Abstract
Tumor-infiltrating immune cells play important roles in metastasis. We have recently revealed the recruitment of a specific myeloid cell subset (CD11b/Gr1mid) to hepatic metastases. Such a recruitment relies on CCL2/CCR2 signaling and acts to sustain metastatic growth. A similar cell subset was identified in patients bearing hepatic metastases of colorectal cancer, highlighting the potential therapeutic relevance of our findings.
Keywords: CCL2, colon cancer, liver metastasis, myeloid cell recruitment
Colorectal cancer (CRC) is a highly prevalent disease characterized by high mortality rates and often complicated by metastatic dissemination, most frequently to the liver. Surgical resection constitutes the most effective therapeutic option for patients with hepatic metastases, although this option is not always feasible due to the extent of metastatic spread. The development of metastases is influenced by intricate interactions between cancer and immune cells. Tumor-associated immune cells, particularly myeloid cells, have been shown to play critical roles in the metastatic cascade. Indeed, the recruitment of inflammatory monocytes,1 macrophages2 and granulocytes3 to the metastatic microenvironment is essential for the development of pulmonary metastases from breast carcinomas and other solid tumors. Although the role of myeloid cells in the metastatic dissemination of CRC to the liver has been less extensively studied, a tumor-infiltrating myeloid cell population (CD11b+/CD34+/Gr1- cells) has been shown to promote the hepatic expansion of CRC cells in the liver.4
Using murine models, we have recently characterized three myeloid cell populations (CD11b/Gr1high, CD11b/Gr1mid and CD11b/Gr1low) in the hepatic tumor microenvironment, each featuring a distinct morphology, distinct surface marker expression and distinct profile of secreted inflammatory mediators,. Among these myeloid cell subsets, CD11b/Gr1mid cells increased dramatically as metastases progressed. This pattern of myeloid cell accumulation was tumor-specific, as we observed it only in mice bearing metastasis from MC38 CRC and Lewis lung carcinoma (LLC) but not B16F1 melanoma cells.5
We have also demonstrated the functional importance of CD11b/Gr1mid and CD11b/Gr1low myeloid cells in the progression of hepatic metastases. The depletion of these cells (as obtained by the administration of diphtheria toxin A in transgenic mice bearing the diphtheria toxin receptor under the control of the CD11b promoter) in animals bearing established liver metastases caused a striking decrease in hepatic tumor burden. Previous studies have established that tumor-infiltrating myeloid cells can support the metastatic cascade at various levels, including early stages of the metastatic process (i.e., the formation of pre-metastatic niche, invasion and extravasation from blood vessels),1,2 metastatic outgrowth and colonization,4 as well as late steps of the metastatic cascade. In our model, CD11b/Gr1mid and CD11b/Gr1low cells were important for the sustained growth of metastatic tumor cells during the late stages of the metastatic process. Thus, we propose that these cells might be a useful therapeutic target for the treatment of established liver metastases.
We verified the fact that CD11b/Gr1mid cells originate in the bone marrow by adoptive cell transfer and demonstrated that these cells home to the liver tumor microenvironment in response to the release of CCL2 by CRC cells (Fig. 1). The inhibition of CCL2 signaling using a CCL2-specific lentiviral-based short-hairpin RNA (shRNA) as well as the absence of its cognate receptor in Ccr2 knockout mice inhibited the recruitment of CD11b/Gr1mid cells, resulting in a marked reduction of tumor burden. The deregulation of CCL2/CCR2 is common in cancer, and it has been implicated in the progression of a number of different primary neoplasms.6,7 We have analyzed the serum of CRC patients, observing a correlation between the levels of CCL2 and disease stage, in line with previous results.6,7 In addition, the analysis of tissue samples from CRC patients bearing hepatic metastases revealed CD11b+/CCR2+ cells (Fig. 1, inset) sharing features with the tumor-infiltrating CD11b/Gr1mid cells identified in our murine model.
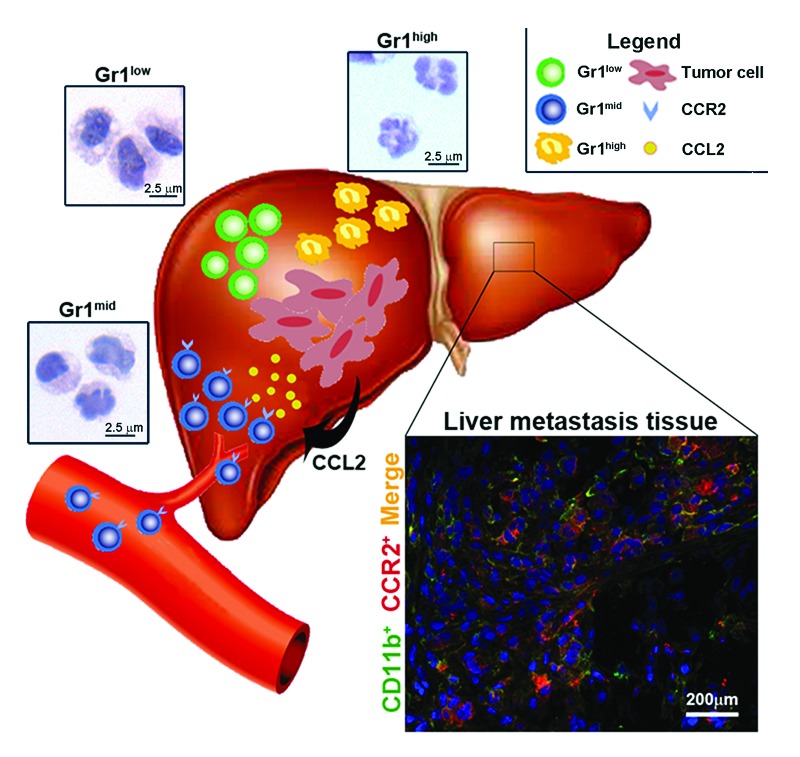
Figure 1. The metastatic liver microenvironment. Three CD11b+ myeloid populations expressing different levels of Gr1 (Gr1low, Gr1mid and Gr1high) were observed in the metastatic liver microenvironment. Metastatic tumor cells secrete CCL2, hence recruiting CCR2-expressing Gr1mid cells from the bone marrow. Cells sharing features with such Gr1mid myeloid population (CD11b+CCR2+) were found in metastatic liver tissues from some colorectal cancer patients (inset).
Chemokine/chemokine receptor pairs represent attractive therapeutic targets, yet trials aimed at inhibiting aberrant chemokine/cytokine networks in cancer patients have provided relatively inconclusive results to date.8 Although our studies demonstrate the importance of CCL2/CCR2 signaling in the development of hepatic metastasis, we have also shown that the inhibition of CCL2 only generate a temporary delay in metastatic growth, suggesting that the redundancy of the chemokine network may limit the efficacy of targeting a single component of this system.
Taken together, our results highlight the importance of myeloid cells in the growth of hepatic metastases. The fact that myeloid cells have been similarly implicated in the development of metastasis from other neoplasms indicates that their recruitment to the tumor microenvironment is a fundamental process, modulating multiple steps in the metastatic cascade, and confirm that metastasizing tumor cells are capable of manipulating the host immune system to their own ends. However, clinical benefits may only be gained by targeting multiple chemokines/chemokine receptors, presumably in a patient-specific manner. A better understanding of the roles of tumor-infiltrating myeloid cells in metastatic dissemination is urgently required to specifically target the mechanisms whereby these cells influence metastatic progression.
Glossary
Abbreviations:
- CRC
colorectal cancer
- KO
knockout
- LLC
Lewis lung carcinoma
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23187
References
- 1.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–55. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A. 2010;107:13063–8. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, et al. Recruitment of a myeloid cell subset (CD11b/Gr1(mid) )via CCL2/CCR2 promotes thedevelopment of colorectal cancer liver metastasis. Hepatology. 2012 doi: 10.1002/hep.26094. In Press. [DOI] [PubMed] [Google Scholar]
- 6.Yoshidome H, Kohno H, Shida T, Kimura F, Shimizu H, Ohtsuka M, et al. Significance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastases. Int J Oncol. 2009;34:923–30. doi: 10.3892/ijo_00000218. [DOI] [PubMed] [Google Scholar]
- 7.Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, et al. Tumor cell-microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15:5485–93. doi: 10.1158/1078-0432.CCR-08-2491. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]


