Abstract
Orchestrating a cytotoxic polymorphonuclear neutrophil (PMN) response strictly focused within the tumor tissue remains a formidable challenge for the successful therapeutic use of these cells. A Salmonella vector carrying an shRNA against indoleamine 2,3-dioxygenase has been shown to recruit PMNs and enhance their activation specifically in the tumor bed, resulting in significant anticancer effects.
Keywords: B16F10 melanoma; indoleamine 2,3-dioxygenase; polymorphonuclear neutrophils; Salmonella; small hairpin RNA
Although polymorphonuclear neutrophils (PMNs) have been contemplated as effectors of antitumor immunity, feasible strategies to focus PMN responses to malignant tissues while avoiding systemic toxic effects, such as those associated with high-dose cytokines or chemotherapy, are still lacking.1 Furthermore, the conditions required to generate PMNs that exert antitumor (N1-polarized) vs. pro-tumor (N2-polarized) effects, are still largely unknown. In a recent study published in Cancer Research,2 we exploited the unique properties of Salmonella as a tumor-homing vector and as a natural attractant for PMNs. As a vector, we used Salmonella to deliver a short-hairpin RNA (shRNA) targeting indoleamine 2,3-dioxygenase (IDO) (shIDO-ST), which is known to operate as a natural suppressor of immune cell function.3 More recently, it has been shown that IDO and its metabolic byproducts induce the apoptotic demise of PMNs, presumably by promoting a consistent depletion of tryptophan in the local microenvironment. Thus, by shutting down IDO expression within the tumor, PMN recruited to clear Salmonella infection are more likely to become activated as compared with PMNs infiltrating IDO-expressing tumors.
As shown in Figure 1, we have found that IDO plays an important role in regulating Salmonella colonization and the recruitment and activation of PMNs within tumors. Inhibiting the expression of tumor-derived IDO presumably lowers the threshold for Salmonella to induce the local production of chemokines involved in PMN infiltration. This notion is supported by clinical studies that have correlated high IDO expression levels in tumors with reduced immune infiltration.4 The downregulation of IDO also exacerbates the propagation of Salmonella within the tumor, and allows for an efficient activation of PMNs to produce reactive oxygen species (ROS), resulting in increased oxidative stress within the tumor microenvironment to cause significant tumor-cell apoptosis and the subsequent clearance of shIDO-ST. We believe that the antitumor activity of shIDO-ST represents a byproduct of ROS-producing PMNs originally recruited to the tumor to clear shIDO-ST infection.
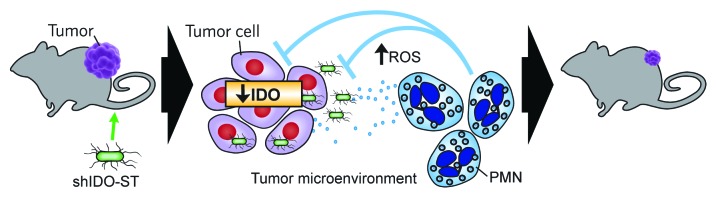
Figure 1. Indoleamine 2,3-dioxygenase silencing by shIDO-ST results in the recruitment and activation of polymorphonuclear neutrophils in tumors. Proposed mechanism of action of shIDO-ST. Left to right: In a B16F10 melanoma-bearing mouse, shIDO-ST is injected intravenously. ShIDO-ST accumulates within tumors and is cleared from peripheral organs within 48–72 h. Indoleamine 2,3-dioxygenase (IDO) silencing, which occurs in infected tumor cells, combined with the presence of Salmonella, results in a cascade of signaling events that recruits polymorphonuclear neutrophils (PMNs) into the tumor tissue. The presence of Salmonella presumably activates PMNs to begin the clearance of infection through the production and secretion of reactive oxygen species (ROS), which make the microenvironment toxic for both bacteria and tumor cells. This mechanism has already been observed in murine models of melanoma and pancreatic ductal adenocarcinoma and could be applicable to a variety of other IDO-expressing tumors.
This work provides further evidence that inactivating immunosuppressive molecules, such as IDO, is a viable strategy for the development of novel immunotherapeutic strategies against cancer. We have previously demonstrated that a combination of a vaccine targeting tumor-associated antigens with the inactivation of the immunosuppressive molecule signal transducer and activator of transcription 3 (STAT3) results in greater tumor control than either intervention alone.5 As shIDO-ST elicits an innate PMN response, it is difficult to imagine that this approach might generate a long-lasting memory component that would prevent relapse. However, the use of shIDO-ST (which inhibits immunosuppression) in combination with a cancer vaccine could provide a prolonged synergistic antitumor effect, stemming from the coalescence of innate and adaptive immune responses.
At least theoretically, shIDO-ST can be used in several IDO-expressing tumors. In this sense, we have already shown that shIDO-ST successfully controls tumor growth in a murine model of pancreatic ductal adenocarcinoma (PDAC).2 Advanced PDAC is resistant to many chemotherapies, at least in part due to extreme degrees of desmoplasia (which decreases the efficiency of drug delivery). In this setting, shIDO-ST constitutes a prime candidate therapy, due to the ability of Salmonella to penetrate and colonize PDAC by virtue of its motility and affinity for hypoxic tissues. More recently, tumor-derived IDO has been implicated in regulatory T cell (Treg) recruitment and has been associated with poor prognosis in glioblastoma multiforme (GBM) patients.6,7 Agents such as D-1-methyl-tryptophan (D-1MT), imatinib, and acyclovir could be used to modulate IDO activity. However, immunotoxic and off-target effects created by a systemic administration, especially in the brain, may put patients at a high risk for complications. As we have shown, shIDO-ST can offer a good degree of specificity against tumor-derived IDO and is able to induce an innate immune response that is not suppressed by the presence of Tregs. This may prove to be an advantage, as it may be difficult to induce an adaptive immune response in an area of the brain (for instance affected by GBM) that is already heavily populated by Tregs. In fact, many tumors are characterized by an influx of mature dendritic cells (DCs) that express copious amounts of IDO, resulting in the generation of Tregs that suppress T-cell proliferation, as well as T-cell responses to tumor antigens.8
Although the phenotypes of N1 and N2 PMNs have been studied in great detail, the factors that drive PMN polarization are not yet fully understood. Recently, Fridlender et al. have shown that the immunosuppressive cytokine transforming growth factor β (TGFβ) can drive the polarization of neutrophils toward a pro-tumor N2 phenotype, while inhibiting the generation of antitumor N1 neutrophils.9 Interestingly, TGFβ has also been implicated in the long-term expression of IDO by DCs, thus exerting additional pro-tumor effects through the generation of immunosuppressive Tregs. Another cytokine, interferon β (IFNβ), has been shown to reduce the angiogenic activity of neutrophils and increase their cytotoxicity, thus exerting antitumor functions.10 We would predict that shIDO-ST might induce a cytokine profile skewed toward antitumor functions (i.e., characterized by low TGFβ and increased IFNβ levels) to produce an increased frequency of N1 neutrophils.
The finding that IDO regulates the infiltration and antitumor functions of PMNs may help to develop strategies to improve current immunotherapeutics for cancer patients. Further studies of neutrophils as elicited by shIDO-ST are required and may reveal phenotypes that are still—at least in part—pro-tumor. Thus, modifications to shIDO-ST such as the co-expression of cytokines that would drive PMNs to a completely N1-polarized phenotype may improve its antitumor effects. Overall, the ability of shIDO-ST to control tumor growth in a variety of models through the recruitment and activation of PMNs supports a role for innate immunity in antitumor therapy.
Glossary
Abbreviations:
- IDO
indoleamine 2,3-dioxygenase
- PMN
polymorphonuclear neutrophil
- shRNA
small-hairpin RNA
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23322
References
- 1.Carey PD, Wakefield CH, Guillou PJ. Neutrophil activation, vascular leak toxicity, and cytolysis during interleukin-2 infusion in human cancer. Surgery. 1997;122:918–26. doi: 10.1016/S0039-6060(97)90333-0. [DOI] [PubMed] [Google Scholar]
- 2.Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, et al. Systemic Delivery of Salmonella typhimurium Transformed with IDO shRNA Enhances Intratumoral Vector Colonization and Suppresses Tumor Growth. Cancer Res. 2012;72:6447–56. doi: 10.1158/0008-5472.CAN-12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKenzie CR, Heseler K, Müller A, Däubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–44. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 4.Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008;14:2310–7. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 5.Manuel ER, Blache CA, Paquette R, Kaltcheva TI, Ishizaki H, Ellenhorn JD, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res. 2011;71:4183–91. doi: 10.1158/0008-5472.CAN-10-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO Expression in Brain Tumors Increases the Recruitment of Regulatory T Cells and Negatively Impacts Survival. Clin Cancer Res. 2012;18:6110–21. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi BD, Fecci PE, Sampson JH. Regulatory T Cells Move in When Gliomas Say “I DO”. Clin Cancer Res. 2012;18:6086–8. doi: 10.1158/1078-0432.CCR-12-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–63. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]


