Abstract
Unlike other tumors, lung cancer appears to be poorly sensitive to immunotherapy. We have recently demonstrated an alternative pathway of lung cancer immunosurveillance. Our data indicate a failure of the adaptive immune system to mediate the immunosurveillance of lung cancer and emphasize the prominent role of natural killer cells in this setting.
Keywords: natural killer cells, lung cancer, mucosal antigen presentation, immunotherapy, adoptive immune system
Principles of Anticancer Immunotherapy
A documented clinical effort to employ immunotherapy against malignancies can be traced back to the late 1800s, when the New York surgeon William Coley started to treat patients with a concoction of inactivated bacterial products known as “Coley’s toxin.”1 Over the last century the immunotherapeutic approaches against both solid and hematological tumors have become more refined, due to an improved understanding of the tumor immune response. The recognition that malignant cells (over)express antigenic proteins that are not present, or are present in relatively low amounts, in normal tissue has brought forth immunotherapeutic strategies focusing on T lymphocytes that target such tumor-associated antigens (TAAs). TAAs are products of mutated cellular genes or viral proteins that are not expressed in physiological conditions by somatic cells. Such antigens are expressed in herpesvirus- or retrovirus-induced malignancies such as Burkitt’s lymphoma.2 Cancer-testes antigens are expressed on germ (but not on somatic) cells, except in the context of malignant transformation. NY-ESO-1 is a prototypic cancer-testes antigen originally identified in esophageal adenocarcinoma,3 and melanoma-associated antigen A3 (MAGE-A3) is a similar antigen that has been actively studied as a target for immunotherapy.4 Alternatively, normal cellular proteins such, as glycoprotein 100 (gp100) and melanoma antigen recognized by T cells 1 (MART-1), can be overexpressed on malignant cells and induce a tumor-specific immune response.5 Incidental observations indicating that a selected group of individuals with circulating antibodies against melanoma-associated antigens may have a more indolent disease course3 suggest that manipulating the immune system of cancer patients may offer therapeutic applications.
In a series of elegant clinical trials, Rosenberg and colleagues demonstrated that autologous T cells, be they derived from tumor-infiltrating lymphocytes or engineered to express a melanoma-reactive T-cell receptor (TCR)—expanded ex vivo and then reinfused into patients—can mediate tumor regression and, sometimes, a durable tumor-specific immune response.6,7 It has recently been demonstrated that the ex vivo modification and reinfusion of autologous T cells expressing a chimeric TCR targeting a B-cell antigen I is particularly efficient for the treatment of chronic lymphocytic leukemia.8 Other immunotherapeutic strategies rely on tumor “vaccines,” resulting in the expansion of TAA-reactive T lymphocytes in vivo. Sipuleucel-T (Provenge™) is a therapeutic vaccine recently approved by FDA for use in metastatic hormone-refractory prostate cancer patients. It relies on the isolation of autologous dendritic cells, their loading with the prostate-specific antigen prostatic acid phosphatase (PAP) ex vivo, and their re-administration to patients. Such a treatment has been shown to provide a survival advantage due to the activation of PAP-reactive T lymphocytes.9 Other strategies for activating TAA-reactive T lymphocytes, such as the administration of MAGE-A3-derived peptides along with an adjuvant, have also demonstrated some degree of efficacy in malignant melanoma patients.10
Limited Success of Immunotherapy Against Lung Cancer
In contrast to melanoma and prostate cancer, limited success has been reported for immunotherapeutic strategies against lung cancer, in particular for approaches targeting T lymphocytes, including vaccination. Mucin 1 (MUC1) is a transmembrane protein expressed on epithelial cells and overexpressed in some lung cancers.11 A randomized Phase IIB trial testing a liposomal vaccine targeting MUC1 in patients with stage IIIB and IV non-small cell lung carcinoma (NSCLC) has recently been completed, demonstrating only a marginal, non-statistically significant improved in survival as compared with control patients.12 A vaccine targeting MAGE-A3 has recently been evaluated in a Phase II trial enrolling lung cancer patients affected by Stage Ib or II tumors after resection.13 Despite the fact that MAGE-A3 was expressed by all patients, no statistically significant increase in survival was noted in this study. Unlike the case of malignant melanoma,14 immunotherapeutic approaches based on the blockade of immune checkpoint demonstrated minimal efficacy in NSCLC patients,15 albeit in an early phase trial. Such a reported lack of efficacy of immunotherapy against lung cancer may stem from a significant discrepancy in the number of trials enrolling NSCLC patients as compared with individuals affected by other tumors, such as melanoma, but it may also reflect the fact that vaccines against lung cancer, or lung cancer immunotherapy in general, is not efficacious. The second possibility seems unlikely, as one of the earliest trials based on ex vivo expanded peripheral blood-derived leukocytes or lymphokine activated killer cells (LAKs) reported tumor regression in a lung adenocarcinoma patient.16
Immunosurveillance of Lung Cancer
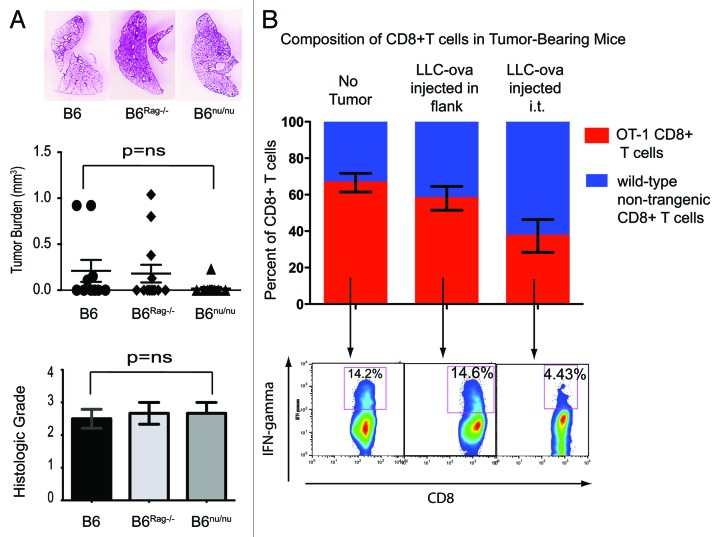
All clinical trials successfully testing immunotherapeutic strategies in cancer patients have been based on initial studies in small animal models. The recent clinical success of immunotherapy in the treatment of malignant melanoma,14 for example, can be directly linked to preclinical studies demonstrating the success of such therapy in rodents.17 Alternatively, it can be argued that animal models of immunotherapy are developed as a result of observational studies focusing on naturally-occurring mechanisms of immunosurveillance. Surprisingly, the immunosurveillance of lung cancer has been poorly investigated. To address this deficiency, we have recently undertaken a series of studies focusing on the mechanisms of immunosurveillance and immunoregulation of lung cancer.18 Using a well-established model of urethane-induced primary lung cancer, we have demonstrated that bone marrow-derived cells play a critical role in the elimination of lung cancer cells. However, to our surprise, T cells played no role in this process. Identical tumor burdens characterized indeed wild-type and mice lacking the adaptive immune system (Fig. 1A). This situation differs significantly from that observed in other types of cancer, such as fibrosarcoma or gastrointestinal stromal tumor, two settings in which T lymphocytes play a critical role in both immunosurveillance and therapeutic responses.19,20 The reasons for this are difficult to understand since lung cancer, just like melanoma and fibrosarcoma, expresses TAAs11 that should, at least in theory, activate antigen-specific T lymphocytes. One possibility is that lung cancer contains a high prevalence of regulatory T lymphocytes, especially CD4+CD25+ regulatory T cells (Tregs). In fact, the earliest description of tumor-infiltrating Tregs was made in human lung cancer.21 It is thus possible that the rapid infiltration of lung tumors with Tregs may inhibit all antitumor immune responses, giving the false impression that this malignancy remains “invisible” to the adaptive immune system. An alternative hypothesis to explain the aforementioned discrepancy may relate to immunoregulation associated with mucosal antigen presentation.
Figure 1. Involvement of the adaptive immune system in the control of lung cancer. (A) No difference in lung tumor burden is evident in B6 wild-type, T cell-deficient nude or Rag2−/− mice treated with urethane. (B) Analysis of splenocytes from chimeric mice demonstrates a relative deficiency of OT-1 ovalbumin-reactive CD8+ T cells in mice bearing lung tumors as compared with those subcutaneous tumors in the flank. A decreased production of interferon γ (IFNγ) is also evident among OT-1 CD8+ T cells in lung tumor-bearing mice. (A) is modified from ref. 18.
Mucosal tolerance plays an important role in normal physiology and homeostasis of barrier organs, preventing exaggerated immune responses to innocuous environmental antigens.22 Multiple experimental models have demonstrated that mucosal antigen delivery results in the generation of CD4+ Tregs,23 but the effects on cytotoxic T lymphocytes (CTLs), most of which are CD8+ T cells, remain poorly defined. Some authors have reported that mucosal antigen presentation can activate CD8+ T lymphocytes,24 while others have demonstrated a downregulation of CD8+ T-cell responses under similar conditions.25 In order to directly address this issue in a model of lung cancer-specific antigen presentation, we generated murine bone marrow chimeras with animals bearing a mixture of CD8+ T cells derived from OT-1 ovalbumin-specific transgenic mice (on a CD45.2+ background) and wild-type CD45.1+ congenic mice. Such a system allowed us to identify ovalbumin-reactive T cells as CD8+CD45.2+CD45.1- cells. After an engraftment period of 3 mo, mice were randomized into 3 groups. Group #1 was injected with saline while Group #2 was s.c. administered with 1 x 106 cells derived from ovalbumin-expressing Lewis lung carcinoma cells (LLC-ova), kindly provided by D. Gabrilovich.26 Group #3 received an intratracheal injection of LLC-ova cells. Such a system, relying on ovalbumin as a surrogate TAA, allows for tracking tumor-specific immune responses. All mice were sacrificed two weeks later and splenocytes analyzed by flow cytometry. Of CD8+ T cells from mixed bone marrow chimeras in Group #1, 66.5% were of OT-1 origin with a non-statistically significant decrease to 58% in animals injected with tumor cells s.c. (Group #2). Mice injected with intratracheal LLC-ova cells (Group #3), however, demonstrated a significant decrease in the proportion of OT-1 T cells to 37.4% (Fig. 1B). Furthermore, upon overnight stimulation with ovalbumin, OT-1 T cells from mice in Group #3 demonstrated decreased interferon γ (IFNγ) production, suggesting the development of anergy to antigen-specific stimulation. These data are in line with previous reports demonstrating that the mucosal delivery of antigens can downregulate systemic CD8+ T-cell immune responses.25,27 It is thus possible that the poor efficiency of TAA-based vaccines in lung cancer patients may be due to either the absence of TAA-specific CD8+ cytotoxic T lymphocytes as a result of deletion or sequestration, or to alternative forms of T-cell inactivation associated with mucosal antigen presentation.
The Innate Immune System Plays a Dominant Role in the Immunosurveillance of Lung Cancer
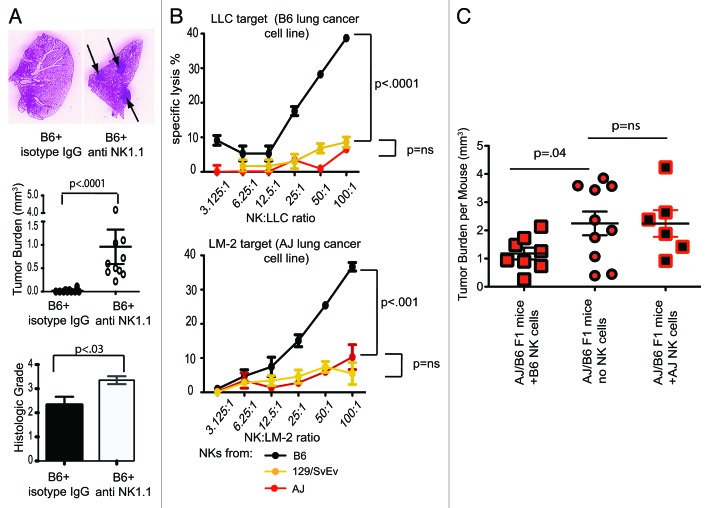
Since we determined that the adaptive immune system fail to control the growth of lunf cancer, we focused our attention on innate immune mechanisms. Unlike T cell-deficient mice, mice lacking natural killer (NK) cells exhibited a statistically significant increase in lung cancer incidence and overall tumor burden as compared with wild-type animals (Fig. 2A).18 Furthermore, we observed a negative correlation between NK-cell cytotoxicity and the susceptibility of mice to carcinogen-induced lung cancer. NK cells from AJ and 129 mice, for example, demonstrated minimal cytolytic activity against lung cancer cells in vitro, and these two strains of mice were found to be highly susceptible to the development of carcinogen-induced lung cancer.18 NK cells from the B6 mouse strain, conversely, exhibited high levels of lung cancer-specific cytotoxicity and B6 mice were resistant to lung cancer carcinogenesis (Fig. 2B).18 These data suggest that NK cells rather than T lymphocytes constitute the main immunological barrier to the development of lung cancer, and might therefore constitute a better targer for lung cancer immunotherapy. In order to directly address this question, we generated AJ/B6 F1 chimeric mice, which have a susceptibility to lung cancer that lays somewhat in between highly susceptible AJ and resistant B6 mice. Upon treatment with urethane, AJ/B6 F1 mice were randomized into three groups. Group #1 was injected with 1 x 106 freshly isolated NK cells from B6 mice, while Groups #2 and #3 were treated with saline or NK cells isolated from AJ mice, respectively. All animals were injected i.p. every two weeks for total of four months and tumor burden was determined at sacrifice as previously described.18 Mice in Group #1 had the lowest tumor burden, suggesting that exogenously administered NK cells can control the development and/or progression of lung cancer, and individual differences in NK cytotoxicity could be utilized as a therapeutic application (Fig. 2C). Very early studies by Rosenberg’s group demonstrated that at least one patient affected by primary lung adenocarcinoma exhibited a partial response to the reinfusion of autologous LAKs expanded ex vivo, which are highly enriched in activated NK cells.16 Most subsequent clinical trials by the same group, however, have focused on melanoma. One recent Phase I clinical trial from Greece demonstrated that the administration of haploidentical NK cells expanded ex vivo may offer clinical efficacy against unresectable lung cancer.28 However, unlike the case of hematologic malignancies,29 NK cell-based immunotherapeutic approaches against lung cancer are poorly explored.
Figure 2. Involvement of natural killer cells in the control of lung cancer. (A) The depletion of natural killer (NK) cells leads to an increased incidence of carcinogen-induced lung cancer and increased tumor burden in mice. (B) NK cells from lung cancer-resistant B6 mice demonstrate more robust lung-cancer specific cytotoxicity than cells from lung cancer-susceptible 129 and A/J animals. (B) The adoptive transfer of NK cells from B6 mice decreases tumor burden in urethane-treated mice. (A and B) are modified from ref. 18.
Conclusions
Lung cancer is the number one cancer-related cause of death in the Western world, being associated with a higher disease-specific mortality then prostate, colon and breast cancer combined.30 Despite this fact, the development of immunotherapeutic approaches against lung cancer lags far behind that of strategies to exploit the immune system against tumors, such as malignant melanoma. Our data and those of others27 demonstrate that the adaptive immune system plays a limited role in the control of lung cancer growth. This may be due to the highly immunosuppressive environment created by lung cancer cells as well as to the unique immunological properties of the lung, which alter TAA-reactive T lymphocytes. Nevertheless, we present compelling data suggesting that immunotherapeutic strategies that are (at least in part) successful against other solid tumors may not be applicable to lung cancer without significant refinement. We also demonstrate a prominent role for NK cells in the primary immunosurveillance of lung cancer as well as their potential as an immunotherapeutic tool against this dreadful disease. We therefore suggest that a different immunotherapeutic approach, perhaps closer to that employed against hematological tumors such as acute myeloid leukemia,29 may offer an improved efficacy for the treatment of lung cancer.
Acknowledgments
Supported by ATS/Lungevity Foundation, the Alvin Siteman Cancer Center Internal Research Grant by The American Cancer Society, NIH K08CA131097, NIH R01HL094601, R01HL113931, and NIH F32HL114270, the Barnes Jewish Research Foundation, and Thoracic Surgery Foundation for Research and Education
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23563
References
- 1.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with report of ten original cases. Am J Med Sci. 1893;105:487–511. doi: 10.1097/00000441-189305000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 3.Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, et al. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 4.van der Bruggen P, Bastin J, Gajewski T, Coulie PG, Boël P, De Smet C, et al. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24:3038–43. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 5.Chang GY, Kohrt HE, Stuge TB, Schwartz EJ, Weber JS, Lee PP. Cytotoxic T lymphocyte responses against melanocytes and melanoma. J Transl Med. 2011;9:122. doi: 10.1186/1479-5876-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 10.Kruit W, Suciu S, Dreno B, Chiarion-Sileni V, Mortier L, Maio M, et al. Active immunization toward the MAGE-A3 antigen in patients with metastatic melanoma: Four-year follow-up results from a randomized phase II study (EORTC16032-18031) J Clin Oncol. 2011;29:abstr 8535. [Google Scholar]
- 11.Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–51. [PubMed] [Google Scholar]
- 12.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulières D, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–81. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 13.Vansteenkiste J, Zielinski M, Liner A, Dahabre E, Esteban W, Malinowski J, et al. Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25:abs 7554. [Google Scholar]
- 14.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch T, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Phase II trial of ipilimumab (IPI) and paclitaxel/carboplatin (P/C) in first-line stage IIIb/IV non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28:A7531. doi: 10.1200/JCO.2009.21.9618. [DOI] [Google Scholar]
- 16.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–92. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 17.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreisel D, Gelman AE, Higashikubo R, Lin X, Vikis HG, White JM, et al. Strain-specific variation in murine natural killer gene complex contributes to differences in immunosurveillance for urethane-induced lung cancer. Cancer Res. 2012;72:4311–7. doi: 10.1158/0008-5472.CAN-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 20.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 22.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889–99. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makita S, Kanai T, Nemoto Y, Totsuka T, Okamoto R, Tsuchiya K, et al. Intestinal lamina propria retaining CD4+CD25+ regulatory T cells is a suppressive site of intestinal inflammation. J Immunol. 2007;178:4937–46. doi: 10.4049/jimmunol.178.8.4937. [DOI] [PubMed] [Google Scholar]
- 24.Hänninen A, Braakhuis A, Heath WR, Harrison LC. Mucosal antigen primes diabetogenic cytotoxic T-lymphocytes regardless of dose or delivery route. Diabetes. 2001;50:771–5. doi: 10.2337/diabetes.50.4.771. [DOI] [PubMed] [Google Scholar]
- 25.Limmer A, Ohl J, Wingender G, Berg M, Jüngerkes F, Schumak B, et al. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35:2970–81. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 26.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–29. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–9. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 30.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]