm-mediated resistance against erythromycin and the detection of the ribosomal erythromycin binding site by TLR13 might have been two completely unrelated events. However, when bacteria such as Staphylococcus began to invade animals, their previously acquired ability to express erms became a useful mechanism of immune evasion, preventing their recognition by TLR13 and thus enabling bacteria to colonize TLR13+ animals nearly undetected. These colonies might serve as reservoirs of MLS antibiotic-resistant bacteria, being capable of invading—upon exposure—MLS antibiotic-treated farm animals and humans.
Figure 1. Interaction of erythromycin and Toll-like receptor 13 with the bacterial but not the eukaryotic ribosome. The magnification of the ribosome at the central peptidyl exit tunnel highlights the highly conserved sequence “CGGAAAGACC” (highlighted as banner) as macrolides, lincosamides and streptogramin (MLS) antibiotic- as well as TLR13-binding site. The question mark depicts the adenosine A2058/2085. If this site is an unmodified adenosine (left panel) it will allow erythromycin (ery) to bind and block protein biosynthesis. At the same time, this sequence is being detected by Toll-like receptor 13 (TLR13) (indicated with a tick “√”). In erm-driven resistance (middle panel) is the A2058/2085 methylated. The methyl groups prevent binding of erythromycin, causing both antibiotic resistance and preventing detection via TLR13 (indicated with an “X”). The right panel illustrates substitution of A2058/2085 by guanosine as is operative in eukaryotic 28S rRNAs and bacterial 23S rRNAs of some MLS antibiotic-resistant bacteria, which abrogates erythromycin binding as well as bacteria detection via TLR13 (indicated with an “X”). Figure designed by Derek Beggs (Bavarian-Nordic, Martinsried, Germany).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23141
References
- 1.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 2.Oldenburg M, Krüger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337:1111–5. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- 3.Luber CA, Cox J, Lauterbach H, Fancke B, Selbach M, Tschopp J, et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 2010;32:279–89. doi: 10.1016/j.immuni.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Vester B, Douthwaite S. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob Agents Chemother. 2001;45:1–12. doi: 10.1128/AAC.45.1.1-12.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 6.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 7.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 8.Wagner H. The sweetness of the DNA backbone drives Toll-like receptor 9. Curr Opin Immunol. 2008;20:396–400. doi: 10.1016/j.coi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev. 2011;243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]