Abstract
Natural killer (NK) cells possess effector and immunoregulatory functions that are controlled by a myriad of receptor-ligand pairs, including human killer inhibitory receptor (KIR) and mouse Ly49-MHC class I interactions. We have recently shown that the NK cell inhibitory molecule Ly49A binds the non-classical MHC molecule H2-M3, thus regulating host innate immune responses to tumor initiation and metastasis.
Keywords: education, inhibitory, metastasis, tumor immunosurveillance
The immune system is clearly an important regulator of tumor development.1 In contrast to the antigen-specific regulation of adaptive immunity, natural killer (NK) cell-mediated responses are controlled by receptors that convey activating or inhibitory signals. Early studies of NK cell inhibition demonstrated that the absence of MHC class I molecules on the surface of cancer cells resulted in their clearance, leading to the “missing self” hypothesis.2 Molecular data in support of this model was provided with the identification of MHC class I recognition by the inhibitory receptor Ly49A. The “missing self” theory proposed that the absence of MHC class I molecules should render cells more susceptible to NK cell-mediated killing. An extension of this model was the “at least one” proposal, suggesting that - for missing self to be active - each NK cell must express an inhibitory receptor that recognizes self MHC molecules.3 However, neither of these models can completely explain NK-cell tolerance, since not all Ly49 molecules have been shown to bind to host MHC molecules and NK cells from MHC class I-deficient mice do not acquire full effector function. These facts raised some doubt about the fidelity of NK-cell inhibitory receptor interactions with MHC class Ia molecules.
Members of the non-classical MHC class Ib family, most significantly Rae/ULBP (NKG2D) and HLA-E/Qa-1b (NKG2ACE), also regulate the functions of NK cells. Given the importance of these non-classical MHC molecules in NK-cell function, it is surprising to note that whether other members of this family actively regulate NK cell biology has not been intensively investigated. H2-M3 is a relatively non-polymorphic MHC class Ib molecule from the same non-classical region as Qa-1b, and was first identified as a minor histocompatibility antigen. The mRNA encoding H2-M3 can be found in most tissues of all strains of mice at a lower level than that coding for classical MHC class I molecules. However, in contrast to classical MHC molecules, most cells do not express H2-M3 on their surface, B cells being a notable exception. H2-M3 specifically binds N-formylated peptides that contain hydrophobic residues with an affinity 100–1000 times greater than that for standard peptides. Given that prokaryotes and mitochondria are the only sources of N-formylated peptides, H2-M3 appears to have evolved to present peptides of bacterial (or mitochondrial) origin.4 Indeed, in the past 20 years attention has been focused on the of role H2-M3 in the biology of a subset of CD8+ T cells. Intriguingly, the first description of H2-M3-deficient mice reported an impaired capacity of lymphocytes from these mice to kill NK cell-sensitive targets.5
Our recent findings demonstrate that H2-M3 can be recognized by Ly49A, the prototypic NK-cell inhibitory receptor.6 This existence of non-classical MHC class Ib molecule-binding receptors specific for allotypic classical class I indicates that there may be a previously unrecognized crossover between these receptor-ligand families. Potentially, this result provides further support to the “at least one” hypothesis and suggests that ligands for Ly49 (and potentially KIR) may exist outside the classical MHC family. From a functional standpoint, the absence of H2-M3 results in NK-cell hyporesponsiveness (due to the ineffective licensing of the Ly49A+ NK-cell subset) and missing-self rejection (H2-M3 acts a self molecule recognized by NK cells) (Fig. 1). Consequently, H2-M3-deficient mice display increased sensitivity to oncogenesis, tumor progression and metastatic spread, the latter in a Ly49A-dependent fashion. Distinct genetic and phenotypic alterations of tumor cells allow for their escape from the control of the immune system, representing a critical step in cancer progression.7 The ability of inhibitory Ly49A to bind H2-M3, which is constitutively expressed by some malignant cells, suggests that tumors may potentially use this interaction to escape both NK and Ly49A-expressing T cells.
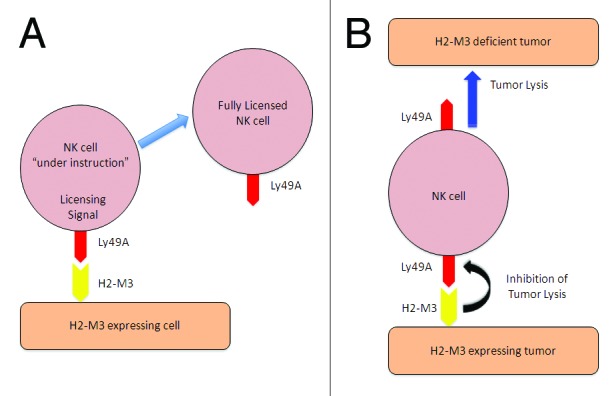
Figure 1. Interactions between H2-M3 and Ly49A regulate natural killer cell education and tumor control. (A) H2-M3 assists in the “licensing” of Ly49A+ natural killer (NK) cells. An interaction between Ly49A on NK cells and H2-M3 on other cells (the identity of the cell conferring the “license” has not been demonstrated) results in a signal for NK cells to become fully mature and recognize self. These NK cells are then fully competent to control infected or neoplastic cells without attacking self. (B) The engagement of H2-M3 on the surface of tumors prevents killing by NK cells. The upregulation of H2-M3 by tumor cells results in the engagement of Ly49A and the subsequent delivery of an inhibitory signal, preventing the NK cell-mediated control of the tumor (bottom). In the absence of H2-M3, Ly49A is not engaged and NK cells are released from such inhibition (top).
Although there is not a direct human homolog of H2-M3, it is possible that a functional homolog exists. Indeed, T-cell responses to N-formylated peptides derived from self (mitochondrial) and foreign (microbial) antigens have been described in humans.8 HLA-F is physiologically expressed by cell types similar to those that express H2-M3, and has been shown to bind inhibitory ILT-2 and ILT-4 receptors. Still, HLA-F lacks an obvious consensus sequence for the binding of N-formylated peptides. HLA-E and HLA-G are the best studied non-classical MHC-like molecules,9 but a better characterization of the functions of MHC-linked class Ib loci in humans and mice (H2-Q, H2-T, and H2-M) is required. Similarly, our work should prompt an examination of whether other Ly49 molecules have non-MHC class Ia binding partners.
The ability of H2-M3 to present mitochondrial peptides raises the interesting possibility that interactions with Ly49A-expressing lymphocytes may be controlled by mitochondrial integrity. A number of tumor cell lines constitutively express H2-M3. In addition, H2-M3 can be induced by a number of potentially lethal stimuli in B16F10 melanoma cells including ionizing radiation, mitomycin C, 5-fluorouracil, and the histone deacteylase inhibitor LBH. This pattern of induction is distinct from that observed for NKG2D ligands, which are mainly upregulated upon the activation of the DNA damage response.10 Whether mitochondrial peptides are exposed on the cell surface in the context of H2-M3 following these insults remains to be established. One might imagine that such exposure may regulate peripheral Ly49A+ lymphocyte subsets in situations of stress or inflammation.
While the ability of classical MHC class Ia molecules to present tumor antigens to CD8+ T cells is a fundamental premise for most modern cancer immunotherapies, the role of non-classical MHC class Ib molecules in regulating the immune response to cancer deserves greater attention.
Glossary
Abbreviations:
- KIR
killer inhibitory receptor
- NK
natural killer
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23336
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Kärre K. How to recognize a foreign submarine. Immunol Rev. 1997;155:5–9. doi: 10.1111/j.1600-065X.1997.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 3.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 4.Colmone A, Wang CR. H2-M3-restricted T cell response to infection. Microbes Infect. 2006;8:2277–83. doi: 10.1016/j.micinf.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J Exp Med. 2006;203:449–59. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews DM, Sullivan LC, Baschuk N, Chan CJ, Berry R, Cotterell CL, et al. Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells. Nat Immunol. 2012;13:1171–7. doi: 10.1038/ni.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Ristori G, Montesperelli C, Fiorillo MT, Battistini L, Chersi A, Sorrentino R, et al. T cell response to N-formylated peptides in humans. Eur J Immunol. 2001;31:2762–70. doi: 10.1002/1521-4141(200109)31:9<2762::AID-IMMU2762>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Bukur J, Jasinski S, Seliger B. The role of classical and non-classical HLA class I antigens in human tumors. Semin Cancer Biol. 2012;22:350–8. doi: 10.1016/j.semcancer.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]


