Abstract
β-glucans are complex, naturally-occurring polysaccharides that prime leukocyte dectin and complement receptor 3. Based on our preclinical findings, indicating that oral barley-derived (1 → 3),(1 → 4)-β-D-glucan (BG) synergizes with the murine anti-GD2 antibody 3F8 against neuroblastoma, we conducted a Phase I clinical study to evaluate the safety of this combinatorial regimen in patients affected by chemoresistant neuroblastoma. In this setting, four cohorts of six heavily pre-treated patients bearing recurrent or refractory advanced-stage neuroblastoma were treated with 3F8 plus BG. Each cycle consisted of intravenous 3F8 at a fixed dose of 10 mg/m2/day plus concurrent oral BG, dose-escalated from 10 to 80 mg/Kg/day, for 10 d. Patients who did not develop human anti-mouse antibodies could be treated for up to 4 cycles. Twenty-four patients completed 50 cycles of therapy. All patients completed at least one cycle and were evaluable for the assessment of toxicity and responses. The maximum tolerated dose of BG was not reached, but two patients developed dose-limiting toxicities. These individuals developed grade 4 thrombocytopenia after one cycle of BG at doses of 20 mg/Kg/day and 40 mg/Kg/day, respectively. Platelet counts recovered following the administration of idiopathic thrombocytopenic purpura therapy. There were no other toxicities of grade > 2. Eleven and 13 patients manifested stable and progressive disease, respectively. Thirteen out of 22 patients with pre-treatment positive 123I-MIBG scans demonstrated clinical improvement on semiquantitative scoring. Responses did not correlate with BG dose or with in vitro cytotoxicity. In summary, 3F8 plus BG is well tolerated and shows antineoplastic activity in recurrent or refractory advanced-stage neuroblastoma patients. Further clinical investigation of this novel combinatorial immunotherapeutic regimen is warranted.
Keywords: 3F8, antibody, immunotherapy, neuroblastoma, β-glucan
Introduction
A majority of patients with neuroblastoma (NB), the most common extracranial solid tumor of children, relapse and die despite aggressive multimodality therapy.1-4 Antibody-mediated anti-GD2 immunotherapy has emerged as an exciting addition to the standard management of NB, as demonstrated in a Phase III study.5 However, for efficacy, this approach requires the concomitant administration of cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-2, the latter being associated with significant toxicity.6 Furthermore, anti-GD2 monoclonal antibodies (mAbs) exert modest antineoplastic activity in patients bearing chemorefractory NB exceeding the minimal residual disease.7 The anti-GD2 murine mAb 3F8 activates the complement system8 and mediates NB cytotoxicity by human lymphocytes,9 cultured monocytes10 and granulocytes.11 3F8 binds the FcγRII and FcγRIII Fc receptors and exploits the GM-CSF-mediated activation of CD11b for eliciting antibody-dependent cellular cytotoxicity (ADCC) by myeloid cells.12,13 3F8 has been studied in Phase I and II trials, with demonstrated safety, and exerts antineoplastic activity in NB patients.14-17
β-glucans are complex glucose polymers containing a backbone of β-1,3-linked and β-1,4-D-glucose molecules.18 β-glucans are found in food including cereals, mushrooms and yeast, as well as in some bacteria. The first clinical studies of β-glucans as antitumor agents were reported by Japanese investigators in the 1980s, although preclinical investigations have been ongoing for nearly 40 y.19 The mushroom-derived (1 → 3),(1 → 6)-β-D-glucans, protein-bound polysaccharide and schizophyllan modestly improved the survival rate of patients with gastrointestinal cancer.20,21 Conversely, barley-derived (1 → 3),(1 → 4)-β-D-glucans have undergone clinical testing primarily for their cholesterol-reducing activity.22
Glucan-binding receptors on human leucocytes include lactosylceramide,23 dectin-124 and the complement receptor 3 (CR3).25 Most recent studies indicate that CR3 is the major receptor mediating the immunological effects of β-glucans.26,27 mAbs allow for the fixation of the complement factor C3b on tumor cells, and the proteolytic fragment iC3b binds CR3 (also known as CD11b/CD18, Mac 1 or αMβ2 integrin), which is expressed on the surface of neutrophils, monocytes, macrophages and natural killer (NK) cells. The activation of CR3, which facilitates diapedesis, phagocytosis and degranulation,28,29 requires the engagement of two sites on its α subunit (CD11b): an iC3b-binding site within the I domain at the N-terminus and a lectin site at the C-terminus, which is bound by β-glucans.30 Of note, iC3b-opsonized tumor cells cannot activate leukocytes unless the C-terminus of CR3 is bound by β-glucans.31,32 Along similar lines, in pre-clinical models, tumors fail to respond to β-glucans in the absence of complement-fixing anti-tumor mAbs (as demonstrated in SCID mice), C3 or leukocyte CR3 (as demonstrated in knockout mouse models), highlighting the essential components of this iC3b-based cytotoxic strategy.32 Although barley-derived (1 → 3),(1 → 4)-β-D-glucan (BG) binds to CR333 and activates ADCC in vitro,25,34,35 most previous studies of the immunomodulatory activity of β-glucans in cancer have focused on mushroom or yeast-derived (1 → 3),(1 → 6)-β-D-glucan.36 In xenograft models, the antitumor effect of complement-activating mAbs was enhanced by oral BG,37,38 as well as by (1 → 3),(1 → 6)-β-D-glucan from yeast or mushrooms.39 Here, we report the results of a Phase I clinical study involving BG in combination with the anti-GD2 mAb 3F8 in patients with chemoresistant metastatic NB.
Results
Patients
Twenty-four heavily-pretreated patients (13 males, 11 females), with a median age of 8 y (range: 35 mo to 19 y), entered the study, eight with progressive disease (PD), and 16 with stable disease (SD) (Table 1). Previous therapeutic regimens included stem-cell transplantation, in 16 patients. A total of 50 cycles of 3F8 plus BG were administered, with patients receiving 1 (n = 12), 2 (n = 4), 3 (n = 2) or 4 (n = 6) cycles. Patients were taken off study due to the development of human anti-mouse antibody (HAMA) responses after cycle 1 (n = 3) or cycle 2 (n = 3); owing to PD after cycle 1 (n = 7), cycle 2 (n = 1) or cycle 3 (n = 2); or upon dose-limiting toxicities (DLTs) (n = 2).
Table 1. Patient characteristics and toxicities.
| Pt. No. | Age at Rx (yrs) | Dose level mg/kg/day |
No. of prior regimens | Pre-Rx status | Pre-therapy extent of disease | No. of cycles | Reason for withdrawal | Unexpected therapy-related toxicities |
|---|---|---|---|---|---|---|---|---|
| 1 |
14 |
10 |
3 |
SD |
MIBG, BM |
2 |
HAMA |
None |
| 2 |
17 |
10 |
5 |
SD |
MIBG, CT |
3 |
PD |
None |
| 3 |
4 |
10 |
3 |
SD |
MIBG, BM, CT |
2 |
HAMA |
None |
| 4 |
5 |
10 |
2 |
SD |
MIBG |
4 |
Completed |
None |
| 5 |
8 |
10 |
3 |
SD |
MIBG, BM |
3 |
PD |
None |
| 6 |
7 |
10 |
3 |
PD |
MIBG, CT |
1 |
PD |
None |
| 7 |
10 |
20 |
3 |
SD |
MIBG, BM |
2 |
HAMA |
None |
| 8 |
15 |
20 |
3 |
SD |
MIBG, BM |
4 |
Completed |
None |
| 9 |
9 |
20 |
6 |
PD |
MIBG, CT, BM |
1 |
HAMA |
None |
| 10 |
4 |
20 |
3 |
PD |
CT, PET |
1 |
PD |
None |
| 11 |
4 |
20 |
2 |
PD |
MIBG, CT, BM |
1 |
PD |
None |
| 12 |
6 |
20 |
3 |
SD |
MIBG, BM |
1 |
DLT |
Grade 4 immune thrombocytopenia |
| 13 |
2 |
40 |
2 |
PD |
MIBG, CT, BM |
1 |
PD |
None |
| 14 |
4 |
40 |
2 |
SD |
MIBG, BM, CT |
4 |
Completed |
None |
| 15 |
6 |
40 |
6 |
SD |
MIBG, CT |
1 |
DLT |
Grade 4 immune thrombocytopenia |
| 16 |
18 |
40 |
3 |
SD |
MIBG, CT |
1 |
HAMA |
None |
| 17 |
17 |
40 |
5 |
SD |
MIBG, BM |
1 |
HAMA |
None |
| 18 |
9 |
40 |
5 |
PD |
MIBG, BM, CT |
2 |
PD |
None |
| 19 |
12 |
80 |
3 |
SD |
MIBG, CT |
4 |
Completed |
None |
| 20 |
5 |
80 |
2 |
SD |
MIBG, BM |
1 |
PD |
None |
| 21 |
3 |
80 |
4 |
PD |
MIBG, CT, BM, PET |
1 |
PD |
None |
| 22 |
14 |
80 |
2 |
SD |
MIBG, BM |
4 |
Completed |
None |
| 23 |
3 |
80 |
2 |
PD |
MIBG, CT |
1 |
PD |
None |
| 24 | 9 | 80 | 2 | SD | MIBG, BM | 4 | Completed | None |
Abbreviations: BM, evidence of disease on bone marrow aspirate and/or biopsy; CT, measurable soft tissue disease on CT; DLT, dose limiting toxicity; HAMA, human anti-mouse antibody response; MIBG, evaluable disease on 123I-MIBG scan; PD, progressive disease; PET, evaluable disease on FDG PET scan; Pt, patient; Rx, 3F8/BG therapy; SD, stable disease; yrs, years.
Toxicities
Most patients tolerated oral BG well. Patients experienced 3F8-related pain, fever and urticaria.14,16,40 In addition, two patients manifested a DLT previously unseen with 3F8-based therapy. Both these patients developed grade 4 acute thrombocytopenia featuring increased bone marrow (BM) megakaryocytes after developing HAMA responses, immediately after cycle one. Neither of these patients was retreated. Thrombocytopenia responded to therapy for idiopathic thrombocytopenic purpura (ITP), consisting of a single administration of dexamethasone, vincristine, anti-D and/or intravenous immunoglobulin (IVIG). One of these two patients had a complete resolution of the thrombocytopenia without recurrence (#15), while the other (#12) developed chronic thrombocytopenia despite initially responding to ITP therapy. To maintain platelet counts, the latter required intermittent ITP therapy until death (owing to progressive NB). There were no other toxicities > grade 2. The maximum tolerated dose (MTD) of BG was not reached: the 2 patients developed thrombocytopenia at BG doses of 20 mg/Kg/day and 40 mg/Kg/day, respectively.
Disease responses
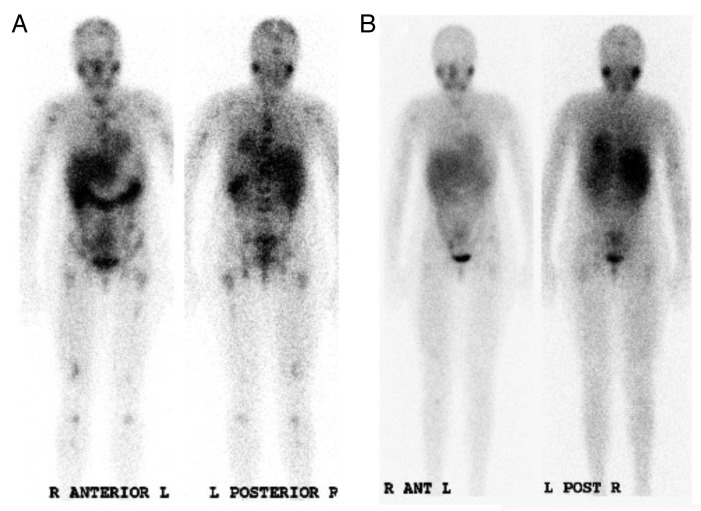
All patients could be assessed for clinical response, which was a secondary objective of this study. Overall, 13/24 (54%) patients demonstrated PD while 11/24 (46%) had stable disease. Although no patient achieved partial response (PR) or better, objective responses (ORs) were observed in several patients. Improvement in 123I-meta-iodobenzylguanidine (MIBG) scans occurred in 13/22 (59%) patients, with mean reductions in Curie extension scores for the entire group and responders being 2.6 and 3.8, respectively (reduction range 1 to 12) (Table 2). There was no dose-response benefit for BG (p = 0.39 for MIBG responses, comparing dose level 1 and 4 by Fisher’s exact test). Two patients had a near complete remission of skeletal MIBG uptake (Fig. 1). Five out of 15 (33%) evaluable patients with BM disease manifested CRs in the BM. These responses were transient, except in one patient (#7). Soft tissue disease reduced slightly in one (7%) out of 14 patients, but otherwise remained unchanged (n = 7) or increased (n = 6). Of 11 patients exhibiting elevated urinary catecholamines at baseline, 6 had this parameter reduced, 2 underwent no significant changes, while 3 manifested further increases. Six out of 8 (75%) patients exhibiting PD prior to therapy had further progression after 1 cycle of 3F8 plus BG. There was no obvious relationship between dose and clinical effect: 4, 3, 2 and 4 patients manifested PD after receiving 10 mg/Kg/day, 20 mg/Kg/day, 40 mg/Kg/day and 80 mg/Kg/day BG, respectively. Response was, however, clearly associated with pretreatment status: 13 out of 16 patients characterized by SD at baseline responded, compared with 1 out of 8 manifesting PD at baseline (p = 0.002, Fisher’s exact test).
Table 2.Responses in patients treated with 3F8+BG.
| Pt. No. | Dose level mg/kg/day |
Best response (after cycle no.) |
Change in semiquantitative MIBG extension score | Overall response (after cycle no.) |
|---|---|---|---|---|
| 1 |
10 |
MIBG improved (1) |
-1 |
PD (2) |
| 2 |
10 |
MIBG improved* (1) |
-1 |
PD (3) |
| 3 |
10 |
MIBG improved |
-12 |
SD (4) |
| 4 |
10 |
MIBG improved (4) |
-7 |
SD (4) |
| 5 |
10 |
MIBG improved, BM not evaluable (1) |
-10 |
PD (3) |
| 6 |
10 |
PD (1) |
0 |
PD (1) |
| 7 |
20 |
MIBG unchanged, BM CR (1) |
-3 |
SD; BM CCR (2) |
| 8 |
20 |
MIBG and BM unchanged |
0 |
PD (4) |
| 9 |
20 |
MIBG improved (1) |
-3 |
SD (1) |
| 10 |
20 |
PD (1) |
+4 |
PD after 1 |
| 11 |
20 |
PD (1) |
NE |
PD after 1 |
| 12 |
20 |
MIBG improved, BM CR (1) |
-4 |
SD (1) |
| 13 |
40 |
PD (1) |
+1 |
PD (1) |
| 14 |
40 |
MIBG, CT improved, BM CR (1) |
-8 |
SD (4) |
| 15 |
40 |
MIBG improved (1) |
-1 |
SD (1) |
| 16 |
40 |
MIBG improved (1) |
-1 |
SD (1) |
| 17 |
40 |
MIBG improved (1) |
-1 |
SD (1) |
| 18 |
40 |
MIBG unchanged; BM CR (1) |
0 |
PD (2) |
| 19 |
80 |
MIBG unchanged; SD (1) |
0 |
PD (4) |
| 20 |
80 |
MIBG unchanged; SD (1) |
0 |
PD (1) |
| 21 |
80 |
PD (1) |
+1 |
PD (1) |
| 22 |
80 |
MIBG improved, BM CR (4) |
-11 |
SD (4) |
| 23 |
80 |
PD (1) |
NE |
PD (1) |
| 24 | 80 | MIBG improved (2) | -4 | SD (4) |
Abbreviations: BM, bone marrow; CCR, continued complete remission; CR, complete remission; MIBG, 123I-MIBG scan; mo, months; NE, not evaluated; PD, progressive disease; SD, stable disease. Durations calculated from start of therapy with 3F8/BG. *Pt No. 2 had received radiation therapy to site of skeletal MIBG uptake about 4 weeks prior to 3F8/BG.
Figure 1. Clinical response of neuroblastoma patients to 3F8 plus barley-derived (1 → 3), (1 → 4)-β-D-glucan. (A and B) 123I-meta-iodobenzylguanidine scans in patient #1 before (A) and after (B) one cycle of 3F8 plus barley-derived (1 → 3), (1 → 4)-β-D-glucan (BG) (administered at dose level 1, i.e., 10 mg/Kg/day).
Survival and late toxicities
The effects of 3F8 plus BG on survival could not be evaluated in this Phase I study and all patients went on to receive subsequent therapy after coming off the study. However, all patients were monitored for late toxicities. Two out of 24 patients (29%) survived for a median duration of 71 mo after enrollment, one with residual disease. Median survival was 23 mo and median time to PD was 4 mo. Two subjects remained alive, both having responded to 3F8 plus BG. The first one (#7) had persistent skeletal MIBG uptake at a follow-up > 110 mo, receiving only 13-cis-retinoic acid after the development of HAMAs. The second one (#24) remained free of disease at a follow-up > 99 mo, having received further consolidation chemotherapy and allogeneic transplantation upon the completion of 4 cycles of 3F8 plus BG. There was no obvious effect of BG dose on progression-free survival (PFS) or overall survival (OS), and the hazard ratio (HR) for a one-level increase in dose was 1.12 (95% CI 0.73, 1.73; p = 0.6). The development of HAMAs was associated with improved OS (HR = 0.32; 95% CI 0, 9.47; p < 0.001), the median survival of patients who did not develop HAMAs (n = 12) being 25 mo and that of patient who did develop HAMAs (n = 8) being 50 mo (4 HAMA-negative patients were not included in this analysis due to death < 4 mo). There were no late toxicities related to 3F8 plus BG. However, one patient developed a rare peritoneal mesothelioma two years after completing therapy, attributable to prior chemo- or radiotherapy.
Correlative studies
Overall, the administration of 3F8 plus BG did not affect in vitro anti-NB immune responses, notably CR3-dependent cytotoxicity from leucocyte fractions in the presence (iC3b-ADCC) or absence (ADCC) of iC3b (p > 0.2 for all endpoints at all dose levels) or the immunophenotype of leukocytes. There was little evidence of a relationship between the dose of BG and immune endpoints. Apart from a weak association between BG dose and CD11b expression in the lymphocyte subpopulation (p = 0.002), there was no correlation between changes in the leukocyte surface receptor repertoire (CD63, CD87 and CD11a) and BG dose. In addition, there was no correlation between BG dose and CD11b expression in granulocytic and monocytic populations. In cytotoxicity assays, GM-CSF slightly decreased iC3b-ADCC, in particular in cells from patients treated with the intermediate (p < 0.01), but not the highest not the lowest BG doses. (1 → 3)β-D-glucan was not detected in the serum of any patient tested by the Fungitell assay (n = 5). Median absolute lymphocyte count (ALC) prior to therapy was 0.8 ± 0.5 /mL. Although ALC at enrollment did correlate with the development of HAMAs (p = 0.048), it did not correlate with OS or PFS. Ten out of 22 patients had mild elevations in circulating IL-10 on day 10 as compared with baseline conditions (mean elevation = 3.98 ± 3.17 pg/mL), whereas no patients manifested elevations in circulating IL-12. IL-10 elevations did not correlative with BG dose or therapeutic responses.
Discussion
Although many phytochemicals have purported immunomodulatory properties, the interaction between immunotherapy and these in anticancer therapy has never been investigated in formal clinical studies. Here, we report the results of the first clinical trial combining a tumor-targeting mAb with a purified immunomodulatory phytochemical. Based on our preclinical data, we chose to test the combination of the anti-NB complement-activating mAb 3F8, whose clinical effects are well characterized, with the CR3-activating glucan BG.15,16,41,42
Therapy was administered on an outpatient basis and was well tolerated even by the youngest individuals enrolled in our study. The MTD of BG was not reached and the study was completed at the planned dose of 80 mg/Kg/day. Two patients developed acute thrombocytopenia as DLT, at different BG doses. The sharp drop in the platelet count of these patients, (1) was associated with an increase in BM megakaryocytes, (2) was paralleled by the development of HAMAs and (3) was responsive to ITP therapy, suggesting an autoimmune phenomenon. This toxicity has never been previously observed with 3F8 and might have resulted from the BG-mediated activation of CR3. However, neither of these patients showed significantly higher ADCC or iC3b-ADCC post-BG. There were no other unexpected adverse events.
Objective responses were documented in 63% (15/24) of patients. All patients had previously received multiple chemotherapy regimens (median 3; range 2–6). Most patients had poor BM reserve (12/24 patients had platelet count < 100,000/μL at baseline) and would have been ineligible for most Phase I/II clinical studies involving conventional chemotherapeutics. Anti-NB responses were recorded for BM disease (histology, MIBG scans) and biochemical markers (urine catecholamines), but, as reported in other mAb-based clinical trials, responses in soft tissue disease were rare. In general, responses were transient and modest. Near-to-complete resolution of extensive MIBG-avid metastases after one cycle of 3F8 plus BG was observed in two chemorefractory patients. The administration of 3F8 plus BG was ineffective at inducing responses in almost all patients with PD at baseline. A dose-response correlation could not be demonstrated.
As a component of bran cereal, the nutritional value of (1 → 3),(1 → 4)-β-D-glucan has been investigated in a number of clinical trials, with variable results. Oat β-glucans lowered blood cholesterol levels in one study,43 though the concentrations of potentially atherogenic lipoproteins were not reduced by modest doses of oat bran in a subsequent study.44 Similarly, barley-derived β-glucans have been associated with variable effects on blood cholesterol level in humans.45,46 In our study, we did not observe a cholesterol-reducing effect from any BG dose (data not shown).
The in vivo mechanism of action of β-glucans has not been adequately investigated. In a murine model, both fluorescein-labeled BG and yeast-derived (1 → 3),(1 → 6)-β-D-glucan could be detected in the spleen, lymph nodes and BM macrophages. Granulocytes with CR3-bound (1 → 3)-β-D-glucan-fluorescein were shown to kill iC3b-opsonized tumor cells following their recruitment to a site of complement activation.47 Since such a mechanism has not yet been demonstrated in humans, a secondary objective of our study was to investigate the immune effects of BG in patients. We observed neither a significant increase in CR3-positive neutrophils or macrophages, nor an enhancement of ADCC or iC3b-ADCC following the administration of 3F8 plus BG, nor did we detect (1 → 3)-β-D-glucan in the serum of 5 patients using the commercial Fungitell assay. It is unclear if these findings point to an alternative in vivo mechanism of action of BG, or to methodological issues. Yeast-derived glucan ingestion has been associated with consistent increases in IL10 mRNA levels in humans.48 However, we did not observe significant elevations in circulating IL-10 in our small patient cohort. This was probably related to the low ALC of most patients at enrollment.
An intriguing finding of this study is that the development of HAMAs is associated with improved survival following the administration of 3F8 plus BG. However HAMA-positive patients continued to have refractory disease despite 3F8 plus BG and subsequent therapies. We first correlated HAMAs with increased survival in patients treated with 131I-3F849 and hypothesized that a 3F8-induced idiotype network contributed to long-term disease control, HAMA being surrogate evidence for the activation of such a network. An idiotype network was demonstrated in a subsequent group of patients treated with 3F8 after chemotherapy on the Memorial Sloan-Kettering Cancer Center (MSKCC) N6 protocol,41 and more recently among patients treated with 3F8 + GM-CSF.17 Although survival was not the primary endpoint of our study, the correlation of OS with HAMAs raises the possibility that the idiotypic network is an important contributor to the survival of NB patients treated with 3F8-based immunotherapy. However, this observation should be tempered by the small number of patients enrolled in our trials, and by the fact that all patients who survived received further anti-NB therapy after completing all cycles of 3F8 plus BG.
mAbs nowadays constitute an established approach to cancer therapy. Yet, there is substantial room for improvements. Antitumor ADCC is Fc-dependent, but CR3-mediated mechanisms also appear to be critical.12,50-52 By activating CR3, β-glucans have been shown to enhance the clinical activity of mAbs in preclinical studies. Natural autoantibodies to a number of self antigens circulate in humans.53,54 Specifically, natural IgM responses to human NB-associated antigens are common among healthy volunteers, but absent or poor among NB patients.55,56 The existence of such natural antibodies may offer us a unique opportunity to exploit plant carbohydrates like β-glucans against cancer. However, as observed in the two patients who developed immune thrombocytopenia, phytochemicals have the potential to elicit autoimmune reactions.57,58 While β-glucans are not used by oncologists, β-glucan-containing natural products such as maitake59 and barley are often consumed by cancer patients. Hence, the role of the patient’s diet and/or the alternative therapies to which he/she is subjected must be carefully considered when evaluating the results of immunotherapy
We have shown that the combination of 3F8 and BG is safe. The encouraging responses observed in a heavily pretreated population support further (Phase II) studies of BG combined to other immunomodulatory agents for the therapy of NB and other tumors amenable to CR3-mediated immunotherapy. Given the low toxicity of BG and the absence of any evidence of dose-response correlations, we recommend a dose of 40–80 mg/Kg/day for future trials.
Patients and Methods
Patient selection
Patients with high-risk NB (stage 4 disease diagnosed at > 18 mo of age or MYCN amplification plus ≥ stage 3 tumor at any age), and a history of PD or chemoresistance were eligible. The presence of evaluable (microscopic BM metastases, elevated tumor markers, abnormal scintigraphic studies) or measurable (by CT or MRI) NB ≥ 4 weeks after completion of systemic therapy was required for eligibility. Patients with life-threatening infections or > grade 2 toxicity according to the National Cancer Institute’s Common Toxicity Criteria version 2.0 (CTC v2.0) were excluded. Conversely, patients with the following grade 3 toxicities (all clearly related to previous therapy) were included: hearing loss, fatigue, alopecia, anorexia, nausea, constipation, elevated liver function tests (LFTs) due to total parenteral nutrition (TPN) and hypomagnesemia.
Study design
The protocol was approved by the institutional review board of MSKCC. Written informed consent was obtained from all patients or their guardians. One cycle consisted of oral BG (available as investigational new drug, prepared to a dilution of 20 mg/mL in sterile water in the MSKCC pharmacy) on days 1 through 12 (given about 1–2 h before 3F8) plus i.v. 3F8, prepared as previously described16 and infused over 1–1.5 h at a fixed dosage of 10 mg/m2/day on days 1 through 5, and 8 through 12. Because of expected pain and hives, 3F8 was given with an antihistaminic and an opiate.15 The dosage of BG was escalated in cohorts of 6 patients at each of 4 dosage levels: 10 mg/Kg/day, 20 mg/Kg/day, 40 mg/Kg/day and 80 mg/Kg/day. Toxicities were graded using CTC v2.0. The dose of BG was escalated only if < 2 patients developed DLT at each of the first three dose levels. DLT was defined as any toxicity > grade 2. Toxicities clearly unrelated to BG were not considered DLT: (a) toxicities related to prior therapy including myelosuppression, hearing loss, alopecia, TPN-associated elevated LFTs; (b) well-established 3F8-dependent toxicities: pain, fever, rash, and anxiety; (c) toxicities from co-interventions routinely used with 3F8, such as opioid-associated constipation. During each cycle, blood counts were checked twice weekly, and LFTs, blood urea nitrogen (BUN) and serum creatinine weekly. HAMA responses were quantified as previously described41 after every cycle. Patients were taken off study if they developed DLT, PD or persistently elevated HAMA titers. Otherwise, patients received up to four treatment cycles, administered approximately four weeks apart from each other.
Response assessment
Disease status was assessed after the first cycle and then at least every three months with CT or MRI, MIBG scan, urine catecholamine measurements and BM studies (based on aspirates, and biopsies from two-to-four different sites on the bilateral anterior and bilateral posterior iliac crests). Disease status was defined by International Neuroblastoma Response Criteria:60 complete remission (CR): no evidence of disease; very good partial response (VGPR): primary mass reduced by 90% to 99%, no evidence of distant disease except for skeletal residua, and normal catecholamines; PR: > 50% decrease in measurable disease and ≤ one NB-positive BM site; mixed response (MR): > 50% decrease of any lesion with < 50% decrease in any other; no response (NR): < 50% decrease but < 25% increase in any existing lesion; and PD: new lesion or > 25% increase in an existing lesion. Patients with NR or MR were categorized as manifesting SD. Since most patients had only evaluable MIBG-positive skeletal disease without measurable soft tissue disease, we also recorded objective improvements in MIBG scans using semiquantitative Curie scores.61,62
Correlative immune studies
Patients were monitored for leukocyte priming by BG on days 1, 8, 12 and 15 of cycles 1 and 2 using a 51Cr release assay. Briefly, LAN-1 and NMB7 NB cells labeled with 51Cr at 100 μCi/106 cells were used as targets. Leukocytes were extracted from peripheral blood samples and studied for 3F8-independent and 3F8-dependent cell-mediated cytotoxicity among granulocytes and lymphocyte cell fractions. Target cells were opsonized with iC3b using normal human serum complement. iC3b opsonized cells were then used to assay for CR3-dependent cytotoxicity in leucocyte fractions in the presence (iC3b-ADCC) or absence (ADCC) of iC3b. Sargramostim (Berlex Oncology), and IL-2 (Novartis) were employed in granulocyte (iC3b-ADCC/GM-CSF) and lymphocyte cytotoxicity assays, respectively. Plates were centrifuged at 200 × g for 4 min at 20°C, and incubated at 37°C for 4 h. Supernatants were harvested using harvesting frames (Skatron). 51Cr released in the supernatant was counted in a universal γ counter. Percentage of specific release was calculated using the Equation 100% × (experimental cpm – background cpm)/(10% sodium dodecyl sulfate (SDS)-releasable cpm – background cpm), where cpm are counts per minute of released 51Cr. Total release was assessed by cell lysis with 10% SDS (Sigma-Aldrich) and background release was measured in the absence of cells. The background was < 20% of total for all cell lines. Lytic units were calculated as previously described.12 Whole blood cells were analyzed for the expression of CD11b, CD63, CD87 and CD11a by flow cytomotetry using specific immunofluorescent antibodies (Becton-Dickinson) before the administration of 3F8 plus BG and on days 4, 8 and 12 of cycle 1, following previously described methods.13
Serum levels of IL-10 and IL-12 were measured prior to BG and 10 d later by Quantikine enzyme-linked immunoassays (R&D Systems). Serum collected from day 8 of cycle 1 on from patients receiving the highest BG dose was tested for the presence of (1 → 3)-β-D-glucan by the commercially available Fungitell modified Limulus Amebocyte Lysate assay (Associates of Cape Cod Inc.).63
Statistical methods
The primary objective of the study was to determine a dosage of BG to take forward to Phase II testing. Since BG is a naturally occurring substance, we expected its toxicity to be low. We therefore required criteria other than toxicity to determine optimal dose, in the event that a MTD might not be reached. Accordingly, we analyzed immune system-, survival- and tumor response-related endpoints. The association between BG dose and immune function was explored using a general estimating equations approach, with day of treatment as co-variate and dose level as predictor. Results from immune assays were entered as continuous variables. As the relationship between dose and response may be non-monotonic, a quadratic term for dose was added to the model. Given the large number of immune endpoints, we used p < 0.005 as the cut-off for further data analysis. For the OS and PFS, logistic and Cox models, were used, respectively, with both the linear and quadratic term for dose. In a separate analysis, we explored a possible association between the development of HAMAs and OS using a landmark analysis. We used a landmark of four months, corresponding to a time point when patients would have been expected to have completed four cycles of 3F8 plus BG therapy. Patients who died before the landmark were thus excluded from the analysis. All analyses were conducted using Stata 9.2 (Stata Corp.).
Acknowledgments
This paper is supported in part by grants from the National Cancer Institute (CA96321), Bethesda, MD; Hope Street Kids, Alexandria, VA; the Katie’s Find A Cure Fund, New York, NY; the Gerber Foundation, Fremont, MI; Pediatric Cancer Foundation, NY; and the Robert Steel Foundation, New York, NY. We thank Junyu Chen, Yi Feng and Hong-Fen Guo for their assistance in performing and analyzing in vitro cytotoxicity, immunophenotype and HAMA assays; Karen Danis for assistance with data management; and Alessandro Jenkner for careful review of the manuscript.
Disclosure of Potential Conflicts of Interest
N-K.V.C. is the inventor of the use of glucan to enhance antibody therapy for cancer.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23402
References
- 1.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Children’s Cancer Group Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 2.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–58. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 3.Ceschel S, Casotto V, Valsecchi MG, Tamaro P, Jankovic M, Hanau G, et al. Survival after relapse in children with solid tumors: a follow-up study from the Italian off-therapy registry. Pediatr Blood Cancer. 2006;47:560–6. doi: 10.1002/pbc.20726. [DOI] [PubMed] [Google Scholar]
- 4.Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D, European Neuroblastoma Study Group. Children’s Cancer and Leukaemia Group (CCLG formerly United Kingdom Children’s Cancer Study Group) High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9:247–56. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 5.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilman AL, Ozkaynak MF, Matthay KK, Krailo M, Yu AL, Gan J, et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children’s Oncology Group. J Clin Oncol. 2009;27:85–91. doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozkaynak MF, Sondel PM, Krailo MD, Gan J, Javorsky B, Reisfeld RA, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children’s Cancer Group Study. J Clin Oncol. 2000;18:4077–85. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 8.Cheung NKV, Walter EI, Smith-Mensah WH, Ratnoff WD, Tykocinski ML, Medof ME. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest. 1988;81:1122–8. doi: 10.1172/JCI113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munn DH, Cheung NK. Interleukin-2 enhancement of monoclonal antibody-mediated cellular cytotoxicity against human melanoma. Cancer Res. 1987;47:6600–5. [PubMed] [Google Scholar]
- 10.Munn DH, Cheung NK. Antibody-dependent antitumor cytotoxicity by human monocytes cultured with recombinant macrophage colony-stimulating factor. Induction of efficient antibody-mediated antitumor cytotoxicity not detected by isotope release assays. J Exp Med. 1989;170:511–26. doi: 10.1084/jem.170.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73:1936–41. [PubMed] [Google Scholar]
- 12.Kushner BH, Cheung NK. Absolute requirement of CD11/CD18 adhesion molecules, FcRII and the phosphatidylinositol-linked FcRIII for monoclonal antibody-mediated neutrophil antihuman tumor cytotoxicity. Blood. 1992;79:1484–90. [PubMed] [Google Scholar]
- 13.Cheung IY, Hsu K, Cheung NK. Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2012;30:426–32. doi: 10.1200/JCO.2011.37.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung NK, Kushner BH, Yeh SD, Larson SM. 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: a phase II study. Int J Oncol. 1998;12:1299–306. doi: 10.3892/ijo.12.6.1299. [DOI] [PubMed] [Google Scholar]
- 15.Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–40. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 16.Kushner BH, Kramer K, Cheung NKV. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–94. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 17.Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–70. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller A, Raptis J, Rice PJ, Kalbfleisch JH, Stout RD, Ensley HE, et al. The influence of glucan polymer structure and solution conformation on binding to (1-->3)-beta-D-glucan receptors in a human monocyte-like cell line. Glycobiology. 2000;10:339–46. doi: 10.1093/glycob/10.4.339. [DOI] [PubMed] [Google Scholar]
- 19.Diller IC, Mankowski ZT, Fisher ME. The effect of yeast polysaccharides on mouse tumors. Cancer Res. 1963;23:201–8. [PubMed] [Google Scholar]
- 20.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J, Study Group of Immunochemotherapy with PSK for Gastric Cancer Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Lancet. 1994;343:1122–6. doi: 10.1016/S0140-6736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 21.Ogoshi K, Satou H, Isono K, Mitomi T, Endoh M, Sugita M, Cooperative Study Group for Esophageal Cancer in Japan Immunotherapy for esophageal cancer. A randomized trial in combination with radiotherapy and radiochemotherapy. Am J Clin Oncol. 1995;18:216–22. doi: 10.1097/00000421-199506000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Nicolosi R, Bell SJ, Bistrian BR, Greenberg I, Forse RA, Blackburn GL. Plasma lipid changes after supplementation with beta-glucan fiber from yeast. Am J Clin Nutr. 1999;70:208–12. doi: 10.1093/ajcn.70.2.208. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman JW, Lindermuth J, Fish PA, Palace GP, Stevenson TT, DeMong DE. A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J Biol Chem. 1998;273:22014–20. doi: 10.1074/jbc.273.34.22014. [DOI] [PubMed] [Google Scholar]
- 24.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton BP, Vĕtvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–46. [PubMed] [Google Scholar]
- 26.van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, Chapel H, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47:575–81. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, et al. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J Immunol. 2012;189:312–7. doi: 10.4049/jimmunol.1200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross GD. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit Rev Immunol. 2000;20:197–222. doi: 10.1615/CritRevImmunol.v20.i3.20. [DOI] [PubMed] [Google Scholar]
- 29.Li B, Allendorf DJ, Hansen R, Marroquin J, Cramer DE, Harris CL, et al. Combined yeast beta-glucan and antitumor monoclonal antibody therapy requires C5a-mediated neutrophil chemotaxis via regulation of decay-accelerating factor CD55. Cancer Res. 2007;67:7421–30. doi: 10.1158/0008-5472.CAN-07-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross GD, Cain JA, Myones BL, Newman SL, Lachmann PJ. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- 31.Vetvicka V, Thornton BP, Wieman TJ, Ross GD. Targeting of natural killer cells to mammary carcinoma via naturally occurring tumor cell-bound iC3b and beta-glucan-primed CR3 (CD11b/CD18) J Immunol. 1997;159:599–605. [PubMed] [Google Scholar]
- 32.Yan J, Vetvicka V, Xia Y, Coxon A, Carroll MC, Mayadas TN, et al. Beta-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18) J Immunol. 1999;163:3045–52. [PubMed] [Google Scholar]
- 33.Xia Y, Vetvicka V, Yan J, Hanikýrová M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–90. [PubMed] [Google Scholar]
- 34.Di Renzo L, Yefenof E, Klein E. The function of human NK cells is enhanced by beta-glucan, a ligand of CR3 (CD11b/CD18) Eur J Immunol. 1991;21:1755–8. doi: 10.1002/eji.1830210726. [DOI] [PubMed] [Google Scholar]
- 35.Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvador C, Li B, Hansen R, Cramer DE, Kong M, Yan J. Yeast-derived beta-glucan augments the therapeutic efficacy mediated by anti-vascular endothelial growth factor monoclonal antibody in human carcinoma xenograft models. Clin Cancer Res. 2008;14:1239–47. doi: 10.1158/1078-0432.CCR-07-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung NK, Modak S. Oral (1-->3),(1-->4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin Cancer Res. 2002;8:1217–23. [PubMed] [Google Scholar]
- 38.Modak S, Koehne G, Vickers A, O’Reilly RJ, Cheung NK. Rituximab therapy of lymphoma is enhanced by orally administered (1-->3),(1-->4)-D-beta-glucan. Leuk Res. 2005;29:679–83. doi: 10.1016/j.leukres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Cheung NK, Modak S, Vickers A, Knuckles B. Orally administered beta-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol Immunother. 2002;51:557–64. doi: 10.1007/s00262-002-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung NK, Kushner BH, Cheung IY, Kramer K, Canete A, Gerald W, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–60. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 41.Cheung NK, Guo HF, Heller G, Cheung IY. Induction of Ab3 and Ab3′ antibody was associated with long-term survival after anti-G(D2) antibody therapy of stage 4 neuroblastoma. Clin Cancer Res. 2000;6:2653–60. [PubMed] [Google Scholar]
- 42.Cheung NK, Sowers R, Vickers AJ, Cheung IY, Kushner BH, Gorlick R. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2006;24:2885–90. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 43.Braaten JT, Wood PJ, Scott FW, Wolynetz MS, Lowe MK, Bradley-White P, et al. Oat beta-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. Eur J Clin Nutr. 1994;48:465–74. [PubMed] [Google Scholar]
- 44.Lovegrove JA, Clohessy A, Milon H, Williams CM. Modest doses of beta-glucan do not reduce concentrations of potentially atherogenic lipoproteins. Am J Clin Nutr. 2000;72:49–55. doi: 10.1093/ajcn/72.1.49. [DOI] [PubMed] [Google Scholar]
- 45.Biörklund M, van Rees A, Mensink RP, Onning G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with beta-glucans from oats or barley: a randomised dose-controlled trial. Eur J Clin Nutr. 2005;59:1272–81. doi: 10.1038/sj.ejcn.1602240. [DOI] [PubMed] [Google Scholar]
- 46.Poppitt SD, van Drunen JD, McGill AT, Mulvey TB, Leahy FE. Supplementation of a high-carbohydrate breakfast with barley beta-glucan improves postprandial glycaemic response for meals but not beverages. Asia Pac J Clin Nutr. 2007;16:16–24. [PubMed] [Google Scholar]
- 47.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, et al. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 48.Kohl A, Gögebakan O, Möhlig M, Osterhoff M, Isken F, Pfeiffer AF, et al. Increased interleukin-10 but unchanged insulin sensitivity after 4 weeks of (1, 3)(1, 6)-beta-glycan consumption in overweight humans. Nutr Res. 2009;29:248–54. doi: 10.1016/j.nutres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Cheung NK, Cheung IY, Canete A, Yeh SJ, Kushner B, Bonilla MA, et al. Antibody response to murine anti-GD2 monoclonal antibodies: correlation with patient survival. Cancer Res. 1994;54:2228–33. [PubMed] [Google Scholar]
- 50.Trottein F, Nutten S, Papin JP, Leportier C, Poulain-Godefroy O, Capron A, et al. Role of adhesion molecules of the selectin-carbohydrate families in antibody-dependent cell-mediated cytoxicity to schistosome targets. J Immunol. 1997;159:804–11. [PubMed] [Google Scholar]
- 51.Metelitsa LS, Gillies SD, Super M, Shimada H, Reynolds CP, Seeger RC. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood. 2002;99:4166–73. doi: 10.1182/blood.V99.11.4166. [DOI] [PubMed] [Google Scholar]
- 52.van Spriel AB, van Ojik HH, Bakker A, Jansen MJ, van de Winkel JG. Mac-1 (CD11b/CD18) is crucial for effective Fc receptor-mediated immunity to melanoma. Blood. 2003;101:253–8. doi: 10.1182/blood.V101.1.253. [DOI] [PubMed] [Google Scholar]
- 53.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10:364–8. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 54.Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–10. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 55.David K, Ollert MW, Juhl H, Vollmert C, Erttmann R, Vogel CW, et al. Growth arrest of solid human neuroblastoma xenografts in nude rats by natural IgM from healthy humans. Nat Med. 1996;2:686–9. doi: 10.1038/nm0696-686. [DOI] [PubMed] [Google Scholar]
- 56.David K, Ollert MW, Vollmert C, Heiligtag S, Eickhoff B, Erttmann R, et al. Human natural immunoglobulin M antibodies induce apoptosis of human neuroblastoma cells by binding to a Mr 260,000 antigen. Cancer Res. 1999;59:3768–75. [PubMed] [Google Scholar]
- 57.Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, et al. β-glucan triggers spondylarthritis and Crohn’s disease-like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–22. doi: 10.1002/art.34423. [DOI] [PubMed] [Google Scholar]
- 58.Hida S, Miura NN, Adachi Y, Ohno N. Effect of Candida albicans cell wall glucan as adjuvant for induction of autoimmune arthritis in mice. J Autoimmun. 2005;25:93–101. doi: 10.1016/j.jaut.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Mayell M. Maitake extracts and their therapeutic potential. Altern Med Rev. 2001;6:48–60. [PubMed] [Google Scholar]
- 60.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 61.Messina JA, Cheng SC, Franc BL, Charron M, Shulkin B, To B, et al. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer. 2006;47:865–74. doi: 10.1002/pbc.20777. [DOI] [PubMed] [Google Scholar]
- 62.Naranjo A, Parisi MT, Shulkin BL, London WB, Matthay KK, Kreissman SG, et al. Comparison of ¹²³I-metaiodobenzylguanidine (MIBG) and ¹³¹I-MIBG semi-quantitative scores in predicting survival in patients with stage 4 neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;56:1041–5. doi: 10.1002/pbc.22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. Evaluation of a (1->3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–62. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]