Abstract
Bone metastases are a common feature of advanced genitourinary malignancies and a prominent cause of morbidity and mortality. Clinical manifestations can include pain, hypercalcemia, pathologic fractures, and spinal cord compression. Optimal systemic therapy for the skeletal component of these cancers often features a combination of disease-specific therapy and bone-targeted therapy. Some agents such as the radiopharmaceutical radium-223 blur the line between those two categories. Osteoclast inhibition is a validated strategy in the management of selected patients with bone metastases and can best be accomplished with one of two agents. Zoledronic acid is the most potent available bisphosphonate and is approved for the prevention of skeletal events due to solid tumors metastatic to bone. Denosumab is a fully human monoclonal antibody that binds and inactivates receptor activator of nuclear factor-kappa-B ligand and is approved for the same indication. Radiopharmaceuticals represent a distinct strategy. Beta-emitters such as strontium-89 and samarium-153 can be effective for the palliation of pain due to bone metastases, but their use is often limited by bone marrow suppression. The alpha-emitting radiopharmaceutical radium-223 has recently been shown to improve overall survival and prevent skeletal events in selected men with castration-resistant prostate cancer metastatic to bone. Multiple ongoing clinical trials are designed to examine the potential for therapeutic inhibition of additional targets such as Src and hepatocyte growth factor (MET). This review discusses the incidence, pathophysiology, and management of bone metastases in the most prevalent genitourinary malignancies.
Introduction
Prostate, kidney, and bladder/urothelial cancers are the most common genitourinary malignancies. The natural history of each can feature bone metastases.
Prostate cancer is the second leading cause of cancer death in men (see Table 1). Bone metastases are by far the most prominent metastatic site, particularly within the axial skeleton.1 In the docetaxel registration program in men with castration-resistant prostate cancer (CRPC), 90% of the patients had bone metastases and less than 25% visceral metastases.2, 3 In non-metastatic castration resistant patients, bone is the first metastatic site 80% of the time.4 This peculiar epidemiology may explain why bone metastases are a major cause of morbidity and mortality this disease. Prostate cancer bone metastases generally appear dense/blastic on plain films but cause structural compromise and greatly elevate the risk for fractures. They are often detectable by technetium-99m methylene diphosphonate (99mTc MDP) bone scan, an established component of disease assessment in prostate cancer clinical trials.5 Other imaging modalities (computed tomography, or positron emission tomography with 18F-sodium fluoride, 18F-acetate, 11C-acetate, 18F-choline, 11C-choline, or others) may also detect bony metastases.6 Without bone-targeted therapy, the rate of skeletal-related events (SREs; pathologic fracture, spinal cord compression, surgery to bone, or radiation to bone) in men with CRPC metastatic to bone in one trial was approximately 44% (fracture rate of 22%) at 15 months.7, 8
Table 1.
Incidence, mortality, and skeletal complications due to genitourinary cancers in Europe and the U.S.
| Europe86 | United States87 | Approximate incidence of skeletal-related events (SREs) when metastatic to bone | |||
|---|---|---|---|---|---|
| New Cases | Deaths | New Cases | Deaths | ||
| Prostate | 382,300 | 89,300 | 241,740 | 28,170 | Castration-resistant prostate cancer: 44% for SRE; 22% for fracture7, 8 |
| Kidney | 88,400 (36.6% women) | 39,300 (36.9% women) | 64,770 (37.8% women) | 13,570 (36.2% women) | 74% for SRE; 40% for fracture9, 12, 13 |
| Bladder | 139,500 (21.4% women) | 51,300 (24.6% women) | 73,510 (24.4% women) | 14,880 (29.4% women) | >50% for SRE15 |
| Testicular | 18,300 | 1,700 | 8,590 | 360 | Poorly described |
Skeletal related events (SREs): pathologic fracture, spinal cord compression, surgery to bone, or radiation to bone.
Kidney cancer is the sixth to ninth most common cancer, depending on the region. Bone is second only to lung as a prevalent site of metastases.9 In patients with metastatic disease, the incidence of bone metastases is approximately 30%.9-11 Radiographically, bone metastases typically appear lytic, but can appear blastic or mixed. They are often but not always detectable by bone scan. Without bone-targeted therapy, the rate of SREs in patients with renal cell carcinoma metastatic to bone in one trial was 74% at one year.12, 13 Longer term, the rate of long-bone fractures has been estimated at approximately 40%.9
Bladder cancer is the fourth to sixth most common cancer, depending on the region. Among patients with metastatic disease, incidence of bone metastases is approximately 30%.14 As with kidney cancer, bone metastases can be radiographically blastic, lytic, or mixed. The rate of SREs in patients with urothelial cancer metastatic to bone is greater than 50% at one year.15
Bone metastases are very rare in patients with testicular cancer. Due to this rarity, their specific natural history is poorly described. They are associated with a poor prognosis according to the International Germ Cell Cancer Collaborative Group (IGCCCCG) classification, with a chance for cure of less than 50%.16
Normal and Pathologic Bone Physiology
Skeletal integrity is maintained by a balance between new bone formation by osteoblasts and bone resorption by osteoclasts. Osteoblasts are derived from stromal stem cells.17 They synthesize and secrete organic matrix that is then mineralized to form new bone. Osteoclasts are specific to bone but are derived from macrophage precursors.18 They bind bone and create an acidified resorption vacuole into which they secrete bone-resorbing enzymes.19 Resultant breakdown of bone matrix liberates numerous factors that can in turn stimulate osteoblast activity (e.g. transforming growth factor-β, insulin-like growth factors I and II, fibroblast growth factors, platelet-derived growth factors).20
Osteoclast regulation is complex but prominently features receptor activator of nuclear factor kappa B (RANK) signaling.19 RANK is a cell surface receptor that is present on osteoclasts throughout much of their lifecycle. RANK ligand (RANKL) binding to RANK promotes differentiation of osteoclast precursors. It is also important to activation and survival of mature osteoclasts. Major sources of RANKL within the bone microenvironment include stromal cells, osteoblasts, and activated T-cells.21-23 Parathyroid hormone (PTH), 1,25-dihydroxyvitamin D3, and other factors produce their effects on osteoclasts by increasing RANKL expression within the bone microenvironment.24, 25 Osteoprotegerin is an endogenous receptor to RANKL that can down-regulate osteoclast activity by serving as a sink for RANKL.26
Calcium homeostasis is important to normal bone mineralization. The vast majority of total body calcium is stored in bone. Serum levels are under strict hormonal regulation at the levels of intestinal absorption, mobilization from the skeleton, and resorption in the kidneys. The active form of vitamin D (1,24-dihydroxycalciferol) promotes absorption of calcium in the gut. PTH regulates resorption of calcium by the kidneys and mobilization from the bones by osteoclasts. Parathyroid hormone related protein is a PTH mimetic that is secreted by some tumors and can cause hypercalcemia of malignancy.
Bone metastases are clearly associated with an increase in bone turnover. This has been demonstrated by changes in actual osteoid volume27 and by changes in serum and urine bone turnover markers.28-30 Two widely-studied bone turnover markers are urinary N-telopeptide (NTx) and bone specific alkaline phosphatase (BAP). uNTx reflects collagen breakdown by osteoclasts. BAP is the bone-specific isoform of alkaline phosphatase (AP) and is elevated in the presence of bone formation by osteoblasts. Correlation is strong between total serum AP and BAP levels.29
Osteoclasts contribute greatly to the pathophysiology of bone metastases due to solid tumors. Osteoclast-mediated bone resorption can weaken the structural integrity of bone and can liberate growth factors that may stimulate osteoblasts and tumor cells. Elevated markers of elevated osteoclast activity are associated with adverse clinical outcomes.30-34 Osteoclast inhibition is therefore a rational therapeutic strategy. Two classes of osteoclast-targeted drugs are approved for this indication. Radiopharmaceuticals represent a third class of approved bone-targeted therapy. Finally, multiple additional classes of agents are in clinical development.
Classes of Available Bone-Targeted Therapies
Bisphosphonates
Bisphosphonates are a class of chemically-simple organic pyrophosphate analogs that inhibit osteoclast function. They are characterized by a central carbon atom, two methyl groups, and two organic side-chains. The composition of the organic side-chains is responsible for differences in relative potency.
Bisphosphonates that contain nitrogen (e.g. zoledronic acid) are far more potent than those that do not (e.g. clodronate). The agents are taken up by osteoblasts and deposited within areas of active bone remodeling. Once incorporated within bone, they likely exert long-lasting effects on osteoclasts that encounter them. Bisphosphonates are not metabolized. Unbound drug is renally eliminated. Serum half lives of most bisphosphonates are on the order of days (e.g. 146 hours for zoledronic acid). Zoledronic acid can be dose-reduced for stable renal dysfunction (glomerular filtration rate or GFR 30-60) but is not recommended for GFR < 30.
RANKL inhibitors
RANK is a central regulator of differentiation, activation, and survival of osteoclasts. Denosumab is a fully human monoclonal antibody that binds and inactivates RANKL. It binds avidly (Kd 3 × 10-12 M) and specifically to RANKL. Bioavailability is high with subcutaneous administration. Typical of a monoclonal antibody, its half-life is on the order of one month. In healthy subjects, a single dose rapidly and lastingly suppresses osteoclast activity as reflected by uNTx.35 Dosing varies by indication. It has been used at 60 mg every 6 months for the management of osteoporosis and at 120 mg every 4 weeks for the management of bone metastases. Dosing is not affected by renal insufficiency.
Radiopharmaceuticals
Radiopharmaceuticals represent another strategy for bone-targeted therapy. Conceptually, they are systemically-administered bone precursors that emit radiation or are linked to a radioactive emitter. This enables the delivery of radiation preferentially to areas of high bone turnover. Beta-emitting radiopharmaceuticals strontium-89, EDTMP-samarium-153, and rhenium-186 HEDP are similar in their abilities to palliate pain due to bone metastases and are approved for this purpose.36 One frequent dose-limiting toxicity is marrow suppression due to beta-particle penetration to adjacent marrow. Radium-223 is a newer alpha-emitting agent that is not yet approved. Alpha-particle penetration (≤100 μm) is far less than that of beta particles (several millimeters), making cytopenias less common.37 In addition, alpha particles are larger than beta particles and produce high linear energy transfer (LET) radiation that may lead to more DNA double-strand breaks. Radium-223 has a half-life of 11.4 days.38
Clinical Trial Endpoints
Clinical trials that study bone-targeted therapies generally feature endpoints that include time to first bone metastasis, skeletal related events (SREs), bone turnover markers, and overall survival. SREs are a composite endpoint that is typically defined as any of the following: pathologic fracture, spinal cord compression, surgery to bone, or radiation to bone. Osteoclast-targeted therapies such as zoledronic acid and denosumab have gained regulatory approval on the basis of their abilities to prevent or delay SREs.
Some studies, therefore, have used a standardized definition of SREs as a regulatory endpoint for osteoclast-targeted therapies. Other studies have examined some version of SREs as an exploratory endpoint. When used in this context, SRE has often been defined differently. New hormonal agents such as abiraterone acetate and enzalutamide (MDV3100) have demonstrated reductions in SREs39, 40, providing evidence that control of tumor growth can reduce the risk of bone complications. The incidence of SREs was not an endpoint in the phase III trials of several disease-modifying systemic therapies that improved overall survival (e.g. docetaxel, sipuleucel-T, cabazitaxel); therapeutic impact on SREs by those agents is therefore difficult to discern.
Many trials examine and report the effect of bone targeted therapy on overall survival. Completed trials of the most potent available osteoclast inhibitors have shown that this strategy does not impact overall survival.8, 41 In contrast, the radiopharmaceutical radium-223 demonstrated an ability to both prevent SREs42 and improve overall survival37.
It is common for trials of bone-targeted agents to formally examine bone turnover markers such as uNTx and BAP; this is discussed below.
Osteoclast Inhibition for CRPC Metastatic to Bone
Among men with prostate cancer, the population at highest-risk for skeletal events is those with CRPC metastatic to bone. Several trials have examined osteoclast inhibition in this setting. The comparatively weak bisphosphonates clodronate and pamidronate did not significantly reduce the incidence of SREs. Zoledronic acid and denosumab have each been shown to produce benefit and are approved for this indication. See Table 2 for a summary of notable trials of osteoclast inhibition for prostate cancer.
Table 2.
Notable completed clinical trials of osteoclast inhibition in advanced prostate cancer
| Study | N | Population | Study Arms | Endpoints | Outcome/Notes |
|---|---|---|---|---|---|
| National Cancer Institute of Canada (NCIC) Clinical Trials Group (CTG) Pr0688 | 209 | CRPC with symptomatic bone metastases | All received mitoxantrone (12 mg/m2 every 3 weeks) 1:1 randomization to clodronate (1,500 mg IV) or placebo every 3 weeks |
Primary: palliative response as assessed by present pain intensity index Secondary: symptomatic PFS, overall survival, quality of life |
No significant difference in palliative response (46% with clodronate vs. 39% with placebo, p = 0.54) or in secondary endpoints such as symptomatic PFS, overall survival, and quality of life |
| CGP 032 & INT-05 (combined analysis)89 | 378 | CRPC with symptomatic bone metastases | 1:1 randomization to pamidronate (90 mg IV) or placebo every 3 weeks for 27 weeks | Self-reported pain score, analgesic use, incidence of SREs, mobility | No significant difference in pain, analgesic use, or skeletal-related events. Urinary bone resorption markers such as NTx were significantly suppressed with therapy. |
| Trial 0397, 8 | 643 | CRPC with bone metastases | 1:1:1 randomization to zoledronic acid (4 mg or 8 mg) or placebo every 3 weeks | Proportion of patients with SREs, time to first SRE, skeletal morbidity rate, pain and analgesic scores, and disease progression | Significant decrease in skeletal related events (33.2 % with zoledronic acid 4 mg vs. 44.2% with placebo), trend toward improved survival. Zoledronic acid 8 mg was modified due to nephrotoxicity. |
| Trial 10341 | 1,904 | CRPC with bone metastases | 1:1 randomization to denosumab (120 mg SC) vs. zoledronic acid (4 mg IV) every 4 weeks | Primary: time to first on-study SRE and was assessed for non-inferiority Secondary: superiority in time to first SRE, overall survival |
Denosumab lengthened time to first on-study SRE (20.7 months vs. 17.1 months, hazard ratio or HR 0.82, 95% CI 0.71 to 0.95; p = 0.0002 for non-inferiority, p = 0.008 for superiority). |
| Medical Research Council (MRC) Pr0543, 44 | 311 | Prostate cancer with bone metastases, starting or responding to first-line ADT | 1:1 randomization to oral clodronate (2,080 mg) vs. placebo daily; maximum 3 years treatment | Primary: symptomatic bone progression free survival Secondary: overall survival, performance status |
Non-significant trend toward improved bone progression-free survival (HR 0.70, 95% CI 0.61 to 1.02; p = 0.066). Long term follow-up revealed an improvement in overall survival with clodronate treatment (HR 0.77, 95% CI 0.60 to 0.98; p = 0.032)43, currently regarded as hypothesis-generating. |
| MRC Pr0443, 45 | 508 | Nonmetastatic prostate cancer, within 3 years of diagnosis | 1:1 randomization to oral clodronate (2.080 mg) vs. placebo daily for up to 5 years | Symptomatic bone metastasis-free survival | There was no improvement in symptomatic bone metastasis free survival (HR 1.22; 95% CI 0.88 to 1.68) or survival (HR 1.02; 95% CI 0.80 to 1.30). |
| Trial 70446 | 201 (closed early) | Nonmetastatic CRPC | 1:1 randomization to zoledronic acid (4 mg IV) or placebo every 4 weeks | Bone metastasis-free survival | Halted early for futility due to lower-than-expected rate of bone metastases. With placebo, median bone metastasis-free survival was 30 months; PSA > 10 ng/mL and PSA doubling time were significantly associated with risk. |
| Trial 14748 | 1,432 | Nonmetastatic CRPC with PSA ≥ 8 μg/L or PSA doubling time ≤10.0 months | 1:1 randomization to denosumab (120 mg SC) or placebo every 4 weeks | Bone metastasis-free survival | Denosumab increased bone-metastasis-free survival by 4.2 months (median 29.5 months with denosumab vs. 25.2 months with placebo; HR 0.85, 95% CI 0.73 to 0.98, p = 0.028). It is not approved for this indication. |
Zoledronic acid was the first drug to reduce SREs in this clinical setting in the “039” trial.7, 8 That study enrolled 643 men with CRPC and bone metastases. Participants were randomized to every-3-weeks treatment with zoledronic acid (4 mg or 8 mg) or placebo. The trial was positive as SREs occurred in a greater proportion of those who received placebo (33.2 % with zoledronic acid 4 mg vs. 44.2% with placebo, 95% confidence interval or CI -20.3% to -1.8%, p = 0.021). Median time to first SRE was also significantly longer with zoledronic acid 4 mg (488 days with zoledronic acid vs. 321 days with placebo, p = 0.009).31 There were no significant differences in endpoints such as disease progression, overall survival, performance status, or quality of life.
The zoledronic acid “039” trial was also notable for nephrotoxicity with zoledronic acid. This observation led to two mid-trial changes. The 8 mg treatment arm was dose-reduced to 4 mg and the infusion time was lengthened from 5 minutes to 15 minutes. These changes have shaped subsequent use of the drug on and off of trials.
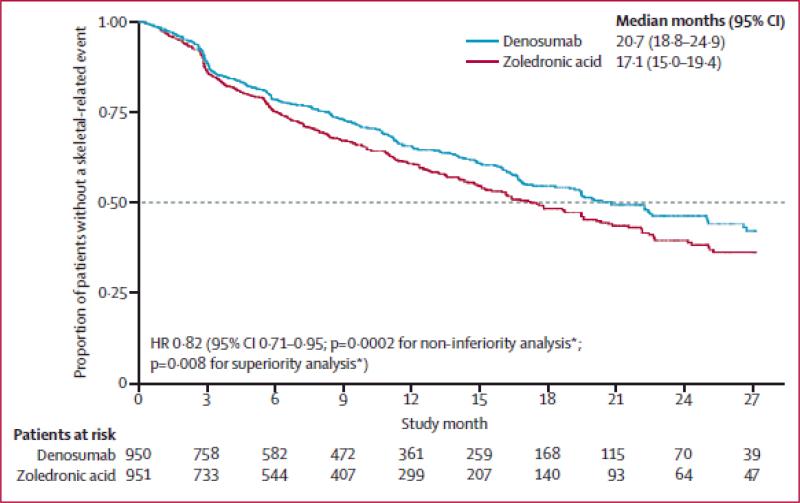
Denosumab was later compared directly to zoledronic acid and shown to be superior in the “103” phase III trial.41 That trial enrolled 1,904 men with metastatic CRPC. They were randomized to denosumab (120 mg SC) or zoledronic acid (4 mg IV) every 4 weeks. The trial was positive as denosumab lengthened time to first on-study SRE (20.7 months vs. 17.1 months, hazard ratio or HR 0.82, 95% CI 0.71 to 0.95; p = 0.0002 for non-inferiority, p = 0.008 for superiority; see Figure 1). Osteonecrosis of the jaw (ONJ) was observed in 1-2% of the study cohort (12 cases with zoledronic acid, 22 cases with denosumab; p = 0.09). Overall survival did not differ.
Figure 1.
Kaplan-Meier estimates of time to first on-study skeletal-related event for men with CRPC metastatic to bone. Subjects were assessed from baseline to the primary analysis cutoff date. HR, hazard ratio. *p values were adjusted for multiplicity.41
Zoledronic acid and denosumab have each been shown to reduce the incidence of SREs in men with CRPC metastatic to bone and are approved in this setting. We recommend use of one of the two agents in men with CRPC metastatic to bone who do not have contra-indications to therapy. In this setting, the optimal timing to start treatment has not been directly addressed in clinical trials. It is reasonable to consider therapy in patients at high-risk for SRE (e.g. those with multiple bony lesions, those with lesions at risk because of their anatomic location, or those with a previous history of SRE).
National Comprehensive Cancer Network guidelines state that “choice of agent may depend on underlying co-morbidities, whether the patient has been treated with zoledronic acid previously, logistics, and/or cost considerations.” What factors most compellingly cause clinicians to choose one over the other? Availability and cost are important factors that are beyond the scope of this review. Three factors favor denosumab in certain settings. First, denosumab produced superior time to first SRE (20.7 months vs. 17.1 months; HR 0.82, 95% CI 0.71 to 0.95; p = 0.008 for superiority).41 This is a modest but significant advantage. Second, zoledronic acid is not recommended for patients with a GFR < 30. Denosumab has not been formally studied in patients with GFR < 30 but is a reasonable option in this population. Third, a subcutaneous injection is usually more convenient than an intravenous injection.
Osteoclast Inhibition with First Line ADT for Prostate Cancer
Osteoclast inhibition in combination with first line androgen deprivation therapy (ADT) for metastatic prostate cancer is not an established strategy to prevent skeletal events. Clodronate failed to demonstrate a clinical benefit in this setting.43, 44 Zoledronic acid has not shown a benefit in this setting but is under study for men with hormone-sensitive bone metastases from prostate cancer in two ongoing phase III trials designed to evaluate SREs (NCT00242567 and NCT00079001).One of those, the CALGB/CTSU “90202” trial was prematurely closed to new accrual in April 2012 due to lack of sufficient study drug. Though follow-up is ongoing, this early closure may compromise an ability to detect a clinically important difference between early vs. standard use of zoledronic acid.
Regulatory approvals for denosumab and zoledronic acid are broader than that which is supported by level 1 evidence. They are European Medicines Agency (EMA) and U.S. Food & Drug Administration (FDA) approved for patients with solid tumors metastatic to bone. Osteoclast inhibition has never been shown to produce benefits in men with prostate cancer who have not yet developed castration-resistance. Metastatic hormone naïve prostate cancer is unique in that it is so frequently responsive to first-line disease-modifying therapy. Further, the relatively long natural history would lead to durations of every-4-weeks therapy that far exceed those that have been studied in trials. This would likely lead to an increase in treatment-related morbidity, particularly ONJ. We argue against use of either agent prior to the development of CRPC.
Osteoclast Inhibition for Prostate Cancer Metastasis Prevention
Osteoclast inhibition for the prevention of bone metastases is not an approved strategy. Clodronate43, 45 and zoledronic acid46 have thus far failed to demonstrate benefits in this setting. Denosumab was the first agent to produce a statistically significant delay the initial onset of bone metastases but was not approved for this indication.
Zoledronic acid is under ongoing study in the Zometa European Study (“ZEUS”)47 and STAMPEDE (NCT00268476) trials. The ZEUS trial has enrolled 1,433 men with nonmetastatic CRPC and at least one of the following high risk factors: PSA ≥20 ng/mL, lymph node positive disease, or Gleason ≥8 cancer. They are randomized 1 to zoledronic acid or placebo every 3 months for 48 months. The primary endpoint is the proportion of men with at least one bone metastasis. STAMPEDE is a seven-arm phase II/III trial that is planned to enroll 4,000 men with high risk localized, metastatic, or relapsed prostate cancer. It examines a number of combinations of ADT, zoledronic acid, docetaxel, abiraterone, and celecoxib. The primary outcome is overall survival.
In the “147” trial, denosumab was the first agent to demonstrate a statistically significant delay in time to first bone metastasis.48 That study enrolled 1,432 men with nonmetastatic CRPC and at least one of the following factors that are associated with risk for bone metastases: PSA ≥8.0 μg/L or PSA doubling time ≤10.0 months. Participants were randomized to denosumab (120 mg SC) or placebo every 4 weeks. The primary endpoint was bone-metastasis-free survival. The trial was positive as denosumab increased bone-metastasis-free survival by 4.2 months (29.5 months vs. 25.2 months; HR 0.85, 95% CI 0.73 to 0.98, p = 0.028). Symptomatic bone metastases were significantly less common with denosumab (69 cases vs. 96 cases; HR 0.67, 95% CI 0.49 to 0.92, p = 0.01) but were relatively uncommon as they occurred in only approximately 12% of the overall study population. Overall survival did not differ. Exploratory analysis indicated a larger effect on bone-metastasis free survival among men with PSA doubling time ≤ 6 months.49
The FDA Oncology Drug Advisory Committee reviewed the results of the denosumab “147” trial recommended against approval for metastasis prevention. The briefing document cited the lack of impact on survival, pain, and health-related quality of life. It also cited the 5% incidence of ONJ in the treatment group. Thus, the use of denosumab in the metastasis prevention setting was not felt to be of sufficient clinical benefit to outweigh the risks of its early and prolonged use. The FDA later issued a Complete Response Letter stating that the application could not be approved in its present form.
Clinical Use of Bone Turnover Markers
The role of bone turnover markers such as uNTx and total and bone AP in clinical practice is presently not well defined. Marker levels are clearly prognostic as they correlate with meaningful clinical outcomes such as SREs, cancer progression, and survival. 30-34, 50-52 They have been widely used in clinical trials as evidence of on-target effects in bone, but their use outside of trials is more limited. Professional guidelines are largely silent on their clinical use. Turnover markers are not clearly predictive as no systemic therapy has been convincingly shown to be more or less effective based on marker levels, though recent data preliminarily suggest greater benefit with radium-223 among patients with high baseline BAP levels37. We argue that prognostic information alone does not justify the widespread use of these markers in clinical practice. In specific circumstances, however, they may rationally guide the escalation of osteoclast-targeted therapy.
Escalation
Sub-optimal marker suppression could be taken as a cue to escalate therapeutic intensity. As neither zoledronic acid nor denosumab has been studied at a dose more often than every 3 to 4 weeks, shortening the dosing interval can not be safely pursued outside of a trial. Neither agent has been extensively studied at above-typical doses (denosumab 120 mg or zoledronic acid 4 mg) or in combination with the other. Change of agent is presently the only available strategy to escalate intensity.
Denosumab appears to be the more potent inhibitor of osteoclast function. It is superior in suppressing bone turnover markers41, 53-55, superior at preventing SREs due to breast cancer54 or CRPC41, and produces higher rates of hypocalcemia41, 54, 55. In patients receiving zoledronic acid, therefore, a switch to denosumab represents an escalation in therapeutic intensity. It is rational to consider such a switch in the presence persistently elevated uNTx levels (e.g. >50 nmol/L BCE/mM) despite ongoing zoledronic acid. In the phase III “039” trial in metastatic CRPC, approximately 20% of the participants receiving zoledronic acid had uNTx levels above this threshold.29 We argue that this is a reasonable clinical use of uNTx. However, given the limited evidence that a switching strategy results in clinical benefit, this strategy should be tested prospectively.
One phase II study made use of this strategy in subjects with at least one bone metastasis and uNTx levels >50 despite IV bisphosphonate treatment.53 They were randomized to continue every-4-weeks bisphosphonate treatment or switch to denosumab 180 mg every 4 weeks or every 12 weeks. Among the 50 subjects with prostate cancer, the primary endpoint (uNTx <50 at week 13) was reached more frequently in the denosumab arms (69% vs. 19%).56 Limitations of that study include the use of pamidronate in some participants, the use of a higher-than-typical denosumab dose, the selection of a “bisphosphonate-refractory” cohort, and the limited number of clinical events (12). Larger studies with clinical endpoints are needed.
De-escalation
Marker suppression beyond the 4-week dosing interval of either agent may provide a rationale for less frequent dosing. The safety of holding treatment until markers rise would need to be established in a large clinical trial designed to demonstrate non-inferiority in incidence of SREs. The BISMARK (BISphosphonate MARKer) trial is an example of this. It will randomize 1,500 women with metastatic breast cancer to receive either typical zoledronic acid dosing or potentially-less-frequent dosing as guided by uNTx levels. That trial is in follow-up. Given the absence of mature clinical trial data, this strategy can not yet be recommended.
Radiopharmaceuticals for Prostate Cancer
Systemically-administered radiopharmaceuticals first demonstrated efficacy in the palliation of pain due to bone metastases from prostate and other cancers. Several beta-emitting radiopharmaceuticals (strontium-89, EDTMP-samarium-153, and rhenium-186 HEDP) are approved for this indication.36 Strontium-89 has also been tested to consolidate chemotherapy in CRPC and showed to improve overall survival in a phase II trial.57 The most prominent limitation of these agents is myelosuppression.
Some studies have suggested potential for the combination of radiopharmaceuticals with other systemic therapies.57, 58 Combination therapy is under study in two notable phase III trials. An NCI-sponsored study combines strontium-89 with either docetaxel/prednisone or the KAVE regimen (ketoconazole, adriamycin, vinblastine, estramustine; NCT00024167). The U.K. TRAPEZE trial (NCT00554918) randomizes men with CRPC metastatic to bone to receive one of four regimens: (1) docetaxel/prednisolone, (2) docetaxel/prednisolone/zoledronic acid, (3) docetaxel/prednisolone/strontium-89, or (4) docetaxel/prednisolone/zoledronic acid/strontium-89.
Given the palliative efficacy of beta-emitters and the theoretical advantages of alpha-emitting agents, the phase III “ALSYMPCA” trial was designed to study the effect of radium-223 on overall survival. That study enrolled 922 men with symptomatic CRPC, at least two bone metastases, and no visceral metastases. Just over half (58%) of the patients had received prior docetaxel treatment. They were randomized 2:1 to receive six monthly treatments with radium-223 (50 kBq/kg IV) or placebo. The trial was positive as median overall survival was significantly longer with radium-223 (14.9 months vs. 11.3 months; HR 0.695, 95% CI 0.581 to 0.832, p = 0.00007).59 Radium also improved time to first SRE (15.6 months vs. 9.8 months; HR 0.658, 95% CI 0.522 to 0.830; p = 0.00037).42 Myelosuppression was slightly more common with treatment than with placebo (grades 3 & 4 neutropenia: 2.2% vs. 0.7%; grades 3 & 4 thrombocytopenia 6.3% vs. 2%). Regulatory review of the radium-223 data is ongoing but will likely result in approval of this agent for men with CRPC and symptomatic bone metastases.
One interesting subgroup analysis of the ALSYMPCA trial found that men with high baseline BAP levels experienced greater relative benefit.37 Another found that men who received concomitant zoledronic acid with radium-223 greater relative benefit. This may be related to the dual inhibition of bone turnover or to the reduced bone turnover and prolonged dwell time for radium in bone when given with osteoclast inhibition. Further study of radium-223 with other concomitant bone targeted and disease-specific therapies is needed to clarify these effects.
It is important to note that external beam radiation can provide effective and tolerable palliation of pain due to individual metastatic lesions or regions. A large majority of patients experience some pain relief with this strategy.60 Although some anatomic locations necessitate fractionation, many studies have made effective use of single-fraction therapy.61
Src Inhibition for Prostate Cancer
Src inhibition is a rational potential strategy for the management of bone involvement by cancer, particularly prostate cancer. Src is one within a family of non-receptor protein tyrosine kinases that are responsible for a diverse range of signal transduction pathways downstream of cell-surface receptors (e.g. growth factor receptors and cytokine receptors). Src is thought to be involved in both the pathogenesis of prostate cancer bone metastases and the regulation of osteoclast function.62, 63
Dasatinib is a potent oral inhibitor of Src family kinases and other kinases and is a prominent agent within this class.64, 65 The combination of dasatinib and docetaxel demonstrated promising safety and activity in a phase II study66 and became the subject of the phase III “READY” trial (NCT00744497). That study completed accrual and was designed to enroll 1,500 men with chemotherapy naïve metastatic CRPC and randomize them to docetaxel and prednisone with or without dasatinib 100 mg daily. The primary endpoint is survival.
MET Inhibition for Prostate Cancer
Hepatocyte growth factor (MET) has emerged recently as a potentially-important target. Cabozantinib (XL184) is an orally-administered tyrosine kinase inhibitor that prominently inhibits vascular endothelial growth factor receptor (VEGFR)-2 (IC50 0.035 nmol/L) and MET (IC50 1.3 nmol/L).67 In early-phase study, it dramatically improved 99mTc MDP bone scan evidence of disease in a high percentage of men with CRPC metastatic to bone.68, 69 This degree of treatment-induced improvements in bone scans has not previously been observed with VEGF-targeted agents70, 71 or with other MET inhibitors. The clinical significance, durability, and mechanisms responsible for these bone scan responses have not been well defined.
On the strength of this preliminary activity, cabozantinib is the subject of two phase III trials among men with prostate cancer. Each will enroll men with CRPC metastatic to bone and progressive despite docetaxel and either abiraterone or enzalutamide (MDV3100). “COMET 1” (NCT01605227) does not require cancer-related pain. Men will be randomized to cabozantinib or prednisone. The primary endpoint is overall survival. “COMET 2” (NCT01522443) requires pain due to bone metastases. Men will be randomized to cabozantinib or mitoxantrone/prednisone. The primary outcome measure is confirmed pain response at week 12 durable since week 6.
Rolotumumab (AMG-102) is a fully human MET-neutralizing antibody72 that did not produced significant benefits in a randomized phase II study.73
ETA Receptor Inhibition for Prostate Cancer
Endothelin A (ETA) receptor inhibition has thus far not yielded clinical benefits in men with prostate cancer. ETA is the receptor for endothelin-1 (ET-1), one of the three peptide members of the endothelin family. Endothelin signaling is important to cell growth and other processes in a diverse array of cell types.74 ET-1 emerged as a potential therapeutic target in cancer due to its role in osteoblast activation and in the pathogenesis of prostate cancer.75, 76 Two oral ETA receptor antagonists (atrasentan77 and zibotentan78, 79) have been the subject of phase III study in men with prostate cancer with both failing to meet their primary endpoints (see Table 3). These data strongly suggest that ETA inhibition alone or in combination with docetaxel is unlikely to substantially prevent or delay metastatic progression. Its role in patients selected based on high bone turnover or in other combinations may still be worthy of study.
Table 3.
Negative randomized phase III trials involving endothelin inhibition
| Agent | N | Population | Study Arms | Endpoints | Outcome/Notes |
|---|---|---|---|---|---|
| Atrasentan4 | 941 | Nonmetastatic CRPC | 1:1 randomization to atrasentan (10 mg PO) or placebo daily | Primary: time to disease progression (onset of metastases) | There was a non-significant (p = 0.288) 93 day improvement in time to disease progression. |
| Atrasentan90 | 809 | Metastatic CRPC | 1:1 randomization to atrasentan (10 mg PO) or placebo daily | Time to disease progression, overall survival | Atrasentan did not reduce the risk of disease progression (HR 0.89; 95% CI 0.76 – 1.04; p = 0.136). |
| Atrasentan SWOG 042191 | 930 planned (closed early) | CRPC with bone metastases | All receive docetaxel and prednisone; 1:1 randomization to atrasentan or placebo for up to 52 weeks | Survival, progression free survival | No differences in median OS (HR 1.01; 95% CI 0.87 – 1.18; p = 0.88), PFS, or response were seen between arms. |
| Zibotentan M0, Study 15 | 1,421 | Nonmetastatic CRPC | 1:1 randomization to zibotentan (10 mg PO) or placebo daily | Progression free survival, overall survival | Halted early due to lack of efficacy (overall survival 40 months with zibotentan, 39 months with placebo). Screen failures due to previously-unidentified metastases were common.92 |
| Zibotentan M1, Study 14 | 594 | CRPC metastatic to bone, mild or no pain | 1:1 randomization to zibotentan (10 mg PO) or placebo daily | Overall survival, progression free survival, time to use of opiates, SREs | Preliminarily reported as negative as the drug did not significantly improve overall survival (24.5 months with zibotentan, 22.5 months with placebo). |
| Zibotentan M1C, Study 33 | 1,052 | CRPC metastatic to bone | All received docetaxel 1:1 randomization to zibotentan (10 mg PO) or placebo daily | Overall survival | Preliminarily reported as negative as the drug did not significantly improve median overall survival (20.0 months with zibotentan, 19.2 months with placebo). |
Renal Cell, Bladder, and Urothelial Cancers
Many clinical trials of bone targeted therapies in advanced solid tumors focus on the most common diseases: prostate cancer and breast cancer. Patterns of drug development outside of breast and prostate cancers have favored single trials with mixed populations or analyses of subsets of patients included in larger phase 3 trials. Nonetheless, clinicians must make rational use of this lower level of evidence.
Zoledronic acid produced benefits in a placebo-controlled phase III trial that enrolled a heterogeneous population of patients with non-breast, non-prostate cancers involving bone.80 Compared to placebo, zoledronic acid was associated with a lower rates of at least 1 SRE at 21 months (39% vs. 46%) and longer median time to first SRE (236 days vs. 155 days, p = 0.009).
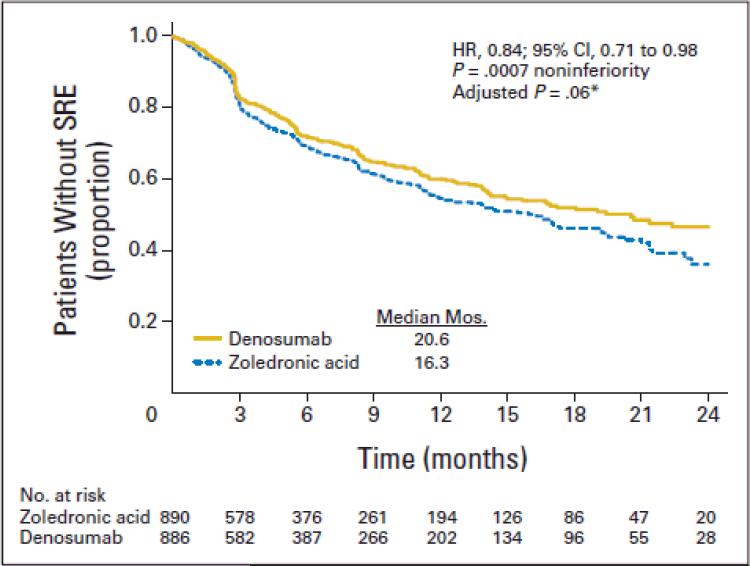
Denosumab and zoledronic acid demonstrated similar efficacy in a more recent phase III trial that enrolled patients with non-breast, non-prostate cancers involving bone.55 Denosumab was non-inferior to zoledronic acid in median time to first SRE (hazard ratio 0.84, 95% CI 0.71 – 0.98, p = 0.0007; see Figure 2). Renal cell carcinoma and urothelial cancers comprised subsets within each of these two pivotal trials.
Figure 2.
Kaplan-Meier estimate of time to first on-study skeletal-related events (SREs) for subjects with multiple myeloma or non-breast, non-prostate solid tumors metastatic to bone. HR, hazard ratio. (*) Adjusted for multiplicity.55
Renal Cell Carcinoma
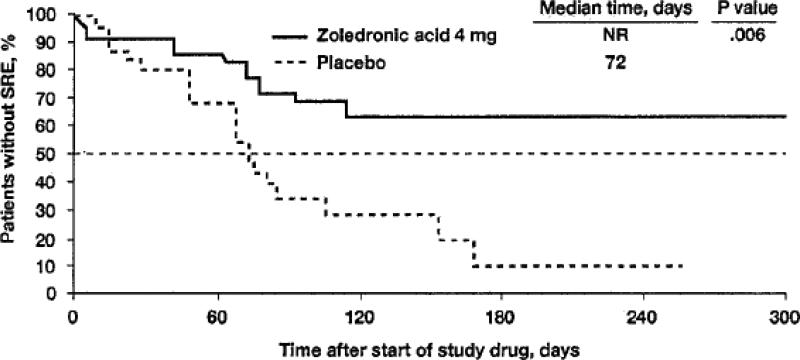
Management of renal cell carcinoma metastatic to bone can reasonably be guided by the zoledronic acid and denosumab trials described above. In particular, retrospective subset analysis of patients with RCC enrolled in the placebo-controlled zoledronic acid trial (n = 74) revealed that zoledronic acid significantly reduced the proportion of patients with an SRE (37% vs. 74% with placebo; p = 0.015; see Figure 3).13 Either of the two agents is reasonable in this clinical setting.
Figure 3.
Kaplan-Meier estimates of time to first skeletal-related event in patients with bone metastases from renal cell carcinoma during a 9-month trial of zoledronic acid. Data presented are for those who received 4-mg zoledronic acid (n = 27) or placebo (n = 19). NR, not reached; SRE.12
Patients with bone metastatic RCC have one of the highest rates of SREs of any solid tumor.81 In the placebo-controlled zoledronic acid trial, the 9-month incidence of an SRE in the placebo arm was 74% with RCC compared to 44% for the overall trial population.13, 80 A reduction in skeletal events is therefore likely to have a greater clinical impact in this group. The reduction in SRE incidence with zoledronic acid was associated with improvements in progression rates, and the relative improvement was particularly high in RCC.12 Thus, zoledronic acid is a reasonable choice to prevent SREs in patients with bone metastatic RCC if renal function is adequate.
Bladder Cancer
Bladder and upper tract urothelial cancers metastatic to bone are also managed as directed by the pivotal phase III trials of zoledronic acid and denosumab. Urothelial cancers are seldom the subject of dedicated phase III study using bone-targeted agents. Zoledronic acid did demonstrate benefits in one small randomized prospective trial (n = 40).15 That study enrolled patients with bone metastases from bladder cancer who were receiving palliative radiation therapy. They were randomized to zoledronic acid or placebo monthly for six months. The primary endpoint was positive as zoledronic acid produced a lower proportion of patients who had developed ≥1 SRE at 12 months follow-up (60% vs. 90% with placebo, p = 0.010). Secondary endpoints such as median time to first SRE and 1-year overall survival were also significantly improved.
Regulatory Approvals
In 2002, the FDA gave broad approval for zoledronic acid in conjunction with antineoplastic therapy for patients with multiple myeloma and with documented bone metastases from solid tumors. The approval stated that in prostate cancer those patients should have progressed after treatment with at least one hormonal therapy. EMA authorized zoledronic acid for “prevention of skeletal related events in adult patients with advanced malignancies involving bone.” More recently, denosumab received broad FDA approval in 2010 for “prevention of SREs in patients with bone metastases from solid tumors”. In 2011, the EMA recommended granting marketing authorization for denosumab “for prevention of skeletal-related events in adults with bone metastases from solid tumors.” Neither agent is approved or recommended for men without bone metastases or for hormone-sensitive bone-metastatic prostate cancer. However, in men who present with a skeletal-related event due to metastatic prostate cancer, the use of one of these agents is reasonable given the high risk nature for subsequent events in this population. Author recommendations are summarized in Table 4.
Table 4.
Author recommendations regarding osteoclast inhibition for genitourinary cancers
| Clinical Setting | Author Recommendations | Notes |
|---|---|---|
| Prostate cancer metastatic to bone and responding to first-line ADT | No osteoclast inhibition | Two ongoing phase III trials are expected to clarify the potential role of zoledronic acid in this clinical setting. |
| CRPC that is not metastatic to bone | No osteoclast inhibition | Denosumab prolonged bone metastasis-free survival in selected patients in this setting but is not approved for this clinical indication for a variety of reasons. |
| CRPC metastatic to bone | In the absence of contraindications, either of the following two options is reasonable: - Denosumab 120 mg every 4 weeks - Zoledronic acid every 4 weeks |
Denosumab is modestly but significantly superior for this indication. Appropriate dental care prior to initiation of therapy is important. Calcium and vitamin D supplementation are recommended. GFR < 30 mL/min is a contraindication for zoledronic acid and requires additional attention to calcium/phosphate monitoring when using denosumab |
| Renal cell or bladder/urothelial carcinoma metastatic to bone | In the absence of contraindications, either of two options is reasonable: - Denosumab 120 mg every 4 weeks - Zoledronic acid every 4 weeks |
Efficacy of the two drugs was similar in head-to-head study within a heterogeneous population of patients with metastatic solid tumors (non-breast, non-prostate). RCC metastatic to bone carries a particularly high risk for SREs, making this a strong indication for osteoclast inhibition. |
Toxicities of Osteoclast-Targeted Therapies
There are a number of potential toxicities of potent osteoclast inhibition (see Table 5). Hypocalcemia is common but is frequently asymptomatic and without clinical consequence. Flu-like acute phase reaction can occur in the wake of intravenous bisphosphonates, but is generally self-limited. Osteonecrosis of the jaw is relatively uncommon but can have substantial negative clinical impact. Nephrotoxicity has been observed with zoledronic acid but can generally be avoided with appropriate dosing, infusion time, and patient selection. Although RANKL plays a role in immune function through the regulation of interactions between T cells and dendritic cells22, 82, 83, infection rates appear to be unaffected.41, 54, 55, 84, 85
Table 5.
Notable toxicities of osteoclast-targeted therapies
| Toxicity | Approximate Incidence | Management/Notes |
|---|---|---|
| Hypocalcemia | Zoledronic acid: approximately 6% (1% grade 3-4) Denosumab: approximately 11-13% (2-5% grade 3-4), higher if impaired renal function |
Many cases asymptomatic Severe/symptomatic cases can lead to hospitalization for calcium repletion We recommend serum 25-OH vitamin D testing and repletion prior to initiation We recommend oral calcium (500-1000 mg daily) and vitamin D3 (600-1000 IU daily) |
| Acute phase reaction | Zoledronic acid: approximately 15-18% Denosumab: approximately 7-8% |
Characterized by flu-like symptoms such as malaise, myalgias, and fever Generally occurs within 24 hours of dosing and resolves without specific intervention |
| Osteonecrosis of the jaw (ONJ) | 1-2% with zoledronic acid or denosumab on phase III trials of metastatic solid tumors41, 54, 55 4-5% over 3-4 years with monthly denosumab for metastasis prevention48 |
Exposed non-healing bone of the jaw93, 94 Key risk factors include drug potency, duration of therapy, and invasive dental procedures95, 96 Published guidelines focus on maintenance of good oral hygiene, avoidance of invasive dental procedures during therapy97-101 |
| Nephrotoxicity | Zoledronic acid: nephrotoxicity was notably observed in the 039 phase III study with 8 mg dose and 5 minute infusion time7; nephrotoxicity is rare with current practice Denosumab: not observed |
Acute tubular necrosis102; severity ranges from mild/reversible to irreversible and requiring hemodialysis Zoledronic acid package insert recommends 15 minute infusion time, 4 mg maximum dose, and specific dose modifications for stable renal dysfunction with creatinine clearance >30 mL/min103 |
Note: Unless otherwise noted, incidence and grade are listed for monthly use of either zoledronic acid (4 mg) or denosumab (120 mg). Estimates are taken from phase III studies involving men with castration-resistant prostate cancer metastatic to bone41 and a mixed population of patients with solid tumors or multiple myeloma involving bone55.
Conclusions
Bone metastases cause considerable morbidity and mortality among patients with genitourinary malignancies. Optimal management requires consideration of bone-targeted therapy as well as disease-specific therapy. Zoledronic acid and denosumab are the most potent and widely-used osteoclast-targeted agents. Multiple additional targets are the subject of ongoing research efforts.
Key Abbreviations
- CRPC
Castration-resistant prostate cancer
- 99mTc MDP
Technetium-99m methylene diphosphonate
- IGCCCCG
International Germ Cell Cancer Collaborative Group
- RANK
Receptor activator of nuclear factor-kappa-B
- RANKL
RANK ligand
- PTH
Parathyroid hormone
- uNTx
Urinary N-telopeptide
- BAP
Bone specific alkaline phosphatase
- AP
Alkaline phosphatase
- GFR
Glomerular filtration rate
- LET
Linear energy transfer
- SREs
Skeletal related events
- CI
Confidence interval
- HR
Hazard ratio
- ONJ
Osteonecrosis of the jaw
- ADT
Androgen deprivation therapy
- EMA
European Medicines Agency
- FDA
Food & Drug Administration
- MET
Hepatocyte growth factor
- VEGFR
Vascular endothelial growth factor receptor
- ETA
Endothelin A
- ET-1
Endothelin-1
References
- 1.Lecouvet FE, Geukens D, Stainier A, Jamar F, Jamart J, d'Othee BJ, et al. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol. 2007;25(22):3281–7. doi: 10.1200/JCO.2006.09.2940. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113(9):2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52(1):81–9. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879–82. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 9.Zekri J, Ahmed N, Coleman RE, Hancock BW. The skeletal metastatic complications of renal cell carcinoma. International journal of oncology. 2001;19(2):379–82. doi: 10.3892/ijo.19.2.379. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 12.Lipton A, Colombo-Berra A, Bukowski RM, Rosen L, Zheng M, Urbanowitz G. Skeletal complications in patients with bone metastases from renal cell carcinoma and therapeutic benefits of zoledronic acid. Clin Cancer Res. 2004;10(18 Pt 2):6397S–403S. doi: 10.1158/1078-0432.CCR-040030. [DOI] [PubMed] [Google Scholar]
- 13.Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98(5):962–9. doi: 10.1002/cncr.11571. [DOI] [PubMed] [Google Scholar]
- 14.Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, Winquist E, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–5. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 15.Zaghloul MS, Boutrus R, El-Hossieny H, Kader YA, El-Attar I, Nazmy M. A prospective, randomized, placebo-controlled trial of zoledronic acid in bony metastatic bladder cancer. Int J Clin Oncol. 2010;15(4):382–9. doi: 10.1007/s10147-010-0074-5. [DOI] [PubMed] [Google Scholar]
- 16.International Germ Cell Cancer Collaborative Group International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 17.Aubin JE. Bone stem cells. J Cell Biochem Suppl. 1998;30-31:73–82. [PubMed] [Google Scholar]
- 18.Roodman GD. Cell biology of the osteoclast. Experimental hematology. 1999;27(8):1229–41. doi: 10.1016/s0301-472x(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 19.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 20.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. The Journal of biological chemistry. 1986;261(27):12665–74. [PubMed] [Google Scholar]
- 21.Kodama H, Nose M, Niida S, Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. The Journal of experimental medicine. 1991;173(5):1291–4. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 23.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofbauer LC, Heufelder AE. Osteoprotegerin and its cognate ligand: a new paradigm of osteoclastogenesis. European journal of endocrinology / European Federation of Endocrine Societies. 1998;139(2):152–4. doi: 10.1530/eje.0.1390152. [DOI] [PubMed] [Google Scholar]
- 26.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 27.Clarke NW, McClure J, George NJ. Osteoblast function and osteomalacia in metastatic prostate cancer. Eur Urol. 1993;24(2):286–90. doi: 10.1159/000474311. [DOI] [PubMed] [Google Scholar]
- 28.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88(12 Suppl):2919–26. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Cook RJ, Coleman R, Brown J, Lipton A, Major P, Hei YJ, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12(11 Pt 1):3361–7. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 30.Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97(1):59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 31.Costa L, Demers LM, Gouveia-Oliveira A, Schaller J, Costa EB, de Moura MC, et al. Prospective evaluation of the peptide- bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol. 2002;20(3):850–6. doi: 10.1200/JCO.2002.20.3.850. [DOI] [PubMed] [Google Scholar]
- 32.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23(22):4925–35. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 33.Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE. Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer. 2003;89(11):2031–7. doi: 10.1038/sj.bjc.6601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajpar S, Massard C, Laplanche A, Tournay E, Gross-Goupil M, Loriot Y, et al. Urinary N-telopeptide (uNTx) is an independent prognostic factor for overall survival in patients with bone metastases from castration-resistant prostate cancer. Ann Oncol. 2010;21(9):1864–9. doi: 10.1093/annonc/mdq037. [DOI] [PubMed] [Google Scholar]
- 35.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19(7):1059–66. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 36.Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Seminars in nuclear medicine. 2010;40(2):89–104. doi: 10.1053/j.semnuclmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Parker C, Heinrich D, O'Sullivan JM, Fossa SD, Chodacki A, Demkow T, et al. Overall survival benefit and safety profile of radium-223 chloride, a first-in-class alpha-pharmaceutical: Results from a phase III randomized trial (ALSYMPCA) in patients with castration-resistant prostate cancer (CRPC) with bone metastases. J Clin Oncol. 2012;30(suppl 5) abstr 8. [Google Scholar]
- 38.Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11(12):4451–9. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 39.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Bono J, Fizazi K, Saad F, Taplin M, Sternberg C, Miller K, et al. Primary, secondary, and quality-of-life endpoint results from the phase III AFFIRM study of MDV3100, an androgen receptor signaling inhibitor. J Clin Oncol. 2012;30(suppl) abstr 4519. [Google Scholar]
- 41.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sartor AO, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, Chodacki A, et al. Radium-223 chloride impact on skeletal-related events in patients with castration-resistant prostate cancer (CRPC) with bone metastases: A phase III randomized trial (ALSYMPCA). J Clin Oncol. 2012;30(suppl 5) abstr 9. [Google Scholar]
- 43.Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872–6. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson AC, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst. 2003;95(17):1300–11. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 45.Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, Sokal M, et al. Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J Natl Cancer Inst. 2007;99(10):765–76. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 46.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 47.Wirth M, Tammela T, Debruyne F, Tubaro A, Witjes W, Patel A, et al. Effectiveness of Zoledronic acid for the prevention of bone metastases in high-risk prostate cancer patients. A randomised, open label, multicenter study of the European Association of Urology (EAU) in Cooperation with the Scandinavian Prostate Cancer Group (SPCG) and the Arbeitsgemeinschaft Urologische Onkologie (AUO). A report of the ZEUS study.. 2008 Genitourinary Cancers Symposium; 2008. Abstract No: 184. [Google Scholar]
- 48.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2011;379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saad F, Smith M, Shore N, Oudard S, Miller K, Tombal B, et al. Effect of denosumab on prolonging bone-metastasis free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol. 2012;30(suppl) abstr 4510. [Google Scholar]
- 50.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21(7):1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13(21):6396–403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16(1):203–11. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 53.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 54.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 55.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic Acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2010;29(9):1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 56.Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. J Urol. 2009;182(2):509–15. doi: 10.1016/j.juro.2009.04.023. discussion 15-6. [DOI] [PubMed] [Google Scholar]
- 57.Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357(9253):336–41. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 58.Fizazi K, Beuzeboc P, Lumbroso J, Haddad V, Massard C, Gross-Goupil M, et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009;27(15):2429–35. doi: 10.1200/JCO.2008.18.9811. [DOI] [PubMed] [Google Scholar]
- 59.Parker C, Nilsson S, Heinrich D, O'Sullivan J, Fossa S, Chodacki A, et al. Updated analysis of the phase III, double-blind, randomized, multinational study of radium-223 chloride in castration-resistant prostate cancer (CRPC) patients with bone metastases (ALSYMPCA). J Clin Oncol. 2012;30(suppl) abstr LBA4512. [Google Scholar]
- 60.Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the Study by the Radiation Therapy Oncology Group. Cancer. 1982;50(5):893–9. doi: 10.1002/1097-0142(19820901)50:5<893::aid-cncr2820500515>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 61.Jeremic B. Single fraction external beam radiation therapy in the treatment of localized metastatic bone pain. A review. Journal of pain and symptom management. 2001;22(6):1048–58. doi: 10.1016/s0885-3924(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 62.Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36(2):177–84. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Fizazi K. The role of Src in prostate cancer. Ann Oncol. 2007;18(11):1765–73. doi: 10.1093/annonc/mdm086. [DOI] [PubMed] [Google Scholar]
- 64.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15(23):7421–8. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu EY, Massard C, Gross ME, Carducci MA, Culine S, Hudes G, et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77(5):1166–71. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1-2 study. Cancer. 2012;118(1):63–71. doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Molecular cancer therapeutics. 2011 doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 68.Hussain M, Smith MR, Sweeney C, Corn PG, Elfiky A, Gordon MS, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29(suppl) abstr 4516. [Google Scholar]
- 69.Smith DC, Smith MR, Small EJ, Sweeney C, Kurzrock R, Gordon MS, et al. Phase II study of XL184 in a cohort of patients (pts) with castration-resistant prostate cancer (CRPC) and measurable soft tissue disease. J Clin Oncol. 2011;29(suppl 7) abstr 127. [Google Scholar]
- 70.Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial Comparing Docetaxel and Prednisone With or Without Bevacizumab in Men With Metastatic Castration-Resistant Prostate Cancer: CALGB 90401. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michaelson MD, Oudard S, Ou Y, Sengelov L, Saad F, Houede N, et al. Randomized, placebo-controlled, phase III trial of sunitinib in combination with prednisone (SU+P) versus prednisone (P) alone in men with progressive castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2011;29(Suppl) doi: 10.1200/JCO.2012.48.5268. abstr 4515. [DOI] [PubMed] [Google Scholar]
- 72.Gordon MS, Sweeney CS, Mendelson DS, Eckhardt SG, Anderson A, Beaupre DM, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16(2):699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 73.Ryan CJ, Rosenthal M, Ng S, Alumkal JJ, Picus J, Gravis G, et al. A multicenter, randomized phase II study of rilotumumab (R) (AMG 102) or placebo (Pbo) plus mitoxantrone (M) and prednisone (P) in patients (pts) with previously treated castrate-resistant prostate cancer (CRPC). J Clin Oncol. 2012;30(suppl 5) abstr 115. [Google Scholar]
- 74.Battistini B, Chailler P, D'Orleans-Juste P, Briere N, Sirois P. Growth regulatory properties of endothelins. Peptides. 1993;14(2):385–99. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- 75.Nelson JB, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56(4):663–8. [PubMed] [Google Scholar]
- 76.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature medicine. 1995;1(9):944–9. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 77.Carducci MA, Nelson JB, Bowling MK, Rogers T, Eisenberger MA, Sinibaldi V, et al. Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetics. J Clin Oncol. 2002;20(8):2171–80. doi: 10.1200/JCO.2002.08.028. [DOI] [PubMed] [Google Scholar]
- 78.James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol. 2009;55(5):1112–23. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Warren R, Liu G. ZD4054: a specific endothelin A receptor antagonist with promising activity in metastatic castration-resistant prostate cancer. Expert opinion on investigational drugs. 2008;17(8):1237–45. doi: 10.1517/13543784.17.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21(16):3150–7. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 81.Woodward E, Jagdev S, McParland L, Clark K, Gregory W, Newsham A, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48(1):160–6. doi: 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 82.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 83.Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. The Journal of experimental medicine. 1999;189(7):1025–31. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watts NB, Roux C, Modlin JF, Brown JP, Daniels A, Jackson S, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int. 2012;23(1):327–37. doi: 10.1007/s00198-011-1755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 86.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 87.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 88.Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335–42. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 89.Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277–84. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 90.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110(9):1959–66. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 91.Quinn DI, Tangen CM, Hussain M, Lara PN, Jr., Goldkorn A, Garzotto M, et al. SWOG S0421: Phase III study of docetaxel (D) and atrasentan (A) versus docetaxel and placebo (P) for men with advanced castrate resistant prostate cancer (CRPC). J Clin Oncol. 2012;30(suppl) abstr 4511. [Google Scholar]
- 92.Yu EY, Miller K, Nelson J, Gleave M, Fizazi K, Moul JW, et al. Detection of Previously Unidentified Metastatic Disease as a Leading Cause of Screening Failure in a Phase III Trial of Zibotentan Versus Placebo in Patients with Nonmetastatic, Castration Resistant Prostate Cancer. J Urol. 2012 doi: 10.1016/j.juro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 94.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23(6):826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2011;23(5):1341–7. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 97.AAOMS American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369–76. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 98.Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1):117–20. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 99.Edwards BJ, Hellstein JW, Jacobsen PL, Kaltman S, Mariotti A, Migliorati CA. Updated recommendations for managing the care of patients receiving oral bisphosphonate therapy: an advisory statement from the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association (1939) 2008;139(12):1674–7. doi: 10.14219/jada.archive.2008.0110. [DOI] [PubMed] [Google Scholar]
- 100.Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20(1):137–45. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 101.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 102.Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int. 2003;64(1):281–9. doi: 10.1046/j.1523-1755.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 103.Novartis. Zometa full prescribing information. Package insert. ; revised 10/2009.