Abstract
Regulatory T cells (Tregs) and plasmacytoid dendritic cells (pDCs) that infiltrate primary breast tumors impair patient survival. The ICOS-mediated interaction between tumor-infiltrating CD4+ T cells and pDCs leads to the amplification of Tregs and interleukin-10 secretion. Importantly, ICOS+ cell infiltration correlates with adverse patient prognosis, identifying ICOS as a new target for cancer immunotherapy.
Keywords: breast cancer, ICOS, immunosuppression, regulatory T cells, plasmacytoid dendritic cells
Breast cancer is one of the main causes of death among women in Western countries, and constitutes an important challenge for health care systems worldwide. Breast carcinomas are considered immunogenic, as adaptive responses against tumor-associated antigens have been described in both mouse models and patient. Nevertheless, breast tumors most often escape the cytotoxic activity of immune cells by hijacking the immune system. Recent successes of anti-CTLA-4 monoclonal antibodies (mAbs) (e.g., ipilimumab) in melanoma patients1 anti-PD-1 or anti-PD-L1 mAbs in patients bearing various solid tumors (encompassing melanoma, renal cell carcinoma and lung cancer)2 highlight cancer immunotherapy as a promising strategy for the identification of new therapeutic targets and agents that may promote antitumor immune responses, hence prolonging patient survival.
High numbers of tumor-associated (TA)-Tregs constitute an independent factor of poor prognosis for breast carcinoma patients.3 Furthermore, breast cancer-infiltrating CD4+FOXP3- T cells (TA-Tconvs) exhibit a memory phenotype (CD45RO+) and very few are activated, contrasting with highly activated TA-Tregs (CTLA-4+GITR+HLA-DR+), which proliferate in situ (Ki67+) and exert immunosuppressive functions in vitro.3 The inducible co-stimulatory molecule (ICOS), which belongs to the CD28 protein family, is highly expressed on Tregs infiltrating various tumors, including melanoma and ovarian cancers. The infiltration of breast carcinoma by TA-pDCs, which is also associated with poor patient prognosis,4 correlates with TA-Treg infiltration, and these cell subsets have been shown to co-localize within primary breast cancer lesions.5 While pDCs from healthy donors express high levels of the unique ligand of ICOS (ICOSL) upon in vitro activation via Toll-like receptor 7 (TLR7) or TLR9, TA-pDCs isolated from tumor cell suspensions fail to express ICOSL, in spite of an activated phenotype (CD40+HLA-DR+CD86+) . Interestingly, the engagement of ICOSL by ICOS+ T cells in vitro, leads to its downregulation at the membrane of TLR-activated pDCs, and ICOSL expression on TA-pDCs can be restored by short-term (24 h) co-culture with breast cancer cells isolated in the presence of blocking anti-ICOS mAbs.6 Taken together, these data demonstrate that the ICOS/ICOSL interaction occurs during the interaction of TA-Tregs with TA-pDCs in breast carcinoma.
Despite their expression of Ki67 in situ, TA-Tregs do not proliferate in vitro in response to TCR stimulation with anti-CD3/anti-CD28 agonist mAb-coated beads and exogenous interleukin-2 (IL-2), contrarily to circulating Tregs or TA-Tconvs, which rapidly proliferate. Interestingly, allogeneic co-cultures, including TLR-activated pDCs and TA-CD4+ T cells, lead to a consistent expansion of FOXP3+ TA-Tregs and stimulated the secretion of IL-10. Such a pDC-dependent immunosuppressive T-cell response appears to be highly dependent on ICOS, as the addition of a anti-ICOS neutralizing mAb selectively inhibits Treg proliferation and IL-10 secretion in this setting. Furthermore, myeloid DCs (mDCs, Lin-HLA-DR+CD11c+BDCA2-) that do not overexpress ICOSL after TLR stimulation do not promote Treg expansion and IL-10 secretion.6 In line with our observations on breast TA-Tregs, the proliferation of ICOS+ Tregs obtained from ovarian cancer ascites7 as well as that of natural ICOS+ Tregs (obtained from the thymus)8 responding to activated pDCs is also dependent on the ICOS/ICOSL interaction (Fig. 1).
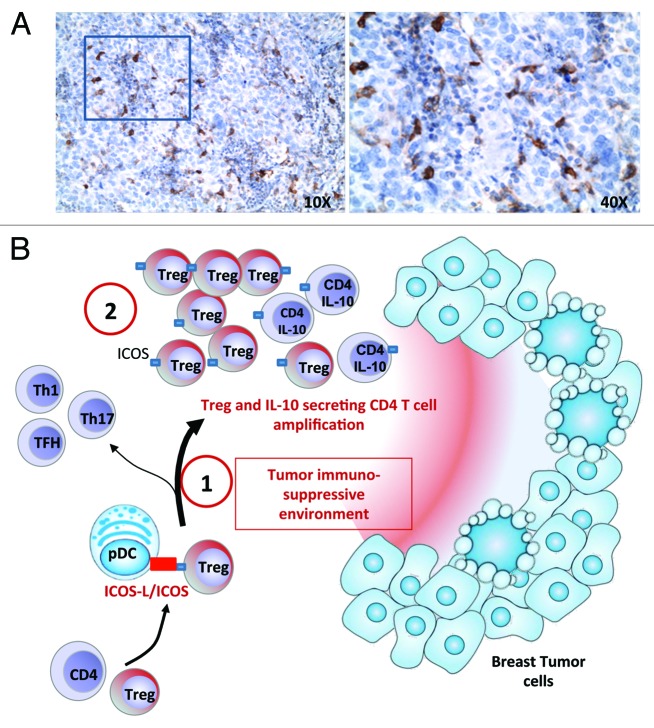
Figure 1. The interaction between ICOS and its ligand (ICOSL) during the contact of plasmacytoid dendritic cells (PDCs) and CD4+ T cells promotes immunosuppression in breast carcinoma through the amplification of regulatory T cell (Tregs) and interleukin-10-secreting CD4+ T cells. (A) A representative immunohistochemical staining of paraffin-embedded breast carcinoma sections with anti-ICOS antibodies (brown) identifies ICOS+ cells inside breast cancer-associated lymphoid structures. Tissues were counterstained with hematoxylin. (B1) By interacting with ICOSL on tumor-associated (TA) pDCs, ICOS participates in the expansion of TA-Tregs and the secretion of interleukin-10 (IL-10) by CD4+ T cells infiltrating breast carcinoma lesions. Moreover, ICOS+ cells are associated with poor patients survival, demonstrating that ICOS participates in T-cell mediated immunosubversion but not in antitumor immunity via TH1,TH17 or follicular helper T (TFH) responses. This activity of ICOS may be linked to immunosuppressive mediators present in the tumor environment such as IL-10 and transforming growth factor β (TGFβ) (B2) Contrarily to circulating Tregs, TA-Tregs are highly dependent on ICOS for their expansion, similar to Tregs from ovarian tumor ascites. Such dependency on ICOS may reflect a particular subpopulation of Tregs that could be targeted with anti-ICOS blocking monoclonal antibodies (mAbs), leading to the restoration of antitumor immunity.
Importantly, neither IL-2, IL-17 or interferon γ (IFNγ) are detectable in dissociated breast carcinoma, in contrast to IL-10 6. The neutralization of ICOS increases the secretion of IL-2 in TA-CD4+ T cells/pDC co-cultures but only slightly reduces the secretion of IFNγ and the proliferation of Tconvs, and does not impact T-cell responses to mDCs. This suggests that ICOS favor TA-Treg expansion and IL-10 secretion but does not participate in the induction of immune effectors against primary breast cancer lesions. Importantly, in a retrospective clinical study involving a cohort of breast carcinoma patients, ICOS expression (as assessed by immunohistochemistry) was mainly detected on TA-Tregs and ICOS+ cell infiltration correlated with reduced disease free and overall survival in univariate analyses,6 in line with results obtained in ovarian cancer patients.7
ICOS constitutes a critical regulator of humoral immune responses, mainly as it stimulates follicular helper T cell activation, as illustrated in ICOS-deficient mice and patients. This said, a few reports also suggest that ICOS may contribute to antitumor cellular immunity. Indeed, in melanoma patients, an increased proportion of IFNγ-producing CD4+ICOS+ T cells has been observed in patients responding to ipilumumab treatment,9 and ICOS-deficient mice bearing B16 tumors do not respond properly to anti-CTLA-4 therapy.10
Interestingly, ICOS+ Tregs have been detected in several human and murine tumors and our in vitro experiments demonstrate that ICOS+ TA-Tregs are strongly dependent on ICOS for their expansion, contrarily to circulating Tregs. Such a dependency on ICOS may reflect a particular subpopulation of Tregs, either linked to a particular origin (similar to thymic ICOS+ Tregs)8 or to a peculiar microenvironment and/or activation status. In this context, it is not clear whether TA-Tregs originate from natural Tregs or from naive T cells in the periphery (inducible Tregs). The expression levels of IKAROS family zinc finger 2 (HELIOS), latency-associated peptide (LAP), glycoprotein A repetitions predominant (GARP) and neuropilin 1(NRP1) and the analysis of FOXP3 gene methylation status may be instrumental to decipher the origin of TA-Tregs. Understanding whether the blockade of ICOS differentially impact natural Tregs, inducible Tregs and TA-Tregs, even if all of them can express ICOS upon activation, could be important for revert TA-CD4+ T cell immunosuppression in breast cancer patients.
Taken together, our data suggest blocking ICOS blockade may constitute a promising strategy to eradicate TA-Tregs and IL-10-producing CD4+ T cells in primary breast carcinoma. The neutralization of ICOS may need to be transient in order to abrogate Treg amplification while leaving unperturbed the restoration of effector cells potentially expressing ICOS. The efficacy of ICOS blockade as a strategy for interfering with in tumor growth is currently under investigation in pre-clinical mouse models of mammary carcinogenesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23185
References
- 1.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517–9. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 3.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–9. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 4.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–74. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 5.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, et al. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:5188–97. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 6.Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, et al. ICOS-Ligand Expression on Plasmacytoid Dendritic Cells Supports Breast Cancer Progression by Promoting the Accumulation of Immunosuppressive CD4+ T Cells. Cancer Res. 2012;72:6130–41. doi: 10.1158/0008-5472.CAN-12-2409. [DOI] [PubMed] [Google Scholar]
- 7.Conrad C, Gregorio J, Wang YH, Ito T, Meller S, Hanabuchi S, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012;72:5240–9. doi: 10.1158/0008-5472.CAN-12-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–80. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Shen S, Gorentla BK, Gao J, Zhong XP. Murine regulatory T cells contain hyperproliferative and death-prone subsets with differential ICOS expression. J Immunol. 2012;188:1698–707. doi: 10.4049/jimmunol.1102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71:5445–54. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]