Abstract
In decerebrated rats, we determined the dose of A803467, a NaV 1.8 antagonist, needed to attenuate the reflex pressor responses to femoral arterial injections of lactic acid (24mM; ~0.1mL) and capsaicin (0.1μg), agents which stimulate thin fiber afferents having NaV 1.8 channels. We also determined whether the dose of A803467 needed to attenuate these reflex responses affected the responses of muscle spindle afferents to tendon stretch and succinylcholine (200μg). Spindle afferents are not supplied with NaV 1.8 channels, and consequently their responses to these stimuli should not be influenced by A803467. Pressor responses to lactic acid and capsaicin were not altered by 500μg of A803467 (n=6). A803467 in a dose of 1mg, however, significantly reduced (p< 0.05; n=12) the pressor responses to lactic acid (23±5 to 7±3ΔmmHg) and capsaicin (47±5 to 31±5ΔmmHg). Surprisingly, we also found that 1 mg of A803467 reduced the responses of 10 spindle afferents to succinylcholine (34±11 to 4±3 Δ imp/s p<0.05) and stretch (83±17 to 0.4±1 Δ imp/s; p<0.05). We conclude that A803467 reduces the reflex response to lactic acid and capsaicin; however, it may be working on multiple channels, including NaV 1.8, other NaVs as well as voltage-gated calcium channels.
Keywords: muscle chemoreflex, A803467, rats, muscle spindles, Aδ and C fibers, arterial blood pressure
Introduction
Stimulation of thin fiber somatic afferents, which are comprised of thinly myelinated Aδ and unmyelinated C fibers, are known to reflexly increase arterial blood pressure and heart rate [24] [25] [4]. In contrast, stimulation of thickly myelinated afferents, which are comprised of Aα and Aβ fibers, does not evoke these increases, and in some instances can evoke reflex decreases in arterial pressure and heart rate [4]. Under normal physiological conditions, thick fiber somatic afferents play important roles in the reflex control of muscle length, in the transduction of non-noxious stimulation of the skin, and in the perception of joint position [15] [5]. Thin fiber afferents, on the other hand, play important roles in evoking reflex pressor–cardioaccelerator responses to exercise [4] [16] and in the transduction of pain arising from the skin, joints, and muscle [5] [26].
Voltage gated sodium channels (NaV) are responsible for the initiation and conduction of action potentials in somatic afferent fibers. The inward currents arising from the opening of sodium channels have been classified as either tetrodotoxin sensitive (TTX-s) or tetrodotoxin resistant (TTX-r). Sodium channels are heteromeric proteins comprised of a pore forming alpha subunit and two beta subunits [23]. Nine alpha subunits have been reported and have been designated NaV 1.1 through NaV 1.9 [23]. Substantial in vitro evidence has shown that the TTX-r alpha subunits NaV 1.8, and NaV 1.9 play important roles in impulse initiation and conduction in thin fiber somatic afferents [20] [12] [27]. TTX-r sodium channels are found on thin fiber somatic afferents [2] [28], which in turn has led to a search for antagonists function as non-opioid analgesics.
One such agent is A803467, which has been shown in vitro to block selectively NaV 1.8 channels [11] [18]. In vivo, intravenous injection of A803467 has been shown to attenuate the responses of rat dorsal horn neurons to probing of their receptive fields in the periphery [11] [18]. In addition, close arterial injection of A803467 has been shown to attenuate the responses of thin fiber afferents to noxious rotation of the knee joint [26]. In these latter experiments, the possibility existed that the attenuation attributed to the blockade of NaV 1.8 channels by A803467 was due to the blockade of CaV 3.2 channels [1]. In addition, there have been no in vivo studies demonstrating the effect of A803467 on autonomic reflexes arising from stimulation of thin fiber somatic afferents. These findings prompted us to test two hypotheses. The first was that A803467 attenuated the reflex pressor responses to stimulation of thin fiber somatic afferents, which are widely believed to be supplied by NaV 1.8 channels [2] [28]. The second was that the attenuation of these pressor reflexes was attributable solely to blockade of NaV 1.8 channels. We chose to stimulate these afferents with femoral arterial injections of capsaicin and lactic acid, substances known to vigorously stimulate thin fiber afferents.
Methods
All procedures were reviewed and approved by the Institutional Care and Use Committee of the Pennsylvania State University College of Medicine.
Surgical preparation
Adult, male Sprague-Dawley rats (n=59, weighing 436 ± 5g) were anesthetized with 2–3% isoflurane gas and 100% oxygen. The trachea was cannulated, and the lungs were ventilated mechanically with the gaseous anesthetic until the decerebration was completed (see below). One carotid artery and jugular vein were cannulated to measure arterial blood pressure and to administer fluids, respectively.
We cannulated the contralateral (I.e., right) iliac artery in a retrograde manner, sectioned this artery and oriented it so that we could pass the tip of the catheter into the left femoral artery. The tip was placed just past the inguinal ligament to the left femoral artery. A snare was placed under the abdominal aorta and vena cava. When tightened, the snare restricted blood flow to and from the left hindlimb.
While the lungs were ventilated with the gaseous anesthetic, we performed a precollicular decerebration using the method described previously [30]. Anesthesia was discontinued after decerebration and the rat was allowed to stabilize for at least 1 hour. Prior to decerebration, Dexamethasone (0.2mg) was injected intravenously to minimize swelling in the brain.
When recording the impulse activity of muscle spindles, we performed a laminectomy to expose the lower lumbar roots (L2–L5). The calcaneal bone of the left hindlimb was cut and the triceps surae muscles were isolated. The free end of the calcaneal tendon was attached to a force transducer (model FT-10C, Grass) to measure tension developed when the triceps surae muscles were stretched.
Experimental protocols
Reflex studies
We injected into the femoral artery capsaicin (0.1μg; 50 μl), a TRPV1 receptor agonist [3] and lactic acid (24mM; 50 μl), an ASIC 3 receptor agonist [10] while measuring arterial pressure and heart rate. Both substances vigorously stimulate thin fiber afferents innervating the hindlimb [14] [13] [6] [22], but have no effect on the discharge of thick fiber afferents [14] [13] [6]. A stock solution of capsaicin (50mg) was dissolved in ethanol (0.5ml) and 5 drops of Tween 80 and then subsequently diluted with saline to a concentration of 2μg/ml. Lactic acid was dissolved in saline. After obtaining pressor-cardioaccelerator responses to injection of capsaicin and lactic acid, we attempted to abolish or attenuate these responses by injecting A803467 (500 μg or 1mg; 100 μl), a NaV 1.8 antagonist [11]. A803467 (Tocris Bioscience) was dissolved in 100% DMSO. While injecting lactic acid or capsaicin, we snared the lower aorta and vena cava snared to limit blood flow to and from the hindlimbs. The order of injection was randomized and was separated by a ten minute interval. Thirty minutes after the second injection, we again snared the descending aorta and vena cava, and injected A803467 into the left femoral artery. After injecting A803467, we maintained the snare for five minutes and then released it for another seven minutes. We used this protocol because A803467 has been shown to reach its peak blocking effect on the responses of thin fiber joint afferents to noxious knee rotation after 11 min [26]; A803467 has a half-life of 4 hours [11]. Twelve minutes after injecting A803467, we tightened the snare and injected lactic acid and capsaicin again in the same order as before injecting A803467. The decerebrated rat was paralyzed throughout the experiment with pancuronium bromide (0.5mg/kg, given every 30min).
Electrophysiological Studies
We recorded the impulse activity arising from muscle spindles before and after injecting either A803467 (1 mg) or mibefradil (1 and 3 mg/kg), a voltage gated calcium 3.2 (T) channel antagonist [29] into the femoral artery. Mibefradil (Tocris Bioscience) was dissolved in saline and was always injected in a volume of 200 μl. A803467 was dissolved in 100% DMSO and was injected in a volume of 100 μl. Single fiber impulse activity arising from muscle spindles was recorded from filaments split from the peripheral cut ends of L4 and L5 dorsal roots. A stimulating electrode was placed on the tibial nerve. Conduction velocities were calculated by dividing the conduction distance between the stimulating and recording electrodes by the conduction time. Each of the afferents had its receptive field plotted in the triceps surae muscles, and each responded to calcaneal tendon stretch, lasting for 30 seconds. Of the 22 spindles tested with either A803467 or Mibefradil, 19 responded to femoral arterial injection of succinylcholine (200 μg; 0.4ml saline) with the abdominal aorta and vena cava snared [8] [32]. Baseline impulse activity of eight spindles was attenuated by DMSO (100 μl), the vehicle for A803467; we did not determine the effect of A803467 on the discharge of these eight spindles.
Data Analysis
Mean arterial blood pressure and heart rate values were expressed as means ± SE. Baseline mean arterial blood pressure and heart rate were measured immediately before a maneuver, and peak mean arterial pressure and heart rate were measured during injection of lactic acid, capsaicin, succinylcholine, and during tendon stretch. Statistical comparisons between pressor and cardioaccelerator responses to capsaicin and lactic acid before and after A803467 were performed using a two-way repeated measure ANOVA with Holm-Sidak’s post hoc test when a significant interaction was found (Graphpad Prism 6.0 software). Statistical comparisons between the responses of muscle afferents to either stretch or succinylcholine before or after A803467 or mibefradil were performed with a two-way repeated measure ANOVA. Again, post hoc comparisons were performed when a significant interaction was found (SAS 9.2 software). The criterion for statistical significance was p<0.05.
Results
Effects of A803467 on cardiovascular reflexes evoked by lactic acid and capsaicin
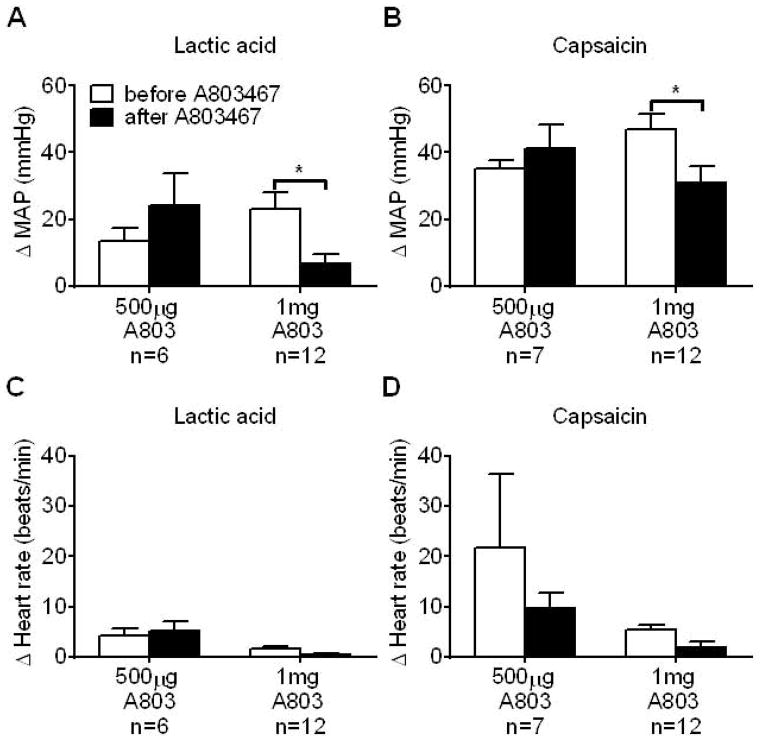
We tested the effect of two doses of the NaV 1.8 channel antagonist, A803467, injected into the femoral artery, on the pressor and cardioaccelerator responses to lactic acid and capsaicin. A dose of 500 μg of A803467 had no effect on the pressor and cardioaccelerator responses to femoral arterial injection of either substance (Figure 1, A through D). In a separate group of rats, we doubled the dose of A803467 (i.e., 1 mg) and found that the pressor responses to femoral arterial injection of lactic acid and capsaicin were significantly reduced by the NaV 1.8 antagonist (Figure 1, A and B) but the cardioaccelerator response was not significantly changed (Figure 1, C and D).
Figure 1.
A803467, in a dose of 1 mg, but not in a dose of 500 μg, significantly attenuated the pressor responses to femoral artery injection of lactic acid (A) and capsaicin (B). Note that neither dose of A803467 attenuated the cardioaccelerator responses to femoral artery injection of either lactic acid (C) or capsaicin (D). Asterisks (*) represent significant differences between the pressor response to either lactic acid or capsaicin before and after A803467, p<0.05.
Electrophysiological experiments
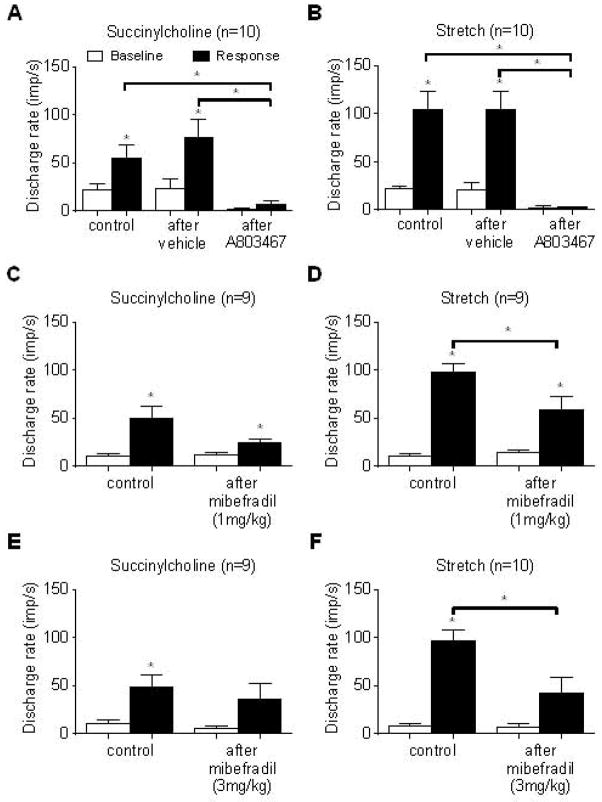
We recorded the impulse activity of 22 muscle spindles with receptive fields in the triceps surae muscles. Of the 22 afferents, we measured the conduction times in 19 and calculated their conduction velocities, which averaged 42.6 ± 2.5 m/s (range: 13.9–60.6 m/s). We found that A803467 (1 mg), injected into the femoral artery, markedly attenuated the responses of ten spindles to both stretch and to succinylcholine (Figure 2, A and B; Figure 3). In addition, A803467 markedly attenuated the baseline firing rate of these afferents (Figure 3). We also determined the effect of two doses of mibefradil, a T type calcium channel (CaV 3.2) antagonist, on the responses of spindles to the two stimuli. The attenuating effect of mibefradil, injected into the femoral artery, on the responses of ten spindles to succinylcholine and to stretch was much smaller than that of A803467 (Figure 2, C through F). Moreover, mibefradil had no effect on their baseline discharge rate.
Figure 2.
Effect of A803467 (1 mg) (A & B) and two doses of mibefradil (C–F) on the responses of spindles to succinylcholine (200 μg), injected into the femoral artery, and to tendon stretch. Note that in A & B that femoral arterial injection of DMSO, the vehicle for A803467, had no effect on the spindles’ responses to either succinylcholine or to stretch. In contrast, injection of A803467 (1 mg) almost abolished these responses as well as baseline activity. Asterisks (*) represent significant differences between baseline discharge and responses to succinylcholine or stretch (p < 0.05). Asterisks (*) over horizontal brackets represent significant differences between the increase in discharge rate in response to succinylcholine or stretch before and after either vehicle (DMSO), A803467 or Mibefradil (p < 0.05).
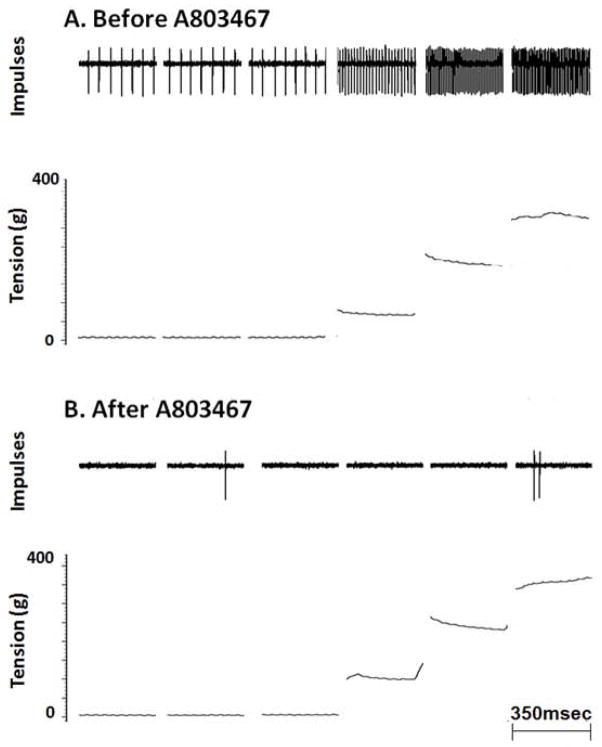
Figure 3.
Effect of A803467 (1mg) on the response of a spindle (CV=43.4 m/s) to tendon stretch. Receptive field of spindle was located in the lateral gastrocnemius muscle.
Discussion
Our goal was to determine if the blocking effect of A803467 on NaV 1.8 channels, which has been demonstrated in vitro, could be translated to in vivo experiments evoking autonomic reflex responses. To accomplish this goal we needed to find an amount of A803467 that attenuated the reflex pressor responses to femoral arterial injections of capsaicin, a TRPV1 receptor agonist, and lactic acid, an ASIC 3 receptor agonist. This amount was found to be 1mg, which translates to approximately to 2mg per kg. The intraarterial dose required to attenuate the reflex pressor responses to capsaicin and lactic acid in our experiments appeared similar, but somewhat smaller, than the intravenous dose of A803467 (10–30 mg/kg) required to attenuate the responses to probing of wide dynamic range neurons in the dorsal horn of anesthetized rats [18] [11].
In our experiments, the dose of A803467 required to attenuate the pressor responses to capsaicin and lactic acid injection markedly reduced the responses of spindle afferents to tendon stretch and succinylcholine. The significance of this latter finding, and the rationale for why we recorded the responses of spindles to these stimuli, is that cell bodies of spindles do not bind the antibody for the NaV 1.8 protein, and as a result are thought not to possess the alpha subunit for this sodium channel [5]. Unless one is willing to postulate that the endings of spindle afferents contain NaV 1.8 channels, whereas cell bodies do not, we must conclude that the dose of A803467 used in our experiments may have blocked additional sodium channels besides NaV 1.8 on these thick fiber afferents as well as on the thin fiber afferents evoking the pressor responses to capsaicin and lactic acid injection. One sodium channel that may have been blocked by A803467 was NaV 1.6, which is present on cell bodies that innervate hindlimb muscles [21] and which can be responsible for bursting activity [23]. In vitro, the selectivity of A803467 for NaV 1.6 is not known, but it is reasonable to speculate that this channel was blocked at least in part by A803467 in our experiments.
In vitro, A803467 is less effective in blocking NaV 1.8 in rats than it is in blocking NaV 1.8 in humans. Specifically, the IC50 for this compound in blocking rat NaV 1.8 was 140nM, whereas the IC50 for blocking human NaV 1.8 was only 8nM [11]. Moreover, the effectiveness of this compound in blocking NaV 1.8 channels in vitro is limited. Specifically, a 1μM concentration of A803467 blocked only 40% of the peak current evoked by depolarizing dorsal root ganglion cells [21]; higher concentrations of A803467 were not used because of concern about the loss of selectivity of the compound for NaV 1.8 channels [21]. These in vitro findings reinforce the possibility that the threshold dose of A803467 required to attenuate the reflex pressor responses to capsaicin and lactic acid injection in our experiments may have blocked other sodium channels in addition to NaV 1.8.
Recently, Bladen and Zamponi reported that low micromolar concentrations of A803467 blocked CaV 3.2 channels in human embryonic kidney cells [1]. These authors suggested that previous reports attributing the analgesic and electrophysiological effects of A803467 to the blockade of NaV 1.8 channels might have been caused partly by the blockade of CaV 3.2 channels. To shed light on this issue, we determined the effect of two doses of mibefradil, a CaV 3.2 antagonist, injected into the femoral artery on the responses of spindle afferents to both succinylcholine injection and tendon stretch. The highest dose of mibefradil used in our experiments (3mg/kg) has been shown to reduce both thermal and mechanical nociceptive responses [33]. We found that mibefradil significantly attenuated the responses of spindles to stretch and to succinylcholine. Nevertheless, the attenuation of these responses by mibefradil paled by comparison with those induced by A803467. As a consequence, our findings with relatively large doses of mibefradil suggest that blockade of CaV 3.2 channels by A803467 was not the only factor in causing the attenuation of the responses of spindles to stretch and succinylcholine.
Our present findings appear to conflict with our previous finding that A803467 applied to the dorsal roots of decerebrated rats had no effect on the pressor response to electrical stimulation of the tibial nerve at current intensities that recruited C-fibers [31]. The conflict may be resolved when one considers the functional location of the NaV 1.8 channel along the sensory neuron. Specifically, NaV 1.8 channels play no role in conducting impulses along axons in the dorsal roots [19] [31] [7] [9], whereas they do play a role in conducting impulses at or near the neuron’s terminal in the periphery as well as in its cell body [21] [12].
One limitation of our study involves assessing the effectiveness in vivo of pharmacological blockade of voltage gated channels. Typically, one demonstrates the effectiveness of a blockade by showing that the antagonist prevents a physiological response to an agonist. This protocol cannot be performed on a channel that is opened by membrane depolarization. Consequently, we cannot exclude the possibility that the doses of the A803467 and mibefradil used in our experiments did not block their intended channels. We also cannot exclude the possibility that mibefradil blocked other calcium channels in addition to CaV 3.2. For example, mibefradil in the doses used in our experiments might have blocked high voltage calcium channels. In that regard, mibefradil has been shown to be 10–30 fold more potent in blocking T-type channels (CaV 3.2) than in blocking L-type channels, which has only a small presence in dorsal root ganglion cells [17]. Likewise, mibefradil has been shown to be 200 fold more potent in blocking T-type channels than in blocking P-type channels [17].
A803467, in vitro, has been reported to be a selective antagonist to NaV 1.8 channels [11] [18]. We attempted to translate these in vitro findings to in vivo experiments by using A803467 to attenuate reflex pressor responses to chemicals that vigorously stimulate thin fiber somatic afferents. We found that the threshold dose of A803467 needed to attenuate these pressor reflexes also abolished the responses of muscle spindles to succinylcholine and stretch. This was a surprising finding because muscle spindles do not have NaV 1.8 channels [5] [9]. We conclude that the threshold dose of A803467 that blocked the pressor reflex responses to capsaicin and lactic acid in our experiments blocked additional channels as well as NaV 1. 8. In vitro evidence suggested that the voltage gated T type calcium channel (CaV 3.2) might be one of these additional channels [1]. Blockade of this calcium channel with mibefradil in our experiments provided evidence that was consistent with this possibility. Nevertheless, the attenuation of the spindles’ responses to stretch and succinylcholine by a large dose of mibefradil in our experiments was considerably less than the abolition of these responses by A803467, raising the possibility that other sodium channels such as NaV 1.6 might have been blocked by this antagonist. In summary, our findings led us to conclude that in vivo A803467 has limited utility as a selective NaV 1.8 antagonist unless one can demonstrate that the dose used has no effect on other channels or receptors.
Highlights.
A803467 (1mg) reduced pressor responses to stimulation of group III & IV afferents
A803467 (1mg) reduced muscle spindle activity to stretch and succinylcholine
The utility of A803467 in vivo is challenged as a selective antagonist for NaV 1.8
Acknowledgments
This work was supported by NIH grants HL-096570 and AR-059397.
Footnotes
Disclosures
No conflicts of interests are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bladen C, Zamponi GW. Common mechanisms of drug interactions with sodium and T-type calcium channels. Mol Pharmacol. 2012;82:481–487. doi: 10.1124/mol.112.079715. [DOI] [PubMed] [Google Scholar]
- 2.Brock JA, McLachlan EM, Belmonte C. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J Physiol. 1998;512(Pt 1):211–217. doi: 10.1111/j.1469-7793.1998.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol. 1970;208:261–278. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol. 2003;550:739–752. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster RW, Ramage AG. The action of some chemical irritants on somatosensory receptors of the cat. Neuropharmacology. 1981;20:191–198. doi: 10.1016/0028-3908(81)90203-3. [DOI] [PubMed] [Google Scholar]
- 7.Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, Lai J. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granit R, Skoglund S, Thesleff S. Activation of muscle spindles by succinylcholine and decamethonium. Acta Physiol Scand. 1953;28:134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 9.Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibers. Brain Res. 1994;639:125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 15.Matthews PBC. Mammalian Muscle Receptors and their Central Actions. Arnold; London: 1972. [Google Scholar]
- 16.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonough SI, Bean BP. Mibefradil inhibition of T-type calcium channels in cerebellar purkinje neurons. Mol Pharmacol. 1998;54:1080–1087. doi: 10.1124/mol.54.6.1080. [DOI] [PubMed] [Google Scholar]
- 18.McGaraughty S, Chu KL, Scanio MJ, Kort ME, Faltynek CR, Jarvis MF. A selective Nav1.8 sodium channel blocker, A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther. 2008;324:1204–1211. doi: 10.1124/jpet.107.134148. [DOI] [PubMed] [Google Scholar]
- 19.Pinto V, Derkach VA, Safronov BV. Role of TTX-sensitive and TTX-resistant sodium channels in Adelta- and C-fiber conduction and synaptic transmission. J Neurophysiol. 2008;99:617–628. doi: 10.1152/jn.00944.2007. [DOI] [PubMed] [Google Scholar]
- 20.Quasthoff S, Grosskreutz J, Schroder JM, Schneider U, Grafe P. Calcium potentials and tetrodotoxin-resistant sodium potentials in unmyelinated C fibres of biopsied human sural nerve. Neuroscience. 1995;69:955–965. doi: 10.1016/0306-4522(95)00307-5. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS. Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J Neurophysiol. 2012;108:2230–2241. doi: 10.1152/jn.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- 23.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato A, Sato Y, Schmidt RF. Changes in heart rate and blood pressure upon injection of algesic agents into skeletal muscle. Pflügers Arch. 1982;393:31–36. doi: 10.1007/BF00582387. [DOI] [PubMed] [Google Scholar]
- 25.Sato A, Schmidt RF. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev. 1973;53:916–947. doi: 10.1152/physrev.1973.53.4.916. [DOI] [PubMed] [Google Scholar]
- 26.Schuelert N, McDougall JJ. Involvement of Nav 1.8 sodium ion channels in the transduction of mechanical pain in a rodent model of osteoarthritis. Arthritis Res Ther. 2012;14:R5. doi: 10.1186/ar3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strassman AM, Raymond SA. Electrophysiological evidence for tetrodotoxin-resistant sodium channels in slowly conducting dural sensory fibers. J Neurophysiol. 1999;81:413–424. doi: 10.1152/jn.1999.81.2.413. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- 29.Todorovic SM, Meyenburg A, Jevtovic-Todorovic V. Mechanical and thermal antinociception in rats following systemic administration of mibefradil, a T-type calcium channel blocker. Brain Res. 2002;951:336–340. doi: 10.1016/s0006-8993(02)03350-4. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol. 2010;299:H106–H113. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuchimochi H, McCord JL, Leal AK, Kaufman MP. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300:H652–H663. doi: 10.1152/ajpheart.00859.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhey BA, Voorhoeve PE. Activation of group IA and group II muscle spindle afferents by succinylcholine and other cholinergic drugs. Acta Physiol Pharmacol Neerl. 1963;12:23–29. [PubMed] [Google Scholar]
- 33.Weng X, Smith T, Sathish J, Djouhri L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Adelta-nociceptors. Pain. 2012;153:900–914. doi: 10.1016/j.pain.2012.01.019. [DOI] [PubMed] [Google Scholar]