Abstract
The Vδ2 and non-Vδ2 (mainly Vδ1) subsets of human γδ T cells have distinct homing patterns and recognize different types of ligands, yet both exert potent antitumor effects. While the T-cell receptor of Vδ2 T cells primarily recognizes tumor cell-derived pyrophosphates, non-Vδ2 γδ T cells preferentially recognize stress-associated surface antigens. Here, we discuss the pros and cons of Vδ2 versus non-Vδ2 γδ T cells as tools for future immunotherapeutic interventions against cancer.
Keywords: Vδ1 T cells, Vδ2 T cells, cancer immunosurveillance, cytotoxicity, γδ T cells
Introduction
γδ T cells are commonly considered to bridge innate and adaptive immunity as they share with cells belonging to the adaptive immune system the expression of clonally rearranged antigen receptors and with cells of the innate immune system the expression of natural killer receptors (such as Natural Killer Group 2 Member D, NKG2D) and pattern recognition receptors.1,2 Moreover, γδ T cells recognize antigens independently of MHC presentation/restriction. In fact, some γδ T-cell receptors (TCRs) such as human Vδ2Vγ9 act like pattern recognition receptors, hence detecting pyrophosphates derived from multiple microbes (and tumor cells) as 'molecular patterns'.2,3 γδ T cells share with conventional αβ T cells many effector functions including cytotoxicity, cytokine production and regulatory activity.4,5 In addition, it appears that human γδ T-cell subsets can also compete with mature dendritic cells in their capacity to take up, process and present foreign antigens to CD4+ and CD8+ αβ T cells.6 The MHC-nonrestricted cytotoxicity of γδ T cells towards tumor cells of epithelial as well as hematological origin has recently raised great interest.7-9 Human γδ T cells come in two major flavors: Vδ2 T cells account for the majority (50-95%) of circulating γδ T cells (in turn constituting only 5% of T cells in the peripheral blood), whereas γδ T cells expressing other Vδ elements ('non-Vδ2') are rare in the blood but appear at increased frequencies in mucosal tissues and in the skin.4,10,11 Although Vδ1 is the second most frequently used Vδ element, γδ T cells expressing one of the few other available Vδ gene segment have been identified. For the purpose of this article, these cells are collectively referred to as non-Vδ2 T cells.
Vδ2 T Cells: Everybody’s Darling
Vδ2 is almost exclusively paired with Vγ9 and Vδ2Vγ9 T cells recognize in a TCR-dependent fashion phosphorylated intermediates of the isoprenoid biosynthesis pathway involved in cholesterol synthesis.12 Such molecules, collectively termed phosphoantigens, are produced by many microbes through the prokaryote-specific non-mevalonate pathway. Microbial phosphoantigens such as (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) operate as extremely potent and selective ligands for Vδ2 T cells, stimulating their activation at pico- to nanomolar concentrations.13 Structurally-related pyrophosphates (such as isopentenyl pyrophosphate, IPP) are also generated by mammalian cells via the mevalonate pathway. Like HMB-PP, IPP is recognized by the Vδ2 TCR, but micromolar concentrations are required for the activation of γδ T cells.14 Vδ2 T cells kill a wide variety of tumor cells including epithelial cancer cells of various origin, acute myeloid leukemia (AML) blasts, lymphoma cells as well as putative cancer stem cells.7,15-18 The stimulation of Vδ2 in vitro with natural or synthetic phosphoantigens (in the presence of interleukin-2, IL-2) triggers a rapid, massive and selective expansion of Vδ2 T cells. Due to the ease whereby Vδ2 T cells are expanded in vitro (even under good manufacturing practice conditions), the adoptive transfer of these cells to tumor patients has been performed in several studies (see below). In addition to phosphoantigens, aminobisphosphonates (N-BPs) can be used to selectively activate Vδ2 T cells. N-BPs such as zoledronic acid (ZOL) are licensed for the treatment of patients with osteoporosis as well as metastatic cancer patients, as they inhibit osteoclastic bone resorption. In addition, ZOL and related N-BPs interfere with the mevalonate pathway by inhibiting the enzymatic processing of the Vδ2-activating metabolite IPP, leading to its accumulation.19 Importantly, N-BPs enhance the susceptibility of multiple tumor cells to γδ T cell-mediated lysis, following increased IPP production.15,16,20 As a consequence, the activation of Vδ2 T cells in vivo by therapeutic applications of N-BPs together with low doses of IL-2 has been explored as an alternative approach to the adoptive transfer of Vδ2 T cells expanded in vitro. The role of tumor- (or microbe-) derived phosphoantigens in the activation of human Vδ2 T cells is undisputed, yet also stress-induced molecules such as homologues of bacterial mismatch repair proteins (human MutS homologue 2, hMSH2) can be recognized by the Vδ2 TCR when ectopically expressed on cancer cells.21,22 Moreover, Vδ2 T cells can kill tumor cells independently of TCR-mediated recognition, for instance upon the activation of NKG2D by tumor cell-expressed NKG2D ligand MHC Class I-related chain A (MICA).23 UL16-binding proteins (ULBPs) constitute a second group of human NKG2D ligands. ULBP1 has been shown to determine the susceptibility of leukemia and lymphoma cell lines to Vδ2 T cells.17,24 As demonstrated by antibody-mediated blocking experiments, the respective contribution of TCR- versus NKG2D-dependent activation to the killing of different tumor cell targets by Vδ2 T cells varies considerably.15 In addition to N-BPs, tumor cell killing by Vδ2 T cells is enhanced by Toll-like receptor (TLR) agonists25 and by antibody-mediated cellular cytotoxicity (ADCC) in the presence of tumor-targeting monoclonal antibodies (mAbs). Therapeutically used mAbs such as trastuzumab (anti-HER2) and rituximab (anti-CD20) enhance the cytotoxicity of CD16-expressing γδ T cells by inducing ADCC.26,27 Superior activity, both in terms of in vitro killing and clinical efficacy, can be expected from bispecific antibodies that cross-link tumor-cell surface antigens with signaling molecules on T cells.28 While bispecific antibody constructs based on CD16 or CD3 do not specifically activate or recruit γδ T cells, we are currently exploring constructs targeting Vγ or Vδ chains, which would selectively engage γδ T cells. Pre-clinical studies have demonstrated the efficacy of adoptively transferred Vδ2 T cells against various hematological and solid tumors.27,29,30 Based on these results, and in view of the ease whereby Vδ2 T cells are activated in vivo and expanded in vitro to large cell numbers (by N-BP or phosphoantigen stimulation), several pilot trials have explored N-BPs plus low-dose IL-2 or the adoptive transfer to cancer patients of Vδ2 T cells expanded in vitro. Favorable responses including survival benefits were observed by Dieli et al. in a Phase I study involving a small cohort of patients affected by hormone-refractory prostate cancer and receiving ZOL plus IL-2, correlating with the activation of Vδ2 T cells in vivo.31 In contrast, no objective clinical responses were reported in a pilot study and a prospective Phase I/II trial involving renal cell carcinoma (RCC) and melanoma patients.32,33 Although Vδ2 T cells are transiently activated in vivo upon the administration of ZOL, γδ T cells rapidly exhaust upon repeated application of N-BPs. In a recent study, we observed a dramatic decline of peripheral blood γδ T cells in osteoporotic patients who were on i.v. or oral N-BP treatment.34 Even though these patients did not receive IL-2 together with N-BPs, a similar reduction was also observed in cancer patients upon repeated administrations of ZOL together with IL-2.32 Therefore, the activation of potentially tumor-reactive Vδ2 T cells in vivo by repeated N-BP plus IL-2 administrations – in the absence of other strategies – does not hold promise as an effective anticancer therapy. Alternatively, the safety and efficacy of the adoptive transfer of Vδ2 T cells expanded in vitro has been assessed in several clinical trials involving patients affected by RCC, non-small cell lung cancer and other solid tumors (see refs. 7 and 9 for recent overviews). Generally, the infusion of γδ T cells expanded in vitro appears to be well tolerated, and no major adverse effects have been observed. So far, however, only limited therapeutic benefits have been reported.7,9,35 Interestingly, objective responses were reported in a recent Phase I/II study enrolling 11 patients with advanced RCC. In this setting, the adoptive transfer of Vδ2 T cells was combined with the administration of ZOL, perhaps accounting for transient adverse reactions but also for beneficial effects.36 Intriguingly, the retrospective analysis of intratumoral γδ T cells and clinicopathological features (i.e., age, gender, tumor size, stage, grade and necrosis) in a large cohort of RCC patients did not reveal any correlation between the abundance of tumor-infiltrating γδ T cells (which were in the 1% range in most cases) and disease outcome.37 While such data might question the role of γδ T cells in RCC, they do not preclude a potential therapeutic efficacy of adoptively transferred γδ T cells.36
Non-Vδ2 γδ T Cells: Vδ1 and Beyond
In the absence of omnipotent ligands for the selective expansion of non-Vδ2 γδ T cells (comparable to phosphoantigens for Vδ2 T cells), it is a demanding task to characterize the potential function of such cells in antitumor immunity. This notwithstanding, there are clear hints for an antitumor function of non-Vδ2 γδ T cells. Vδ1 T cells, which usually constitute a minor proportion of circulating γδ T cells, can exert potent cytotoxic effects against blasts from patients with acute lymphoblastic leukemia (ALL) or AML,38 as well as against chronic lymphocytic leukemia cells39,40 and primary multiple myeloma cells.41 The reactivity of Vδ1 T cells towards hematological malignancies is not limited to cytotoxicity. In fact, a proliferative response associated with IL-4 production was reported for Vδ1 T cells in low-grade non-Hodgkin lymphoma patients.42 The ligands potentially recognized on leukemia/lymphoma cells by the Vδ1 TCR have not been unambiguously identified. However, in addition to TCR-dependent pathways, signals delivered via activating receptors suach as NKG2D, natural cytotoxicity receptors (NCR) like NKp30, and DNAX accessory molecule-1 (DNAM-1) play a prominent role in the recognition of tumor cells by these more ambivalent T cells.40-42 Intriguingly, MICA is recognized not only by NKG2D but also directly via the Vδ1 TCR, thereby possibly enabling a “superstimulation” of Vδ1 T cells by TCR plus NKG2D.43 In fact, MICA is frequently expressed on the surface of AML and ALL cells.44 ULBPs, notably ULBP3, are also expressed on tumor cells of hematological origin and trigger cytotoxicity and/or cytokine production by Vδ1 T cells.42,45 Together with the observation that the inducible NKp30 as well as other NCRs enable Vδ1 T cells to kill cells that are resistant to phosphoantigen-activated Vδ2 T cells,40 it is safe to conclude that Vδ1 have a substantial capacity to attack various leukemia and lymphoma cells, and thus might carry immunotherapeutic potential – provided that efficient large scale expansion would be achievable. Recently, experimental protocols based on the mitogenic stimulation with concanavalin A or immobilized anti-CD3 mAbs have been reported to allow for a robust expansion of Vδ1 T cells (in addition to Vδ2 T cells) when total γδ T cells are used as a starting cell population.39,46,47 Therefore, it appears we are approaching the moment when Vδ1 T cells might also be amenable for adoptive cell transfer studies.
While Vδ1 T cells seemingly have a particular affinity for leukemia and lymphoma cells, other non-Vδ2 γδ T cells might be more prone to kill solid tumors. An exciting example extends the common theme of a shared role of γδ T cells in infection and antitumor immunity, which has been first established for phosphoantigen-reactive Vδ2 T cells, to non-Vδ2 γδ T cells. On the grounds of the previously described selective increase of non-Vδ2 γδ T cells in the blood of renal allograft recipients who developed cytomegalovirus (CMV) infection after transplation,48 Halary and coworkers discovered that these γδ T cells recognize both CMV-infected cells and intestinal tumor cells.49 Moreover, CMV-reactive Vδ2-negative γδ T cells exhibited antitumor activity against colon carcinoma cells in a pre-clinical adoptive transfer model.50 Interestingly, there is also clinical evidence for a role of Vδ2-negative γδ T cells in immunosurveillance of kidney transplanted patients who are at an increased risk to develop cancer. Couzi et al. reported that an increase in Vδ2-negative γδ T cells is significantly associated with a lower incidence of cancer development, but only in patients who experienced CMV infection.51 Recently, Déchanet-Merville’s group could identify the shared ligand of CMV-infected endothelial cells and epithelial tumor cells as the MHC-like endothelial protein C receptor (EPCR).52 EPCR is the newly minted stress-regulated molecule that is specifically recognized by Vδ5 T cells.52 A short summary of major activating ligands for Vδ2 and non-Vδ2 γδ T cells expressed by tumor cells is provided in Table 1.
Table 1. Activating ligands for human Vδ2 and non-Vδ2 γδ T cells expressed by tumor cells: a simplified view.
| |
Ligands for |
|||
|---|---|---|---|---|
| γδ T cell subset | T-cell receptor | NKGD2 | NKp30 | |
| |
Vδ2 |
IPP, hMSH2 |
MICA, ULBP1 |
|
| non-Vδ2: | Vδ1 |
unknown, MICA |
ULBP3 |
B7-H659 |
| Vδ5 | EPCR | n.d. | ||
EPCR, endothelial protein C receptor; hMSH2, human MutS homologue 2; IPP, isopentenyl pyrophosphate; MICA, MHC Class I-related chain A; n.d., not determined; ULBP, UL16-binding protein.
Potential of γδ T Cells in Antitumor Immunity: Beyond Direct Cytotoxicity
Human γδ T cells have additional capacities that are worth exploiting for immunotherapeutic purposes. As previously mentioned, activated Vδ2 T cells can take up and process antigens for subsequent (cross-)presentation to antigen-specific αβ T cells.6 This property can also be extrapolated to tumor-associated antigens. In the tumor microenvironment, Vδ2 T cells might kill tumor cells and subsequently take up antigen by phagocytosis or trogocytosis, followed by presentation to tumor-reactive αβ T cells.53 The coating of tumor cells with antibodies (e.g., by therapeutic mAbs) could increase the efficacy of this process and additionally drive the licensing of γδ T cells for professional antigen presentation.54 In view of the so far limited success of dendritic cell-based antitumor vaccination, it appears unrealistic to expect better results with Vδ2 antigen-presenting cells (APCs). Nevertheless, such an approach might be advantageous if combined to other antitumor strategies. Along these lines, recent data indicate that γδ T cells play a pivotal role in determining the efficacy of anticancer chemotherapy. In several murine transplantable tumor models, anticancer drugs that induced immunogenic cell death (such as oxaliplatin or anthracyclines) triggered the local invasion of IL-17-producing γδ T cells, which occurred before and was required for the subsequent invasion of tumor-reactive cytotoxic T lymphocytes.55 Although it is presently unknown whether such a mechanism also applies to humans (and if so, which γδ T-cell subset is involved), this is an important issue for the future development of combinatorial immunotherapies against cancer.9
Functional Plasticity of γδ T Cells: Beware of the Suppressors
γδ T cells enjoy a remarkable degree of functional plasticity.4,5 As discussed above, circulating Vδ1 T cells exert potent anti-leukemia/lymphoma effector activities. In contrast, Vδ1 T cells infiltrating breast tumors exhibit immunosuppressive functions and inhibit αβ T-cell and dendritic-cell activation, thus supporting immune escape.56 Under the influence of transforming growth factor β (TGFβ), the regulatory activity associated with FOXP3 expression is also inducible in Vδ2 T cells.57 As with CD4+ T cells, the local micromilieu impacts on the functional differentiation of γδ T cells in the course of their activation. Tumor-derived inhibitory cytokines such as TGFβ and IL-10 are decisive factors for driving the development of regulatory γδ T cells. Therefore, an important issue for the development of γδ T cell-based immunotherapies, particularly adoptive cell transfer protocols, is to counteract the inhibitory differentiation pathway in γδ T cells, for instance by co-stimulation with TLR agonists.56
Concluding Remarks
γδ T cells are attractive candidates for anticancer immunotherapy, mainly due to their MHC-non restricted antitumor activity. As discussed here, Vδ2 and non-Vδ2 γδ T cells have a partially redundant antitumor profile. While the features and perspectives of these cell subsets have usually been investigated independently from each other, it seems more than reasonable to exploit their combined activity, at least in certain types of cancer such as acute leukemia and multiple myeloma, two settings in which both Vδ138,41 and Vδ218,58 T cells have been implicated. Moreover, the APC capacity of Vδ2 T cells harbors interesting perspectives for antitumor vaccination. In this regard, it looks as if non-Vδ2 γδ T cells might lose to Vδ2 T cells, but the potential APC function of non-Vδ2 γδ T cells remains to be investigated. Extrapolating the fascinating results on the role of IL-17-producing γδ T cells for successful chemotherapy in mice models to the human setting, it is presently unknown which one of the human γδ T-cell subsets – if any – would mediate a similar functional outcome. Possible activities of human γδ T-cell subsets that can be targeted in immunotherapeutic approaches are summarized in Figure 1. Taken together, all the open questions need to be addressed when pursuing γδ T cell immunotherapy – but there is no discernable reason to put Vδ2 T cells against non-Vδ2 γδ T cells. Mutualism appears indeed to be a innate part of the multi-faceted nature of these cells.
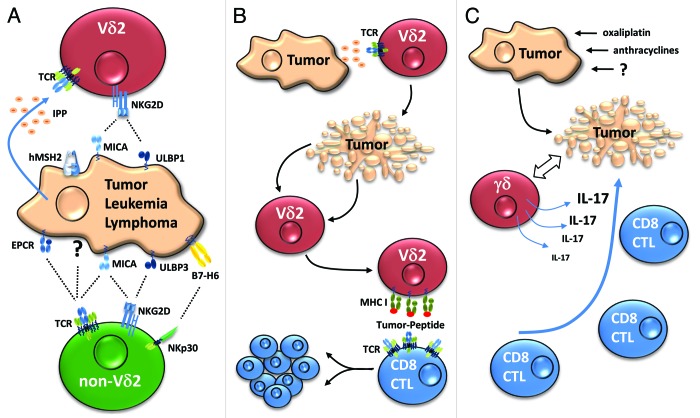
Figure 1. Possible roles of γδ T cells in antitumor immunity. (A) Direct cytotoxic effector activity. The cytotoxic potential of Vδ2 T cells is activated following the T-cell receptor (TCR)-dependent recognition of tumor-associated phosphoantigens (e.g., isopentenyl pyrophosphate IPP) or ectopically expressed molecules, such as human MutS homologue 2 (hMSH2), as well as following the activation of NKG2D by MHC Class I-related chain A (MICA) or UL16-binding protein 1 (ULBP1). The specific ligands of non-Vδ2 TCRs have not been precisely identified, with the exception of MICA for Vδ1 and EPCR for Vδ5. NKG2D on Vδ1 γδ T cells is preferentially activated by ULBP3, which is often expressed on the surface of leukemia and lymphoma cells. (B) Antigen-presenting function of Vδ2 T cells. Activated Vδ2 T cells kill tumor cells (top) and can engulf antigen by phagocytosis, endocytosis or trogocytosis (middle), process such antigens and subsequently present them to tumor-specific CD8+ cytotoxic T lymphocytes (CTLs) (bottom). (C) γδ T cells contribute to effective chemotherapy. Certain chemotherapeutic agents induce immunogenic tumor cell death (top), activating interleukin-17 (IL-17)-secreting γδ T cells (middle) that are required (at least in mice) for the subsequent recruitment and activation of tumor-specific CTLs (bottom).
Acknowledgments
Work from our laboratory is supported by the Pancreatic Cancer Consortium Kiel funded by the Deutsche Forschungsgemeinschaft (WE 3559/2-1). S.K. was supported by a fellowship from the Alexander-von Humboldt Foundation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23304
References
- 1.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–78. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 3.Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human γ δ T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 4.Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136:283–90. doi: 10.1111/j.1365-2567.2012.03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabelitz D, He W. The multifunctionality of human Vγ9Vδ2 γδ T cells: clonal plasticity or distinct subsets? Scand J Immunol. 2012;76:213–22. doi: 10.1111/j.1365-3083.2012.02727.x. [DOI] [PubMed] [Google Scholar]
- 6.Meuter S, Eberl M, Moser B. Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells. Proc Natl Acad Sci U S A. 2010;107:8730–5. doi: 10.1073/pnas.1002769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braza MS, Klein B. Anti-tumour immunotherapy with Vγ9Vδ2 T lymphocytes: from the bench to the bedside. Br J Haematol. 2013;160:123–32. doi: 10.1111/bjh.12090. [DOI] [PubMed] [Google Scholar]
- 8.Siegers GM. Anti-leukemia activity of human γ δ T cells. Oncoimmunology. 2012;1:237–9. doi: 10.4161/onci.1.2.18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannani D, Ma Y, Yamazaki T, Déchanet-Merville J, Kroemer G, Zitvogel L. Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol. 2012;33:199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Kalyan S, Kabelitz D. Defining the nature of human γδ T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol. 2013;10:21–9. doi: 10.1038/cmi.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtmeier W, Pfänder M, Hennemann A, Zollner TM, Kaufmann R, Caspary WF. The TCR-δ repertoire in normal human skin is restricted and distinct from the TCR-δ repertoire in the peripheral blood. J Invest Dermatol. 2001;116:275–80. doi: 10.1046/j.1523-1747.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Fang Z, Morita CT. Vgamma2Vdelta2 T Cell Receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol. 2010;184:6209–22. doi: 10.4049/jimmunol.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001;509:317–22. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 14.Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, et al. Preferential recognition of a microbial metabolite by human Vgamma2Vdelta2 T cells. Int Immunol. 2007;19:657–73. doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- 15.Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, et al. Lysis of a broad range of epithelial tumour cells by human γ δ T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–8. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 16.Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–96. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 17.Gomes AQ, Correia DV, Grosso AR, Lança T, Ferreira C, Lacerda JF, et al. Identification of a panel of ten cell surface protein antigens associated with immunotargeting of leukemias and lymphomas by peripheral blood gammadelta T cells. Haematologica. 2010;95:1397–404. doi: 10.3324/haematol.2009.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gertner-Dardenne J, Castellano R, Mamessier E, Garbit S, Kochbati E, Etienne A, et al. Human Vγ9Vδ2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188:4701–8. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 19.Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benzaïd I, Mönkkönen H, Stresing V, Bonnelye E, Green J, Mönkkönen J, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011;71:4562–72. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, Chen H, Mo C, Cui L, He W. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human γδ T cells to induce innate anti-tumor/virus immunity. J Biol Chem. 2012;287:16812–9. doi: 10.1074/jbc.M111.327650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mo C, Dai Y, Kang N, Cui L, He W. Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem. 2012;287:19242–54. doi: 10.1074/jbc.M112.349936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V γ 9V δ 2 T cells by NKG2D. J Immunol. 2005;175:2144–51. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 24.Lança T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood. 2010;115:2407–11. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 25.Shojaei H, Oberg HH, Juricke M, Marischen L, Kunz M, Mundhenke C, et al. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human gammadelta T cells. Cancer Res. 2009;69:8710–7. doi: 10.1158/0008-5472.CAN-09-1602. [DOI] [PubMed] [Google Scholar]
- 26.Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, So HF, et al. V γ 9 V δ 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs--rituximab and trastuzumab. Int J Cancer. 2008;122:2526–34. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 27.Capietto AH, Martinet L, Fournié JJ. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. . J Immunol. 2011;187:1031–8. doi: 10.4049/jimmunol.1100681. [DOI] [PubMed] [Google Scholar]
- 28.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–7. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 29.Kabelitz D, Wesch D, Pitters E, Zöller M. Characterization of tumor reactivity of human V γ 9V δ 2 γ δ T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767–76. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- 30.D’Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. V γ 9V δ 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–8. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- 31.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G, et al. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–60. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J, et al. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35:205–13. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- 34.Kalyan S, Quabius ES, Wiltfang J, Mönig H, Kabelitz D. Can peripheral blood γδ T cells predict osteonecrosis of the jaw? An immunological perspective on the adverse drug-effects of aminobisphosphonate therapy. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1769. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. . Br J Cancer. 2011;105:778–86. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1075–84. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC, et al. Questionable relevance of γ δ T lymphocytes in renal cell carcinoma. J Immunol. 2008;180:3578–84. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meeh PF, King M, O’Brien RL, Muga S, Buckhalts P, Neuberg R, et al. Characterization of the gammadelta T cell response to acute leukemia. Cancer Immunol Immunother. 2006;55:1072–80. doi: 10.1007/s00262-005-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y, Felizardo TC, et al. Human Vδ1 γδ T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy. 2011;13:753–64. doi: 10.3109/14653249.2011.553595. [DOI] [PubMed] [Google Scholar]
- 40.Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. . Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 41.Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy. 2012;14:1110–8. doi: 10.3109/14653249.2012.700766. [DOI] [PubMed] [Google Scholar]
- 42.Catellani S, Poggi A, Bruzzone A, Dadati P, Ravetti JL, Gobbi M, et al. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood. 2007;109:2078–85. doi: 10.1182/blood-2006-06-028985. [DOI] [PubMed] [Google Scholar]
- 43.Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A. 2011;108:2414–9. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–96. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 45.Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M, et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64:9172–9. doi: 10.1158/0008-5472.CAN-04-2417. [DOI] [PubMed] [Google Scholar]
- 46.Siegers GM, Ribot EJ, Keating A, Foster PJ. Extensive expansion of primary human gamma delta T cells generates cytotoxic effector memory cells that can be labeled with Feraheme for cellular MRI. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1353-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dokouhaki P, Han M, Joe B, Li M, Johnston MR, Tsao MS, et al. Adoptive immunotherapy of cancer using ex vivo expanded human gammadelta T cells: A new approach. Cancer Lett. 2010;297:126–36. doi: 10.1016/j.canlet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–49. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, et al. Shared reactivity of Vδ2(neg) γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. . J Exp Med. 2005;201:1567–78. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devaud C, Bilhere E, Loizon S, Pitard V, Behr C, Moreau JF, et al. Antitumor activity of gammadelta T cells reactive against cytomegalovirus-infected cells in a mouse xenograft tumor model. Cancer Res. 2009;69:3971–8. doi: 10.1158/0008-5472.CAN-08-3037. [DOI] [PubMed] [Google Scholar]
- 51.Couzi L, Levaillant Y, Jamai A, Pitard V, Lassalle R, Martin K, et al. Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol. 2010;21:181–8. doi: 10.1681/ASN.2008101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872–9. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 53.Moser B, Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci. 2011;68:2443–52. doi: 10.1007/s00018-011-0706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol. 2012;188:1708–16. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]
- 55.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing γ δ T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–48. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 57.Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, et al. Cutting edge: TGF-β1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574–7. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
- 58.Burjanadzé M, Condomines M, Reme T, Quittet P, Latry P, Lugagne C, et al. In vitro expansion of gamma delta T cells with anti-myeloma cell activity by Phosphostim and IL-2 in patients with multiple myeloma. . Br J Haematol. 2007;139:206–16. doi: 10.1111/j.1365-2141.2007.06754.x. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Wang Q, Mariuzza RA. Structure of the human activating natural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J Exp Med. 2011;208:703–14. doi: 10.1084/jem.20102548. [DOI] [PMC free article] [PubMed] [Google Scholar]