Abstract
Myeloid-derived suppressor cells (MDSCs) promote tumor growth and metastasis. We have recently demonstrated that the pro-inflammatory cytokine macrophage migration inhibitory factor (MIF) enhances the immunosuppressive microenvironment by increasing the abundance of monocytic MDSCs within the tumor. Our results suggest that MIF is a potential therapeutic target for the prevention of metastasis as it regulates the tumor microenvironment.
Keywords: MDSC, metastasis, MIF, sulforaphane, tautomerase, tumor microenvironment
By the time most cancers become clinically evident, malignant cells can be found in the patient’s circulation.1 In view of this, it is perhaps surprising that the frequency of metastatic recurrence in treated cancer patients does not approach 100%. While some aspects of metastatic colonization are undoubtedly correlated with tumor cell-intrinsic factors, significant contributions to the metastatic process come from the host. Progress toward the goal of reducing metastatic disease will rely on the understanding of the functional interplay between early metastatic cancer cells and the host environment, in particular of the immune mechanisms that malignant cells exploit for their survival during metastasis.
A robust myeloid cell infiltrate is generally a poor prognostic indicator in human tumors.2 Several mechanisms whereby tumor cells manipulate the abundance of myeloid cells or otherwise support tumor progression have been characterized in experimental models. Many cancers stimulate myeloid-derived suppressor cells (MDSCs), a heterogeneous population of immature myeloid cells that suppress anticancer immune responses. Murine MDSCs are positive for the differentiation markers GR1 and CD11b and can be further subdivided into a monocytic and a granulocytic subpopulation, based on Ly6C and Ly6G expression, respectively.3 Both types of MDSCs suppress T-cell responses through partially, but not completely, overlapping mechanisms,3,4 though monocytic MDSCs are generally viewed as the most immunosuppressive subpopulation.3,5 The identification of MDSCs in humans has been slowed by the absence of a similarly simple panel of cell surface markers. Recent efforts to identify human MDSCs have used overlapping panels of markers to isolate cells that suppress T-cell responses in vitro.6 Thus, MDSCs are likely to impact human cancer through the suppression of antitumor immune responses.
The macrophage migration inhibitory factor (MIF) was initially characterized for its role in inflammation. MIF is overexpressed in many cancers, and the degree of expression often correlates with tumor aggressiveness and metastatic potential.7 Further linking MIF to cancer, we identified MIF as a functional target for a class of cancer-preventive compounds.8 We showed that these chemicals (found in food) covalently modify MIF, inhibiting its enzymatic tautomerase activity. Though this enzymatic activity of MIF is highly conserved, no natural substrate has been identified yet, and its contribution to the biological activities of MIF remains controversial. Previous work evaluating the role for MIF in cancer had focused on the impact of MIF on tumor cell-intrinsic aspects, including growth responses, the activation/inhibition of signaling cascades and cell cycle progression.9 However, given its role as an pro-inflammatory cytokine, we hypothesized that MIF might influence the interaction between the tumor cells and the host immune system, rather than impact an intrinsic property of tumor cells.
We have recently investigated the role of MIF in cancer growth and metastasis using a model of highly aggressive breast cancer (4T1 cells).10 We demonstrated that the depletion of MIF has no impact on the growth of cancer cells in vitro. Along similar lines, when cancer cells were orthotopically implanted into the mammary fat pads of immunodeficient SCID/beige mice, MIF-containing and MIF-depleted tumors exhibited similar growth rates and were comparably capable of generating pulmonary metastases. However, when implanted into immunocompetent mice, MIF-depleted tumors grew more slowly and were dramatically compromised in their ability to form metastases in the lung. These observations demonstrate that—at least in this tumor model—MIF does not affect cancer cells directly, but instead influence the interaction between malignant cells and the host immune system.
When we examined tumor infiltrates, we found that MIF-containing lesions contained abundant monocytic MDSCs, while their MIF-deficient counterparts presented fewer such cells. Reconstitution of MIF-depleted cells with wild-type MIF restored tumor infiltration by monocytic MDSCs, as well as growth rates and the ability of cancer cells to generate pulmonary metastases. In contrast, a tautomerase-inactive MIF variant failed to do so, and the abundance of tumor-infiltrating monocytic MDSCs in this setting was similar to that observed in MIF-deficient tumors. This suggests that the tautomerase activity of MIF is important for its effects on MDSCs and tumor metastasis. In support of this notion, the administration of a MIF inhibitor, sulforaphane, decreased the abundance of tumor-infiltrating monocytic MDSCs. Of note, MDSCs purified from MIF-expressing tumors suppressed to comparatively higher extents T-cell proliferation in vitro, presumably reflecting a higher prevalence of monocytic MDSCs in this cell population. We hypothesized that a factor produced by MIF-expressing 4T1 cells might induce the proliferation or the differentiation of monocytic MDSCs, and showed that the co-culture of splenic myeloid cells with media conditioned by MIF-expressing 4T1 cells drives the accumulation of monocytic MDSCs. This accumulation was reduced if conditioned media were obtained from MIF-deficient cells or were treated with the MIF inhibitor sulforaphane.
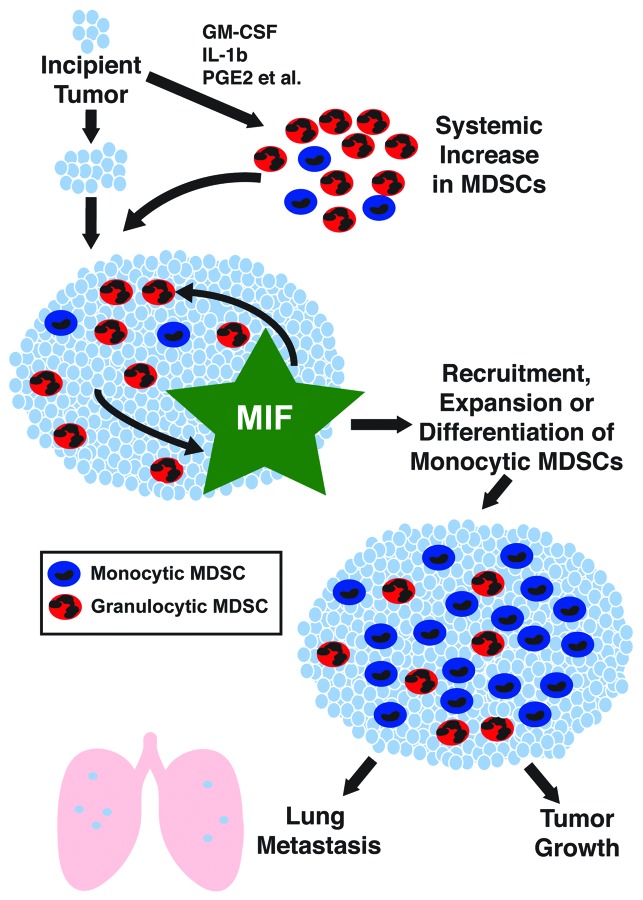
Our findings support a model in which MIF promotes tumor growth and metastasis by influencing the abundance of tumor-infiltrating monocytic MDSCs (Fig. 1). Therefore, we suggest that MIF inhibition might be an effective strategy for reducing the incidence of metastatic recurrence in cancer patients through influence over the host immune response. Our report demonstrates one approach for such control, namely, the neutralization of the tautomerase activity of MIF with the cancer preventive agent sulforaphane. In the future, conventional anticancer therapies may be combined with approaches to make the patient microenvironment less hospitable for the survival of micrometastases. MIF inhibition may be a valuable weapon in this fight.
Figure 1. Mechanisms of MIF-dependent tumor growth and metastasis. Factors produced by incipient tumors induce monocytic and granulocytic myeloid-derived suppressor cells (MDSCs) in the circulation, spleen and within the tumor. The production of macrophage migration inhibitory factor (MIF) by tumor cells leads to an increased abundance of monocytic MDSCs within the tumor, through enhanced recruitment, expansion or local differentiation. Monocytic MDSCs suppress antitumor immune responses, de facto underlying the MIF-induced stimulation of tumor growth and metastasis. MIF inhibition maybe a valuable approach for the prevention of metastatic disease by targeting the host microenvironment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23337
References
- 1.Zhe X, Cher ML, Bonfil RD. Circulating tumor cells: finding the needle in the haystack. Am J Cancer Res. 2011;1:740–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35:107–15. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 7.Grieb G, Merk M, Bernhagen J, Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 2010;23:257–64. doi: 10.1358/dnp.2010.23.4.1453629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross JV, Rady JM, Foss FW, Lyons CE, Macdonald TL, Templeton DJ. Nutrient isothiocyanates covalently modify and inhibit the inflammatory cytokine macrophage migration inhibitory factor (MIF) Biochem J. 2009;423:315–21. doi: 10.1042/BJ20091170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell RA, Bucala R. Tumor growth-promoting properties of macrophage migration inhibitory factor (MIF) Semin Cancer Biol. 2000;10:359–66. doi: 10.1006/scbi.2000.0328. [DOI] [PubMed] [Google Scholar]
- 10.Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 2012;189:5533–40. doi: 10.4049/jimmunol.1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]