Abstract
Objectives
Sarcomatoid variant is a spindle cell phenotype of renal cell carcinoma (RCC), which is associated with a poor prognosis. We reviewed outcomes of systemic therapy for metastatic, sarcomatoid-variant RCC.
Methods
Clinical features, treatment outcome, and survival were evaluated in 63 patients with sarcomatoid-variant metastatic RCC (47 clear cell, 16 nonclear cell). Initial systemic treatment included antiangiogenesis-targeted therapy (n=34), cytokines (n=20), and chemotherapy (n=9).
Results
Five of 63 patients (8%) achieved an objective response to the first systemic treatment: 1 (5%) to cytokine and 4 (12%) to sunitinib-targeted therapy. Median progression-free survival for 63 patients was 3 months (95% confidence interval), and median overall survival was 10 months (95% confidence interval). The median progression-free survival for patients treated with sunitinib versus all others was 4.4 months versus 2 months (P=0.03), and 3 months for patients with clear-cell histology versus 1.6 months for nonclear-cell histology (P=0.004).
Conclusions
Metastatic sarcomatoid-variant RCC was associated with a poor response to systemic therapy. Sunitinib treatment resulted in a modest response rate, but studies to characterize the underlying tumor biology of sarcomatoid-variant RCC, to assess outcome to targeted agents, and to develop novel treatment strategies are warranted.
Keywords: metastatic, sarcomatoid, renal cell carcinoma, sunitinib, targeted therapy
Sarcomatoid-variant represents a spindle cell phenotype of renal cell carcinoma (RCC) that can be present in any subtype (clear cell, papillary, chromophobe or unclassified). Case reports of sarcomatoid-variant RCC highlight a poor prognosis. Most reports comprise less than 20 patients, and lack consensus on treatment outcome and an optimal approach to management.1–8 The reported median survival from initial diagnosis ranges from 9 to 19 months.9,10 Response rates to chemotherapy and cytokines are low across all series.1–3,11
Recent years have brought significant advances in classification and treatment options for metastatic RCC. An improved understanding of the mechanisms involved in RCC growth and angiogenesis has led to the development and implementation of antiangiogenesis-targeted therapies in the treatment paradigm for metastatic RCC. Small molecule-targeted inhibitors sunitinib, sorafenib, temsirolimus, everolimus, and the monoclonal antibody bevacizumab have shown substantial antitumor activity in randomized phase 3 clinical trials,12–17 and have assumed a predominant role in the standard management of first-line, second-line, and third-line treatment for metastatic clear-cell RCC. As a result, the treatment paradigm, prognosis, and overall survival (OS) for patients with metastatic RCC have dramatically improved.
These new agents target angiogenesis by the hypoxia-driven gene pathway, with differences in the targeting profile, mechanisms of action, and pharmacokinetic profiles. For example, sunitinib is a small molecule that blocks activity for the receptors of vascular endothelial growth factor and platelet-derived growth factor, presumably of the endothelium. In contrast, temsirolimus, an mTOR inhibitor, may perform a more central action in tumor cells and endothelium by inhibiting multiple functions, including regulation of cell growth, metabolism, and angiogenesis.
In this era of targeted therapy, individualized care is an important goal in the treatment of RCC. The identification of subsets of patients who may or may not benefit from these agents is of critical importance. With the exception of the temsirolimus phase 3 trial,13 which included any RCC histology, other pivotal phase 3 trials conducted with targeted agents, and formerly with cytokines, provided a limited characterization of RCC histology, or focused primarily on the clear-cell component. In addition, none of these trials included a central pathology review or specified the presence of sarcomatoid features in the tumors. Studies characterizing histology and immunohistochemical (IHC) expression are necessary to identify subsets of patients who either respond or are refractory to treatment, and this information can be used to direct specific drug therapy and guide clinical trial eligibility and design.
Overall, experience with these new, targeted agents is very limited in sarcomatoid-variant RCC, a rare and aggressive RCC tumor type. Nanus et al5 reported antitumor activity in several patients with sarcomatoid-variant RCC who had been treated with the combination of doxorubicin and gemcitabine chemotherapy. This led to an Eastern Cooperative Oncology Group phase 2 trial to formally study the regimen.18 More recently, a retrospective analysis reported objective responses to vascular endothelial growth factor therapy for sarcomatoid-variant RCC, and concluded that these agents were of value in treating this subset of patients.19 On account of the activity observed in this report,19 and earlier studies showing mixed success with chemotherapy and cytokines, we carried out a retrospective analysis on all patients with sarcomatoid-variant metastatic RCC treated at Memorial Sloan-Kettering Cancer Center (MSKCC) over an 11-year interval. This report covers clinical features, treatment outcomes, and survival for patients with sarcomatoid-variant RCC. In addition, we investigated the IHC expression profile of hypoxia-inducible and mTOR pathway markers in patients with tumor specimens available for review.
MATERIALS AND METHODS
Sixty-three patients with metastatic, sarcomatoid-variant RCC, clinically evaluated and treated at MSKCC between January 1997 and October 2008, were included in this retrospective study, which was approved by the MSKCC Institutional Review Board. All patients had findings of sarcomatoid-variant RCC with either clear-cell or nonclear-cell histology by pathologic review at MSKCC, clinical evaluation showing metastases, and follow-up data.
Patients were identified from scanning 3 sources: (a) a database of 651 patients with metastatic RCC treated on a clinical trial, (b) a pathology database of 268 patients with sarcomatoid-variant RCC between January 1997 and June 2008, and (c) a surgical database of 2585 patients undergoing nephrectomy between 1997 and 2008.
The starting point for analysis was defined as the date of first treatment for patients treated on a prospective clinical trial and those treated by standard means outside a clinical trial at MSKCC. Patients who entered into more than 1 clinical trial were evaluated for this study at the time of entry into their first MSKCC trial. Patient demographics, clinical features at study entry, systemic therapy, assessment of response, date of last follow-up, and status were recorded. Systemic therapy was categorized as “targeted” (sunitinib, sorafenib, temsirolimus, or everolimus), “cytokine-based” (interferon-α, interleukin 2, or combination), and “other, ” which included conventional chemotherapy, and miscellaneous novel agents and therapies. New agents in clinical trials included geldanamycin, bortezomib, and cetuximab.20–22 One patient was treated with nonmyeloablative allogeneic bone marrow transplantation on a clinical trial. Data, when available, were obtained for second and third treatments given at MSKCC. Progression-free survival (PFS) was measured in months from the start of therapy to the first documentation of objective disease progression or to death from any cause, whichever occurred first. OS time was measured in months from the start of therapy to the date of death or last follow-up. Survival distributions were estimated by the Kaplan-Meier methodology.23 PFS was compared for “sunitinib treatment” versus “other treatment, ” and “clear cell” versus “nonclear cell” histology using the log-rank test.
Pathology Review and IHC Profile
All pathology materials were reviewed at MSKCC. In a subset of 31 patients with materials available in pathology files, all hematoxylin and eosin-stained slides (range, 10 to 32; average 17) were reviewed, and the percentage of the sarcomatoid and necrosis components in each tumor was estimated. Whenever possible, a representative paraffin block containing both the epithelial and sarcomatoid components of the tumor was chosen for IHC staining for each case. IHC staining was carried out on 28 tumors using antibodies against phospho-S6 (p-S6) and phospho-4E BPI (p-4E BP1) (markers for activated mTOR pathway), and hypoxia-inducible factor-1α (HIF-1α) and carbonic anhydrase IX (CA-IX) (markers for HIF pathway). Positivity was graded as zero to 3+ (0=0% to 5%; 1+ =6% to 25%; 2+ =26% to 50%; and 3+ ≥50% tumor cells positive) for p-S6 (cytoplasmic), p-4E BP1 (nuclear and/ or cytoplasmic), HIF-1α (nuclear), and CA-IX (membranous and cytoplasmic). The immunoreactivity in each tumor was evaluated for both the epithelial and sarcomatoid components. As positivity was similar in both the components as reported earlier,24 final grading was assigned by averaging the results in both areas. The location of immunoreactivity, whether around foci of necrosis (perinecrotic) or more diffuse, was also noted.
RESULTS
Patient Characteristics
The median age of study patients was 60 years. Sixty patients (95%) had their primary tumor resected by nephrectomy. There were a relatively high proportion of patients with lung, lymph node, and bone metastases. Fifty patients (79%) had ≥3 metastatic sites. RCC subtypes included 47 clear-cell tumors (75%) and 16 nonclear-cell tumors (25%). The nonclear-cell tumors consisted of 4 papillary, 5 chromophobe, 1 collecting duct, and 6 unclassified cell types (Table 1).
TABLE 1.
Patient Characteristics (N=63)
| Characteristic | No. Patients | % |
|---|---|---|
| Sex | ||
| Male | 41 | 65 |
| Female | 22 | 35 |
| Age (y) | ||
| Median (range) | 60 (31–81) | |
| Race | ||
| White non-Hispanic | 56 | 89 |
| Black non-Hispanic | 3 | 5 |
| Other | 4 | 6 |
| Earlier nephrectomy | 60 | 95 |
| No. metastatic sites | ||
| 1 | 1 | 2 |
| 2 | 12 | 19 |
| 3 | 25 | 40 |
| ≥4 | 25 | 40 |
| Sites of metastatic disease | ||
| Lung | 51 | 81 |
| Mediastinum | 16 | 25 |
| Retroperitoneum lymph nodes | 23 | 37 |
| Bone | 26 | 41 |
| Liver | 19 | 30 |
| Brain | 6 | 10 |
| Lymph nodes | 36 | 57 |
| Other | 34 | 54 |
| Histology subtype | ||
| Clear cell | 47 | 75 |
| Papillary | 4 | 6 |
| Chromophobe | 5 | 8 |
| Collecting duct | 1 | 2 |
| Unclassified | 6 | 10 |
| Baseline values, median (range) | ||
| KPS | 80 (60–100) | |
| LDH | 154 (91–320) | |
| Hemoglobin | 12.3 (7.8–14.9) | |
| Albumin | 4.2 (2.8–4.9) | |
| Corrected calcium | 8.9 (7.3–12.0) | |
| MSKCC risk group25 | ||
| Favorable | 23 | 37 |
| Intermediate | 37 | 59 |
| Poor | 3 | 5 |
PS indicates Karnofsky performance status; LDH, lactate dehydrogenase; MSKCC, Memorial Sloan-Kettering Cancer Center.
Treatment and Response
Thirty-four patients (54%) received targeted therapy as the first treatment at MSKCC. In most instances, the drug was sunitinib (n=29, 46% patients), but 3 patients were treated with sorafenib and 2 patients with temsirolimus. Twenty patients (32%) received cytokine therapy including interferon (19 patients) and interleukin-2 (1 patient). Nine patients (14%) received miscellaneous therapies, including gemcitabine or novel agents/programs in clinical trials.
All patients were evaluable for response to initial treatment. Five patients (8%) achieved objective responses; 4 partial responses (PRs) were observed to sunitinib therapy and 1 PR to interferon therapy (Table 2). Thirty patients achieved stable disease (SD) (18 to targeted therapies, 8 to cytokine therapies, and 4 to other therapies) as their best response. Twenty-eight patients had progression of disease as their initial response.
TABLE 2.
First Systemic Treatment and Response*
| Treatment | No. (%) | PR, No. (%) | SD, No. (%) | POD, No. (%) |
|---|---|---|---|---|
| Targeted | 34 (54) | 4 (12) | 18 (53) | 12 (35) |
| Sunitinib† | 29 (46) | 4 | 17 | 8 |
| Sorafenib | 3 (5) | 0 | 1 | 2 |
| Temsirolimus | 2 (3) | 0 | 0 | 2 |
| Cytokine based | 20 (32) | 1 (5) | 8 (40) | 11 (55) |
| Interferon | 19 (30) | 1 | 7 | 11 |
| Interleukin-2 | 1 (2) | 0 | 1 | 0 |
| Other | 9 (14) | 0 (0) | 4 (44) | 5 (55) |
| Geldanamycin | 1 (2) | 0 | 1 | 0 |
| Cetuximab | 2 (5) | 0 | 0 | 2 |
| Mini-Allo‡ | 1 (2) | 0 | 1 | 0 |
| Bortezomib | 4 (6) | 0 | 1 | 3 |
| Gemcitabine | 1 (2) | 0 | 1 | 0 |
| Response total | 5 (8) | 30 (48) | 28 (44) |
POD indicates progression of disease; PR, partial response; SD, stable disease.
On the basis initial evaluation at MSKCC.
In combination with gefitinib, bevacizumab, or interferon.
Nonmyeloablative allogeneic bone marrow transplant.
Responses were recorded for 31 patients who received a second treatment at MSKCC. In this group, 19 patients (61%) received targeted therapy: 5 with sunitinib, 6 with sorafenib, 5 with temsirolimus, 2 with everolimus, and 1 with bevacizumab. Two patients achieved objective responses. One complete response was observed with sunitinib therapy and 1 PR was achieved with temsirolimus therapy. SD as best response was achieved in 7 patients treated with targeted agents: 3 with sorafenib, 1 with temsirolimus, 2 with everolimus, and 1 with bevacizumab therapy. An additional patient treated with gemcitabine+docetaxel and 1 patient treated with lenalidomide achieved SD as their best response.
Overall, 34 patients were treated with sunitinib as first-line or second-line therapy. Complete response was observed in 1 (3%), PR in 4 (12%), and SD in 17 (50%) of the 34 patients treated with sunitinib as first-line or second-line treatment. For the 5 patients with objective (complete or partial) responses, the median response duration was 7 months (range, 3 to 16 mo). Overall, 7 patients were treated with temsirolimus as first-line or second-line therapy; best response was a PR in 1 patient, SD in 1 patient, with progression in 5 patients.
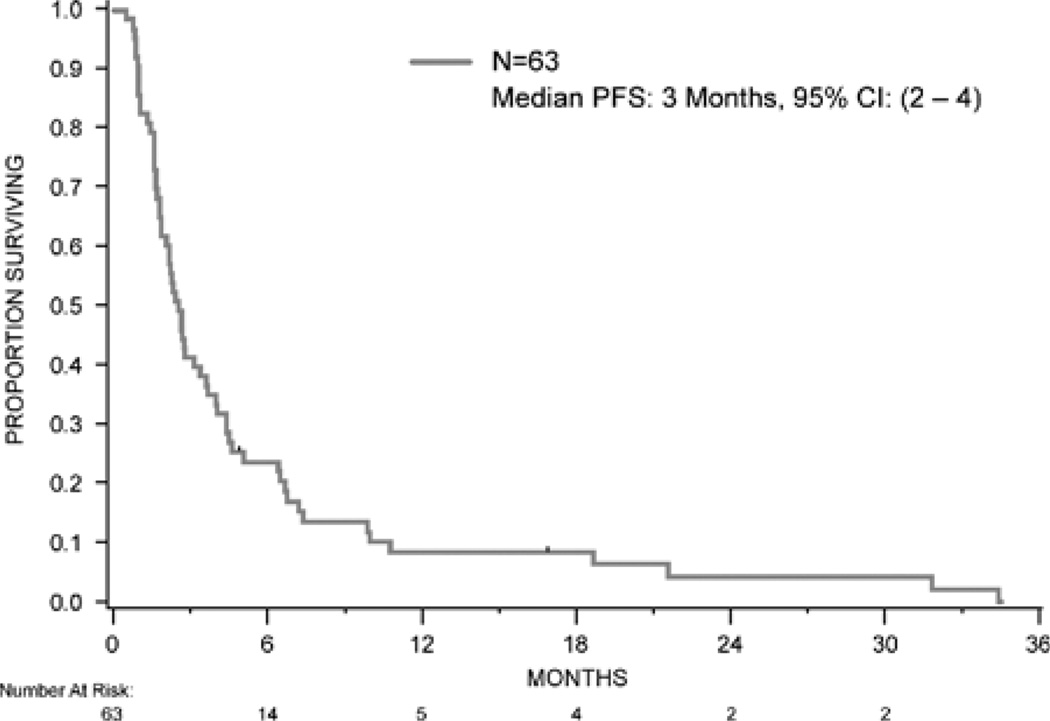
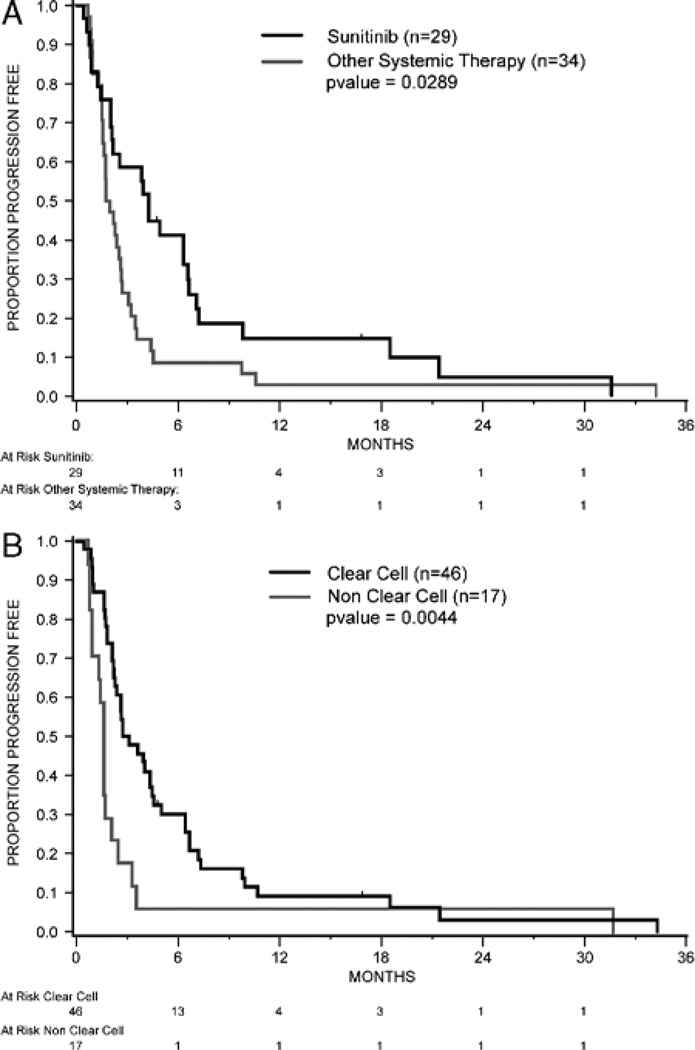
PFS and OS were assessed in the 63 patients from start of the first therapy at MSKCC. The median PFS was 3 months [95% confidence interval (CI), 2–4; Fig. 1]. PFS was compared by selected features including sunitinib therapy (given as first therapy, yes or no), histology (clear cell vs. nonclear cell), and MSKCC risk group (favorable/intermediate/poor). Differences in PFS were observed based on first therapy (sunitinib vs. all other therapies) and histology (clear cell vs. nonclear cell; Figs. 2A, B). The median PFS for patients treated with sunitinib was 4.4 months (95% CI, 2.2–6.7) versus 2 months (95% CI, 1.7–2.7) for all other therapies (P=0.03; Fig. 2A). The median PFS for patients with clear-cell histology was 3 months (95% CI, 2.3–4.5) versus 1.6 months (95% CI, 1.0–2.1) for nonclear-cell histology (P=0.004; Fig. 2B). No difference in PFS was observed according to the MSKCC risk group distribution.
FIGURE 1.
Progression-free survival. CI indicates confidence interval.
FIGURE 2.
A, Progression-free survival by treatment. B, Progression-free survival by histology.
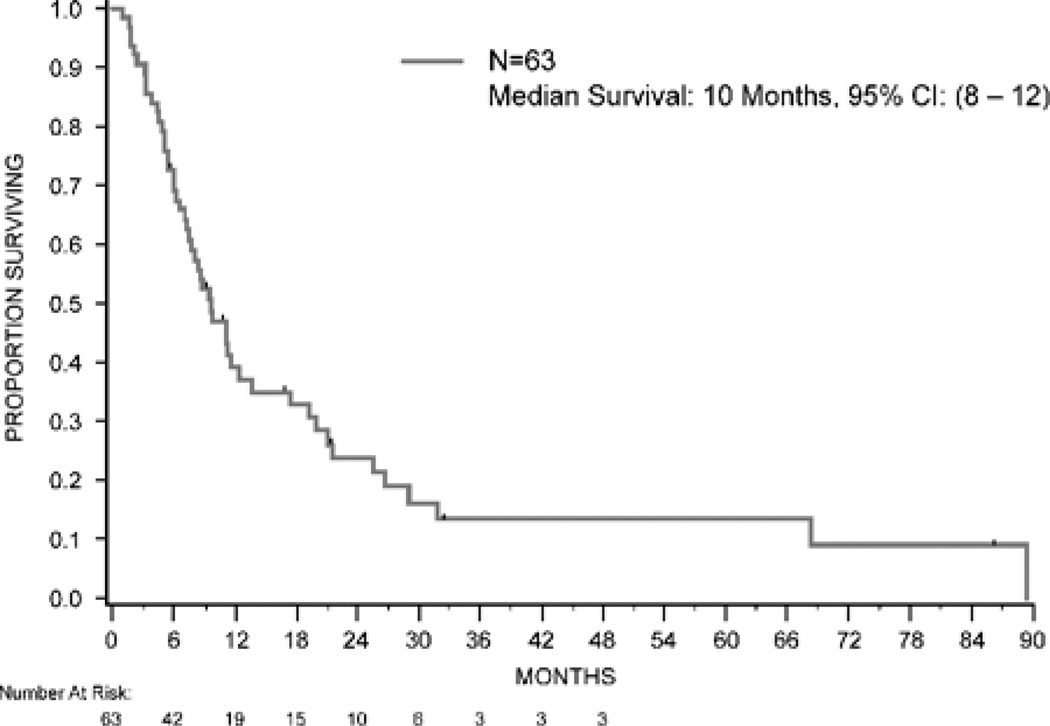
At the time of analysis, 49 of the 63 patients had died, 12 remained alive with disease, and 2 were alive at last follow-up. The median follow-up for survivors was 11 months (range, 2 to 86 mo). Median OS for the entire group was 10 months (95% CI, 8–12; Fig. 3). There was no difference in survival based on sunitinib therapy, histology, or MSKCC risk group. Median survival was 11.3 months in the favorable-risk group, 8.1 months in the intermediate-risk group, and 7.6 months in the poor-risk group (P=0.29).
FIGURE 3.
Overall survival. CI indicates confidence interval.
Pathology Review and Immunohistochemistry Staining
Tissue was available for review in 31 patients (49%). The median sarcomatoid content in the tumors was 20% (range, 2% to 100%). An association between the percentage of sarcomatoid and PFS or OS could not be identified.
Paraffin blocks were available for IHC staining in 28 patients (44%) (Table 3). Most of the clear-cell sarcomatoid RCC samples overexpressed (2+ or 3+) CA9 (87%), HIF-1α (61%), p-4E BP1 (54%), and p-S6 (72%); but no significant relationships could be detected between overexpression and outcome. Nonclear-cell sarcomatoid RCC samples did not overexpress these markers.
TABLE 3.
Immunohistochemical Expression in Clear Cell and Nonclear-cell Sarcomatoid RCC (n=28)
| Staining Grade |
||||||||
|---|---|---|---|---|---|---|---|---|
| Clear-cell RCC (n=24), No. (%) |
Nonclear-cell RCC (n=4), No. (%) |
|||||||
| Antibody | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| CA9 | 2 (8) | 1 (4) | 1 (4) | 20 (83) | 4 (100) | 0 (0) | 0 (0) | 0 (0) |
| HIF-1α | 6 (25) | 3 (13) | 4 (17) | 11 (46) | 4 (100) | 0 (0) | 0 (0) | 0 (0) |
| p-4E BP1* | 8 (35) | 2 (9) | 3 (13) | 10 (43) | 4 (100) | 0 (0) | 0 (0) | 0 (0) |
| p-S6* | 4 (18) | 2 (9) | 3 (9) | 14 (61) | 2 (50) | 2 (50) | 0 (0) | 0 (0) |
Staining not analyzed in 1 of 24 clear-cell tumors.
CA9 indicates carbonic anhydrase IX; HIF-1α, hypoxia-inducible factor-1α; p-4E BP1, phosphorylated eukaryotic initiation factor 4E-binding protein 1; pS6, phosphorylated protein S6 kinase 1; RCC, renal cell carcinoma.
DISCUSSION
The results of this study showed that metastatic sarcomatoid-variant RCC is associated with a poor outcome to systemic therapy. Our findings are consistent with the recent report citing occasional responses to sunitinib-targeted therapy, in which the estimated median PFS and OS were 5.3 and 11.8 months, respectively. Larger prospective trials have reported response rates of 30% to 50% with a median PFS of 8 and 11 months in metastatic, clear-cell RCC.12,26,27 The retrospective analysis of Golshayan et al,19 and our own experience, suggests that the robust response and PFS associated with sunitinib diminishes in the subset of patients with sarcomatoid features. Limitations of both reports include their retrospective nature, single-center experience, the small number of patients, and the inclusion of patients with nonclear-cell RCC histologies. Study of a larger number of patients with sarcomatoid-variant clear-cell RCC in a prospective manner is required.
A phase 3 trial of temsirolimus versus interferon showed survival benefit for patients with “poor prognosis” features.13 The trial allowed accrual for “clear cell” and “other” cell types. Although central pathology review was not available, and further discrimination of “other” cell types was not made, some of these would have likely been “sarcomatoid” RCC. In our series, we observed 1 patient with a PR to temsirolimus and a relatively high level of overexpression of mTOR pathway markers in the IHC staining of patients with sarcomatoid-variant clear-cell RCC. Clinical trials with temsirolimus and everolimus are warranted to further define activity in patients with sarcomatoid-variant RCC and nonclear-cell histologies.
Chemotherapy has been studied extensively in metastatic RCC, with no agent showing clear evidence of clinical benefit. However, 1 series of patients with various “poor prognosis” cell types treated outside a clinical trial has reported responses to combination doxorubicin and gemcitabine cytotoxic chemotherapy. This led to an Eastern Cooperative Oncology Group prospective, multicenter trial using this chemotherapy combination in a similar spectrum of RCC histologies, all containing sarcomatoid features. A response rate of 16% was reported, and the median PFS was 3.5 months.18 Data from this study point to modest activity in patients with sarcomatoid-variant RCC.28,29 Two ongoing phase 2 trials (NCT 00556049 and 00496587) are also currently studying combinations of chemotherapy and sunitinib in patients with sarcomatoid-variant metastatic RCC.
There is a need to explore novel therapeutic agents in prospective clinical trials directed at sarcomatoid-variant and other poor prognostic subsets of patients with RCC. Outside the context of a clinical trial, in general practice, we offer vascular endothelial growth factor-targeted therapy (ie, sunitinib, sorafenib, or bevacizumab) in treating patients with metastatic sarcomatoid RCC and an underlying clear-cell component. Temsirolimus has improved survival in metastatic RCC with poor prognostic features regardless of specific RCC histology.13 We hypothesize that some patients in the temsirolimus phase 3 trial had sarcomatoid features.
The diagnosis of sarcomatoid-variant RCC is made by review of tumor morphology and appropriate IHC staining. Investigation into the molecular signature of the sarcomatoid-variant may clarify the mechanisms of RCC resistance and provide new therapeutic targets. In our study, most clear-cell sarcomatoid-variant RCC overexpressed CA9, HIF-1α, p-4E BP1, and p-S6. The overexpression of markers for the mTOR activation pathway is intriguing, as this has been associated with a poor prognosis phenotype and clinical response to mTOR inhibitor systemic therapy.30
CONCLUSIONS
In summary, although sunitinib resulted in a modest response rate, metastatic sarcomatoid-variant RCC responds poorly to systemic therapy. Studies to characterize the underlying tumor biology of sarcomatoid RCC, to assess outcomes using targeted agents, and to develop novel treatment strategies are warranted in this unusual, aggressive RCC phenotype.
ACKNOWLEDGMENTS
The authors thank Carol Pearce (MSKCC Department of Medicine writer/editor) for her review of the manuscript.
Sources of support: Craig Tifford Fund.
Footnotes
Financial disclosures: Robert J. Motzer---research funding from Pfizer, Wyeth, Novartis, and GlaxoSmithKline; consulting with Novartis. Darren Feldman---research funding from Pfizer. The other authors declare no conflicts of interest.
REFERENCES
- 1.Sella A, Logothetis CJ, Ro JY, et al. Sarcomatoid renal cell carcinoma. A treatable entity. Cancer. 1987;60:1313–1318. doi: 10.1002/1097-0142(19870915)60:6<1313::aid-cncr2820600625>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Caliendo G, Hu XP, et al. Impact of histology on the treatment outcome of metastatic or recurrent renal cell carcinoma. Med Oncol. 1998;15:44–49. doi: 10.1007/BF02787344. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Droz JP, Rolland F, et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: a phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J Urol. 2002;168:959–961. doi: 10.1016/S0022-5347(05)64551-X. [DOI] [PubMed] [Google Scholar]
- 4.Culine S, Bekradda M, Terrier-Lacombe MJ, et al. Treatment of sarcomatoid renal cell carcinoma: is there a role for chemotherapy? Eur Urol. 1995;27:138–141. doi: 10.1159/000475145. [DOI] [PubMed] [Google Scholar]
- 5.Nanus DM, Garino A, Milowsky MI, et al. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer. 2004;101:1545–1551. doi: 10.1002/cncr.20541. [DOI] [PubMed] [Google Scholar]
- 6.Wood L, Amato R, Daliani D, et al. Phase I study of outpatient interferon-A (IFN), doxorubicin (DOXO), and ifosfamide (IFOS) for patients with metastatic sarcomatoid carcinoma; Presented at the 1999 ASCO Annual Meeting; 1999. [Google Scholar]
- 7.Amato RJ, Khan M. A phase I clinical trial of low-dose interferon-alpha-2A, thalidomide plus gemcitabine and capecitabine for patients with progressive metastatic renal cell carcinoma. Cancer Chemother Pharmacol. 2008;61:1069–1073. doi: 10.1007/s00280-007-0568-7. [DOI] [PubMed] [Google Scholar]
- 8.Cangiano T, Liao J, Naitoh J, et al. Sarcomatoid renal cell carcinoma: biologic behavior, prognosis, and response to combined surgical resection and immunotherapy. J Clin Oncol. 1999;17:523–528. doi: 10.1200/JCO.1999.17.2.523. [DOI] [PubMed] [Google Scholar]
- 9.De Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25:275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol. 2004;28:435–441. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kwak C, Park YH, Jeong CW, et al. Sarcomatoid differentiation as a prognostic factor for immunotherapy in metastatic renal cell carcinoma. J Surg Oncol. 2007;95:317–323. doi: 10.1002/jso.20669. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 13.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 15.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal-cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas N, Manola J, Pins M, et al. ECOG 8802: Phase II trial of doxorubicin (Dox) and gemcitabine (Gem) in metastatic renal cell carcinoma (RCC) with sarcomatoid features; Presented at the 2009 Genitourinary Cancers Symposium; 2009. [Google Scholar]
- 19.Golshayan AR, George S, Heng DY, et al. Metastatic sarco-matoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27:235–241. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 20.Kondagunta GV, Drucker B, Schwartz L, et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J Clin Oncol. 2004;22:3720–3725. doi: 10.1200/JCO.2004.10.155. [DOI] [PubMed] [Google Scholar]
- 21.Ronnen EA, Kondagunta GV, Lau C, et al. A phase I study of sunitinib malate (SU11248) in combination with gefitinib in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2006;24:225s. [Google Scholar]
- 22.Motzer RJ, Amato R, Todd M, et al. Phase II trial of antiepidermal growth factor receptor antibody C225 in patients with advanced renal cell carcinoma. Invest New Drugs. 2003;21:99–101. doi: 10.1023/a:1022928612511. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Tickoo SK, Alden D, Olgac S, et al. Immunohistochemical expression of hypoxia inducible factor-1alpha and its downstream molecules in sarcomatoid renal cell carcinoma. J Urol. 2007;177:1258–1263. doi: 10.1016/j.juro.2006.11.100. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaelson MD, Schwarzberg A, Ryan DP, et al. A phase I dose-finding study of sunitinib (SU) in combination with gemcitabine (G) in patients (pts) with advanced solid tumors. J Clin Onc. 2008;26:20. (suppl; abstr 14522). [Google Scholar]
- 29.Staehler M, Schoppler G, Haseke N, et al. Sorafenib is superior to combination therapy with gemcitabine plus doxorubicin for patients with sarcomatoid renal cell carcinoma; Presented at the 2008 Genitourinary Cancers Symposium; 2008. [Google Scholar]
- 30.Pantuck AJ, Seligson DB, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]