Abstract
Multiple events are involved in the development of acute inflammation and injury in the lungs. A progressive rise of oxidative stress due to altered reduction-oxidation (redox) homeostasis appears to be one of the hallmarks of lung pathologies such as injury, inflammation and ischemia/reperfusion. However, despite the growing evidence that alteration of the redox balance in the lungs, antioxidant therapy may attenuate acute lung injury and inflammation. We studied the effect of thiol antioxidant compound, amifostine, on acute lung dysfunction and pulmonary endothelial barrier compromise induced by gram-negative bacterial wall lypopolysacharide (LPS). In vitro, LPS as well as other producers of reactive oxygen spices (ROS), interleukin-6 (IL-6) and hydrogen peroxide (H2O2), induced significant reorganization of actin cytoskeleton accompanied by formation of stress fibers and paracellular gaps and associated with decreased transendothelial electrical resistance, a hallmark of endothelial barrier dysfunction. These disruptive effects were inhibited by pretreatment of endothelial monolayer with amifostine. Moreover, amifostine inhibited LPS-mediated ROS production and significantly suppressed LPS-, IL-6-, and H2O2-induced activation of redox sensitive signaling mechanisms including p38 and Erk1/2 MAP kinases, and NFκB pathway. In the murine model of LPS-induced acute lung injury, intraperitoneal administration of amifostine reduced LPS-induced oxidative stress and neutrophil recruitment to the lungs. These studies demonstrate for the first time that amifostine dramatically reduces endothelial cell barrier dysfunction and acute lung injury caused by bacterial products via inhibition of oxidative stress and redox-sensitive inflammatory pathways, and may therefore be considered for therapeutic treatment of lung inflammation.
Keywords: permeability, endothelium, lung, LPS, ROS, MAPK
INTRODUCTION
Acute lung injury (ALI) is characterized by diffuse inflammation in lung parenchyma. The involvement of inflammatory mediators in ALI has been intensely investigated, and oxidant mediated tissue injury has been assessed with the pathogenesis of ALI (1–3). In response to bacterial wall liposacharide (LPS) which appears as a result of gram-negative bacterial infection, the pulmonary macrophages and endothelium become activated and upregulate surface expression of adhesion molecules (4). This leads to neutrophil adhesion and subsequent transmigration from the intravascular space into the alveolus. The activated neutrophils produce a plethora of inflammatory mediators that include reactive oxygen species (ROS) such as OH− and O2− and nitric oxide, cytokines, chemokines, and cationic proteins. Reactive oxygen and nitrogen species can lead to cell injury by various mechanisms, including: a) direct damage to DNA resulting in strand breaks and point mutations; b) lipid peroxidation with formation of vasoactive and pro-inflammatory molecules; c) oxidation of proteins (primarily at sulfhydryl groups) that alter protein activity (5, 6), leading to release of proteases and inactivation of antioxidant and antiprotease enzymes (7), and d) alteration of transcription factors such as activator protein-1 and nuclear factor NF-κB, leading to enhanced expression of pro-inflammatory genes (8, 9).
One important facet of ALI is oxidative injury to the lung mediated by ROS, the partly reduced derivatives of molecular oxygen (1). Reactive nitrogen species, derivatives of nitric oxide have also implicated in oxidation (nitration) of proteins and lipids (10). Diverse pro-inflammatory compounds including LPS, cytokines, chemokines, complement fragment, clotting fragment, and lipid mediators that are elevated in patients with ALI, are capable of priming and/or activating neutrophils and endothelial cells to generate ROS (11–13). Among various circulating agents increased during inflammation, ROS may cause tissue oxidative stress that may further boost inflammatory signaling and lung vascular barrier dysfunction.
Although the role of anti-oxidant therapies in the ALI treatment is well recognized, effective antioxidant agents for treatments of these pathologies remain to be developed. Amifostine is a phosphorothioate that is converted to its active free thiol form by dephosphorylation by alkaline phosphatase in tissue. It has been approved by the FDA for use as a cytoprotective agent to decrease the incidence of moderate-to-severe xerostomia in patients undergoing postoperative radiation therapy for the treatment of head-and-neck cancer (14, 15). As a reducing agent capable of participating in intracellular reductive/oxidative process, amifostine has the potential to affect redox-sensitive transcription factors and gene expression once inside the cell (16). Its cytoprotective role includes several mechanisms, inactivation of free oxygen or nitrogen radicals, the chemical repair of damage through the donation of hydrogen atoms, and the induction of intracellular hypoxia as a result of auto-oxidation. In this respect, amifostine may ameliorate LPS-induced acute lung injury through suppression of a ROS generation.
In this study we tested this hypothesis and investigated effects of amifostine on endothelial cell (EC) barrier dysfunction induced by inflammatory agonists and oxidants in the cell-based and mouse models of LPS-induced acute lung injury. Our results suggest that the protective effect of amifostine may be mediated by its antioxidant properties resulting in downregulation of oxidative stress and redox-sensitive signaling cascades and lead to attenuation of lung inflammation and vascular leak.
MATERAILS AND METHODS
Reagents and cell culture
Unless otherwise specified, biochemical reagents were obtained from Sigma (St. Louis, MO). Amifostine compounds WR-1065 (unprotected form used for cell culture treatments) and WR-2021 (protected formulation used for intravenous injections in vivo) were obtained from the Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute. WR-1065 [2-(aminopropylamino) ethanethiol] used for in vitro study was dissolved at a concentration of 1 M in phosphate-buffered saline (PBS) (8.1 mM Na2HPO4, 1.5 mM KH2PO4, 140 mM NaCl) immediately before use. LPS from E Coli 055:B5 was obtained from Sigma-Aldrich Inc. (St. Louis, MO), IL-6 and IL-6 soluble receptor (IL6-SR) were obtained from R&D Systems (Minneapolis, MN). The following antibodies were commercially obtained: anti-VE-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-Hsp27 (Stressgen, Ann Arbor, MI), anti-nitrotyrosine (Invitrogen, San Francisco, CA), anti-diphospho-MLC, anti-phospho-p38, anti-phospho-MEK1/2, anti-phospho-Erk1/2, anti-phospho-IκBα, anti-phospho-NFκB-p65, horseradish peroxidase-linked anti-mouse and anti-rabbit IgG antibodies (Cell Signaling, Beverly, MA). Texas-Red phalloidin and Alexa Flour 488 conjugated secondary antibodies were purchased form Molecular Probes (Eugene, OR). Human pulmonary artery endothelial cells (HPAEC) and cell culture basal medium (EBM-2) with growth supplements were obtained from Clonetics (Walkersville, MD), cultured according to the manufacturer’s protocol, and used at passages 5–9.
Endothelial cell imaging
EC monolayers grown on glass coverslips were stimulated with agonist of interest, then washed with warm PBS twice and fixed in 3.7% paraformaldehyde solution in PBS for 10 min at room temperature followed by immunofluorescence staining. EC cytoskeletal organization was analyzed as we have previously described (17).
Measurement of transendothelial electrical resistance (TER) as an index of barrier dysfunction
The cellular barrier properties were analyzed by measurements of transendothelial electrical resistance across confluent endothelial cell monolayers using the electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as previously described (18, 19).
Detection of ROS production in live cells
ROS production was measured using Image-iT LIVE Green Reactive Oxygen Species Detection Kit (Molecular Probes, Inc., Eugene, OR, USA). HPAEC cells were seeded in 12-well plate. Confluent cells were pretreated with 4 mM WR 1065 for 30 min or 5 mM N-acetylcysteine (NAC) for 1 hr followed by LPS stimulation for 3 hrs. After stimulation, cells were gently washed once in warm Hanks buffer and incubated with 1 ml of 25 μM carboxy-H2DCFDA working solution (25 min, 37 °C, protected from light). Then cells were washed gently 3 times in warm Hanks buffer and subjected to microscopy using Nikon video-imaging system (Nikon Inc, Tokyo, Japan) consisting of phase contrast inverted microscope equipped with set of objectives and filters for immunofluorescence and connected to a digital camera and image processor. Ten fields were randomly chosen for each experiment condition.
Immunoblotting
Immunoblot detection of proteins of interest was performed as described previously (19, 20). Briefly, after exposure to different agonists equal amounts of cell or lung tissue extracts were subjected to 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to PVDF membrane (Millipore, Bedford, MA) (100 V for 1 hour), and probed with specific antibodies. Immunoreactive proteins were visualized by enhanced chemiluminescence according to the manufacturer’s protocol (Amersham, Little Chalfont, UK). Protein bands were quantified using ImageQuantR (Molecular Dynamics, Sunnyvale, CA) software.
In vivo model of ALI
All protocols involving the use of animals were approved by the University of Chicago Institutional Animal Care & Use Committee for the humane treatment of experimental animals. Adult male C57BL/6J mice, 8–10 week old, with average weight 20–25 grams (Jackson Laboratories, Bar Harbor, ME) were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg) according to institutional regulations. LPS (0.7 mg/kg body weight, Escherichia coli O55:B5) or sterile water was injected intratracheally in a small volume (20–30 μl) using a 20 gauge catheter Penn-Century Inc., (Philadelphia, PA). Mice were randomized to concurrently receive sterile saline solution or WR-2721 (200 mg/kg) intraperitoneal injection to yield the experimental groups: control, LPS (0.7 mg/kg) only, WR-2721 (200 mg/kg) only, and LPS (0.7 mg/kg) + WR-2721 (200 mg/kg).
Bronchoalveolar lavage (BAL) analysis
After 18 hrs, animals were sacrificed by exsanguination under anesthesia. Tracheotomy was performed, and the trachea was cannulated with a 20 gauge intravenous catheter, which was tied into place. BAL was performed using 1 ml of sterile Hanks Balanced Salt Buffer. The collected lavage fluid was centrifuged at 2500 rpm for 20 min at 4°C, the supernatant was removed and frozen at −80°C for subsequent protein study. The cell pellet was then resuspended in 1 ml of red blood cell lysis buffer (ACK Lysing Buffer, BioSource International) for 5 min and then re-pelleted by centrifugation at 2500 rpm for 20 min at 4°C. The cell pellet was again resuspended in 200 μl of PBS, and 20 μl of cell suspension were used for cell counting by a standard hemocytometer technique. The remaining 180 μl of cell suspension were re-pelleted by centrifugation, and cell pellets were stored at −80°C for subsequent detection of myeloperoxidase activity (MPO). The BAL protein concentration was determined by a modified Lowry colorimetric assay using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). The absorbance was measured at 750 nm, and protein concentration was determined using standard curves.
Myeloperoxidase activity (MPO) measurement
MPO activity was performed as described elsewhere (21). Briefly, cell pellets were subjected to a round of freezing (on dry ice) and thawing (at room temperature), followed by 20-second sonication (Virsonic V60) at full power on ice. Samples were centrifuged at 10,000 X g for 10 min at 4°C, the supernatant was collected into fresh 1.5 ml tubes and placed on ice. Next, 100 μl of supernatant was added to polystyrene cuvette, and the MPO reaction was initiated by adding 2.9 ml of 1 X assay buffer (50 mM potassium phosphate buffer, pH=6.0 containing 0.167 mg/ml of odianisidine and 0.0005% of H2O2). Changes in absorbance at 460 nm were recorded for 1 min with spectrophotometer (Shimadzu UV 1201) and the rate of change in absorbance/min was converted to the MPO activity.
Histological assessment for lung injury
Left animal lungs were harvested without lavage collection and fixed in 4% parafomaldehyde. After fixation, the lungs were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosion (H&E). Sections were evaluated at x 400 magnificence.
Statistical analysis
Results are expressed as means ± SD of three to ten independent experiments. Stimulated samples were compared to controls by unpaired Student’s t-test. For multiple-group comparisons, a one-way variance analysis (ANOVA), followed by the post hoc Fisher’s test, were used. P<0.05 was considered statistically significant.
RESULTS
Effects of amifostine on EC barrier dysfunction induced by H2O2, LPS, and IL-6
To examine effects of amifostine on endothelial permeability induced by agonists involved in oxidative stress, we measured changes in transendothelial electrical resistance (TER) in human pulmonary EC cultures challenged with H2O2, LPS, or IL-6 with or without amifostine pre-treatment. Stimulation of cells with H2O2 (250 μM), LPS (200 ng/ml), or IL-6 (25 ng/ml combined with IL6-SR, 100ng/ml) caused significant increase in EC permeability (Figure 1). Pre-treatment with amifostine WR-1065 for 30 min dramatically attenuated H2O2-indcued barrier disruption in a dose-dependent manner (Figure 1A). Noticeably, at the concentration 4mM WR-1065 exhibited maximal protective effect. These data are in agreement with earlier studies conducted on different models (22, 23). Similarly, amifostine attenuated EC dysfunction induced by LPS and IL-6 (Figure 1BC, respectively). Amifostine alone did not affect basal TER levels in pulmonary arterial EC culture.
Figure 1. Effect of amifostine on H2O2, LPS and IL-6-induced endothelial barrier dysfunction.
Human pulmonary EC were grown on golden microelectrodes. At the time point indicated by first arrow, cells were pretreated with WR-1065 (0.4 mM, 1 mM or 4 mM, 30 min) followed by stimulation with 250 mM H2O2 (Panel A). EC were pretreated with 4 mM WR-1065 for 30 min followed by 200 ng/ml LPS stimulation with LPS (200 ng/ml) (Panel B), or combination of IL-6 (25 ng/ml) and its soluble co-receptor IL-6-SR (10 U/ml) (Panel C) marked by second arrow, and TER reflecting EC permeability changes was monitored over time. Shown are pooled data from three independent experiments.
Effects of amifostine on endothelial cytoskeletal remodeling induced by H2O2, LPS, and IL-6
To assess effects of amifostine on cytoskeletal rearrangement associated with EC dysfunction induced by H2O2, LPS, or IL-6, we performed immunofluorescence staining of human pulmonary EC stimulated with these agonists with or without amifostine WR-1065 pre-treatment. In unstimulated cells, F-actin was primarily organized into actin bundles which were similar in cells treated with WR-1065 (4 mM) alone (Figure 2A). After 30 min of H2O2 (250 μM) stimulation, or 6 hrs of stimulation with LPS (200 ng/ml) or IL-6 (25 ng/ml combined with IL6-SR, 100ng/ml), F-actin was reorganized into thicker stress fibers in the center of the cells, whereas peripheral actin rim was significantly weakened. These changes were associated with appearance of paracellular gaps (shown by arrows) indicating EC barrier compromise. Remarkably, amifostine pretreatment dramatically attenuated stress fibers and gap formation induced by H2O2, LPS, and IL-6. In agreement with these data, pre-incubation of pulmonary EC with amifostine also attenuated H2O2− and LPS-induced disruption of adherens junctions, as detected by immunofluorescence staining for VE-cadherin (Panel B, shown by arrows). These results suggest that the protective role of amifostine may be attributed to its ability to attenuate cytoskeletal remodeling induced by H2O2, LPS, and IL-6.
Figure 2. Amifostine prevents agonist-induced lung EC cytoskeletal remodeling and adherens junction disruption.
EC were grown on glass cover-slips pretreated with amifostine (4 mM, 30 min) or not, followed by H2O2 (250 mM, 15 min), LPS (200 ng/ml, 6 hrs), or IL-6 + IL-6-SR (25 ng/ml and 10 U/ml, 6 hrs) stimulation. Analysis of actin cytoskeletal remodeling was performed by immunofluorescent staining with Texas Red phalloidin. Paracellular gap formation is shown by arrows (Panel A). VE-Cadherin staining was performed, to visualize LPS-induced disruption of adherens junctions (shown by arrows) (Panel B). The panels are representative of the entire cell monolayer. Shown are results of three independent experiments.
Effects of amifostine on ROS production induced by LPS
To test the hypothesis that amifostine may attenuate LPS-induced cytoskeletal remodeling and barrier dysfunction by its ability to scavenge ROS produced by EC upon LPS treatment, we measured ROS production in EC stimulated with LPS with or without amifostine pretreatment. Well-known anti-oxidative agent NAC (24–26) was used as a positive control. LPS treatment (6 hrs) increased ROS production in pulmonary arterial EC in a dose-dependent manner with maximal effect at 500 ng/ml LPS (Figure 3). Pretreatment with 4 mM WR-1065 or 5 mM NAC significantly inhibited ROS production induced by LPS. These results and data shown in Figures 1,2 clearly indicate that oxidative stress is directly involved in the LPS-induced EC barrier dysfunction. These results also suggest that the protective role of amifostine in preventing H2O2, LPS, or IL-6-induced barrier dysfunction may be mediated at least in part by its antioxidant activity.
Figure 3. Amifostine inhibits ROS production induced by LPS.
HPAEC were pretreated with vehicle (top row), NAC (5 mM, 1 h) (middle row) or WR-1065 (4 mM, 30 min) (low row) followed by LPS stimulation (100 ng/ml, 200 ng/ml, 500 ng/ml). ROS was detected by DCFDA fluorescent assay described in “Material and Methods”. The results are representative of the entire cell monolayer and have been reproduced in three independent experiments.
Signaling pathways modulated by amifostine and involved in the regulation of EC permeability
ROS and associated oxidative stress are shown to activate various signaling molecules, such as Erk-1/2, p38, JNK MAP kinases, p53, PI3K/Akt and NF-κB signaling (8, 27–29). Therefore, MAPK signaling cascades are intimately involved in control of EC permeability and inflammatory responses (30, 31) and regulated by phosphorylation on serine/threonine and tyrosine residues. In the next series of experiments we investigated effects of amifostine on the regulation of MAPK- and NF-κB-dependent signaling activated by oxidative stress. HPAEC Exposure of lung endothelium to LPS (200 ng/ml, 2 hrs) caused pronounced phosphorylation/activation of the MEK1/2 - Erk1/2, and p38 MAP kinase signaling cascades (Figure 4AB). Activated p38 MAPK induces MAPKAP-K2-mediated phosphorylation of Hsp27, a regulator of actin dynamics (32, 33). Our results demonstrated significant increase in Hsp27 phosphorylation in response to LPS. Remarkably, pretreatment with WR-1065 (4 mM, 30 min) significantly attenuated the activation of p38 and Erk1/2 MAPK cascades. Analysis of NF-κB-dependent signaling revealed that LPS-induced IκBa and NF-κB activation was significantly attenuated in EC pretreated with WR-1065. Similar results were obtained in experiments with H2O2 (250 mM, 15 min) and IL-6 (25 ng/ml combined with IL6 soluble receptor (IL6-SR), 100 ng/ml, 2 hrs). Amifostine pretreatment (WR-1065, 4 mM, 30 min) dramatically reduced H2O2-mediated phosphorylation of p38 MAPK, Hsp27, IκBa, and IL-6-mediated phosphorylation of Hsp27 (Figure 4 BC). Increased myosin light chain (MLC) phosphorylation is associated with isometric tension development and increased actin polymerization in endothelial cells (34, 35). Additionally, inflammatory mediators may inactivate myosin-associated protein phosphatases (36, 37), further promoting MLC phosphorylation. We next analyzed effects of amifostine on phosphorylation of MLC induced by H2O2, IL-6, and LPS. Pretreatment with WR-1065 significantly inhibited phosphorylation of MLC induced by these agonists (Figure 4). Exposure of the EC culture to medium alone or WR-1065 had no effect on the activation of p38 MAPK, Erk1/2 MAPK, NF-κB, or MLC signaling.
Figure 4. Amifostine attenuates intracellular signaling activated by inflammatory agonists.
HPAEC were pretreated with amifostine (4 mM, 30 min) or vehicle followed by stimulation with H2O2 (250 mM, 15 min) (Panel A), IL-6 + IL-6-SR (25 ng/ml and 10 U/ml, 2 hrs) (Panel B), or LPS (200 ng/ml, 2 hrs) (Panel C). Phosphorylation of p38, MEK1/2, Erk1/2, IκBα, NκB-p65, MLC and Hsp27 was detected with corresponding phospho-specific antibodies. Results are representative of three independent experiments. In vivo, five animals per each experimental group were treated with LPS (0.7 mg/kg) with or without concurrent administration of WR-2721 (200 mg/kg), or with WR-2721 (200 mg/kg) alone.
Effects of amifostine on LPS-induced lung inflammation and barrier dysfunction in vivo
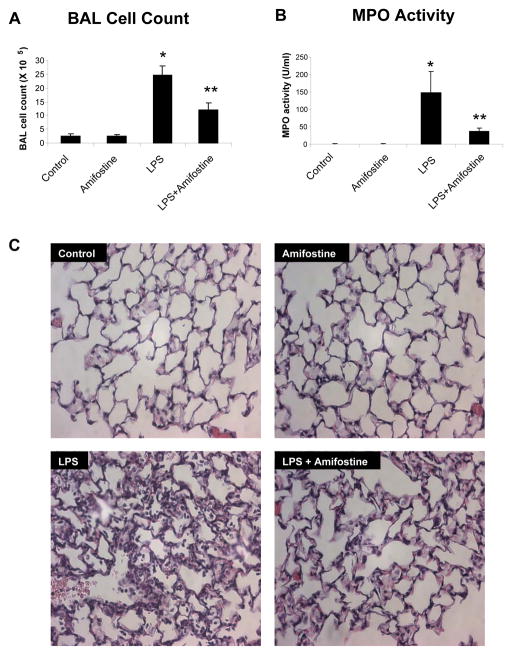
Tu evaluate a role of amifostine in the modulation of lung dysfunction, we used in vivo model of LPS-induced acute lung injury. LPS induced a dramatic acute inflammatory response in the lung with a nearly 10-fold increase in BAL cell counts at 16 hours (2.52 ± 0.76 X 105 cells/ml versus 2.46 ± 0.33 X 106 cells/ml, p<0.001) (Figure 5A). The influx of neutrophils into the lungs in response to LPS was inhibited by treatment with amifostine (2.46 ± 0.33 X 106 cells/ml versus 1.22 ± 0.24 X 106 cells/ml, p<0.001). Correspondingly, the activity of myeloperoxidase, which is the marker of tissue oxidative stress and inflammation induced by activated neutraphis, was dramatically decreased by WR-2721 treatment compared to LPS alone (37 ± 8.5 U/ml versus 148.3 ± 61.4 U/ml, p<0.01) (Figure 5B). Next, using BAL protein content as a marker of barrier disruption and lung injury we examined effects of amifostine on LPS-induced lung vascular leak. Results depicted in Figure 5C show that intratracheal instillation of LPS induced a significant increase in the total protein concentration in BAL fluid, as compared to control animals (0.14 ± 0.04 mg/ml versus 1.01 ± 0.13 mg/ml, p<0.001). Concurrent treatment with intratracheal LPS and intraperitoneal WR-2721 significantly attenuated BAL protein concentrations compared to LPS alone (0.46 ± 0.12 mg/ml versus 1.01 ± 0.13 mg/ml, p<0.001). There was no significant difference in BAL protein in WR-2721-treated animals and controls in the absence of LPS. Histological analysis of lung sections was performed to examine effects of amifostine on LPS-induced lung injury. LPS stimulation (18 hrs) induced neutrophil accumulation in the alveolar space, which was significantly reduced by WR-2721 treatment (Figure 6). Taken together with in vitro experiments, our findings strongly suggest the protective effect of amifostine against LPS-induced lung dysfunction.
Figure 5. Amifostine attenuates LPS-induced neutrophil accumulation in BAL, increased MPO activity and lung barrier dysfunction.
C57BL/6J mice were subjected to treatment of LPS (0.7 mg/kg), with or without concurrent treatment with WR-2721 (200 mg/kg). Control animals were treated with vehicle or WR-2721 (200 mg/kg) alone. After 18 hrs of stimulation, cell count (Panel A), MPO activity (Panel B) and protein concentration (Panel C) were measured in bronchoalveolar lavage fluid taken from control and experimental animals. Results are represented as mean + SE; *p < 0.001 vs. control; **p < 0.001 vs. LPS; n=6–9 per group.
Figure 6. Effects of amifostine on LPS-induced acute lung injury.
Lung specimens were obtained from control mice (A), mice treated with amifostine (B), LPS (C) and LPS + amifostine (D). At the end of experiment, lungs were excised, fixed in 4% paraformaldehyde, embedded in paraffin, and used for histochemical analysis after H&E staining. Images are representative of 6–9 lung specimens for each condition. Original magnification: X200.
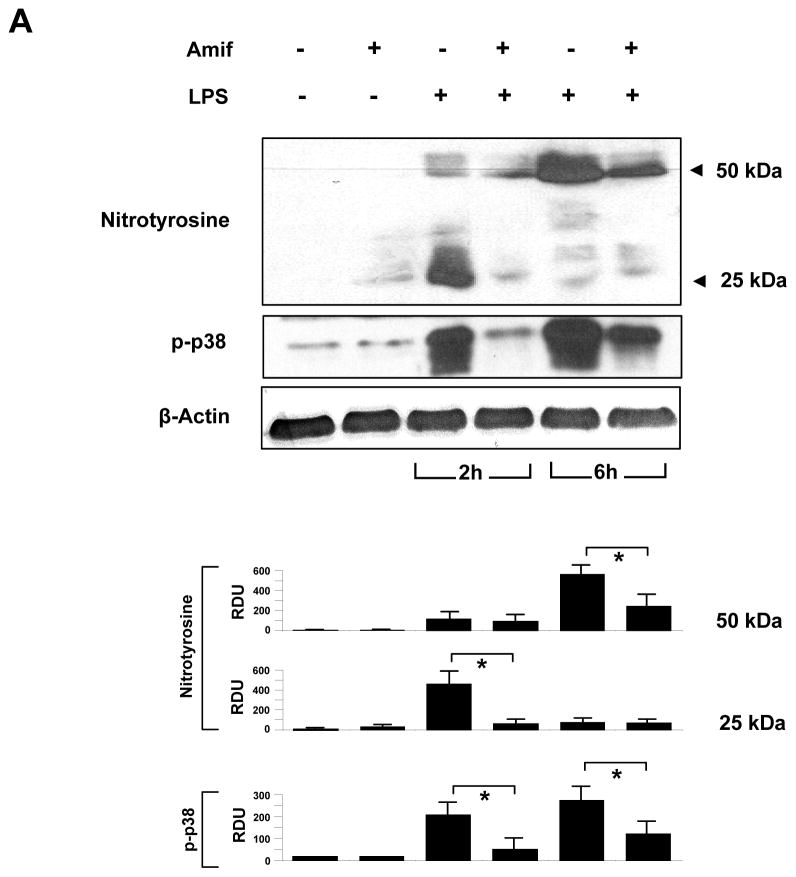
To determine whether the protective effect of amifostine was associated with its anti-oxidative effect in vivo, we performed western blot analysis of the mouse lung tissue samples to detect presence of tyrosine nitrated proteins, which are formed in tissues in the presence of the active metabolites of nitric oxide.
LPS-induced ROS production, NO synthase activation and increased NO generation leads to rapid reaction of NO with superoxide and generation of peroxynitrite. At physiological pH and in the presence of transition metals, peroxynitrite undergoes heterolytic cleavage to form hydroxyl anion and nitronium ion, the latter of which nitrates protein tyrosine residues. Thus, the presence of proteins with nitrated tyrosine residues can be used as a marker of oxidative stress in vivo. Accordingly, the presence of nitrotyrosine has been detected in various inflammatory processes including vascular atherogenesis (38).
LPS treatment increased the content of nitrotyrosine-containing proteins in the lung tissue samples with major bands observed in the 25 kDa and 50 kDa range, which was significantly attenuated by amifostine treatment (Figure 7, upper panel). After 6 hrs of LPS challenge, nitrotyrosine-containing proteins were mainly detected in the 50 kDa range. Likewise, amifostine treatment significantly reduced protein nitration. In addition, amifostine dramatically attenuated LPS-induced phosphorylation of p38 MAPK (Figure. 7, middle panel). These results strongly suggest that the protective effect of amifostine is associated with the reduction of LPS-induced oxidative stress.
Figure 7. Effects of amifostine on LPS-induced p38 activation and nitrotyrosine accumulation in mouse lungs.
Mouse lungs were harvested after 2 hrs and 6 hrs of LPS instillation, and tissue samples were prepared for western blot analysis of p38 MAP kinase phosphorylation and accumulation of tyrosine nitrated proteins. Results are representative of three independent experiments.
DISCUSSION
Although ROS generated under physiologic conditions play an important role in cell homeostasis and cell signal transduction (39), their excessive generation at sites of inflammation become detrimental and may cause tissue injury. The vascular endothelium, which regulates the passage of macromolecules and circulating cells from blood to tissue, becomes readily exposed to oxidative stress and plays a critical role in the pathophysiology of several vascular disorders. Furthermore, accumulating evidence suggests that strategies directed to reduce the cellular oxidative stress modulate cell activation in inflammatory states (6, 40). The main finding of this study is demonstration that amifostine, which acts as a donor of active free thiol, inhibited LPS-induced endothelial monolayer disruption and attenuated LPS-induced mouse lung injury via downregulation of ROS and tissue oxidative stress, as examined in vitro and in situ.
Antioxidant therapy, by altering the redox balance in the lungs, has been shown to be an effective method to attenuate lung injury in several models (41–43). LPS is an important trigger of the lung inflammation involved in pathogenesis of lung edema and ARDS. Through binding to its receptor, CD14, on the cell membrane, LPS induces the release of proinflammatory cytokines. As a result of LPS challenge, activated neutrophils migrate into the lung interstitium from the blood circulation and produce significant amounts of ROS leading to further escalation of inflammation. The activation of endothelial cells also generates ROS and may also contribute to the formation of oxidant-rich environment at the sites of inflammation.
Disruption of endothelial barrier during inflammation is important pathogenic mechanism of ALI. Functionally, pro-oxidant molecule H2O2 and inflammatory mediators LPS and IL-6 induced stress fiber formation and adhesion junction (VE-Cadherin) disruption, which was prevented by amifostine. The barrier protective effect of amifostine observed in this study was further linked to its ability to inhibit ROS production and attenuate redox-dependent pro-inflammatory signaling. Our results show that amifostine attenuated LPS-induced ROS production and oxidant stress both in the EC cultures and in the murine model of LPS-induced acute lung injury. Western blot detection of nitrotyrosinated proteins, a footprint of oxidative stress, showed increased lung tissue levels of nitrotyrosinated protein immunoreactivity after 2 hrs and 6 hrs of LPS exposure, which was dramatically attenuated by amifostine.
NF-kB is one of redox-sensitive transcription factors activated by MAP kinase and PKC pathways upon LPS stimulation, which regulates expression of proinflammatory cytokines. Previous studies identified that NAC, the classical antioxidant, suppressed ROS-mediated lung injury and NF-kB activation (44). Our data show that potent inhibitory effects of amifostine on the LPS-induced NF-kB activation. Erk-1,2 and p38/MK-2 MAP kinase pathways may activate NF-kB in a redox-sensitive manner (45). In addition, p38 MAPK is also involved in actin filament reorganization in response to oxidative stress via MAPK-dependent phosphorylation of actin-binding effector Hsp27 (34, 46). This study shows that phosphorylation of p38 and Hsp27 phosphorylation caused by LPS or H2O2 was dramatically attenuated by amifostine in vitro and in vivo. In addition, amifostine abolished LPS-, H2O2-, and IL-6-induced stimulation of regulatory MLC, which triggers EC contraction and barrier dysfunction.
Antioxidant strategies for attenuation of lung inflammatory injury have been tested in previous studies. For example, N-acetyl cysteine (NAC), a thiol antioxidant compound, exhibited antioxidant effects and suppressed ROS-mediated lung injury (24, 25, 47). However, NAC application in clinical settings did not prove its efficiency. For example, in fibrose alveolitis, where activated inflammatory cells induce oxidative stress in a lower respiratory tract, high doses of NAC (1.8 g daily for 12 weeks in addition to immunosuppressive therapy) did not significantly suppress inflammatory cell activation (48). A Nordic Multi-Centre Controlled Trial showed that a 6-day course of intravenous NAC during the first week of life does not prevent bronchopulmonary dysplasia or death, or improve lung function at term in infants with extremely low birth weight (49, 50). Our results indicate potent protective effects of amifostine in vitro and in the animal models of LPS-induced ALI. Further studies in our lab are aimed at more detailed characterization of amifostine effects in the in vivo models of ALI.
Several lines of evidence suggest that endothelium is a major site of amifostine absorbance and conversion to active form. WR-2721 is presumably modified by membrane-bound alkaline phosphatase, which is highly expressed in the endothelium, and transferred into the thiol metabolite WR-1065, which quickly penetrates into cell, where the thiol groups act as free-radical scavengers and protect cells from oxidative damage (51, 52). On the other hand, pulmonary endothelium and epithelium are the major sources of oxidants and possibly significant contributors in maintaining the oxidant-rich environment at the inflammatory locus (29). Based on our data, we speculate that the potent protective effects of amifostine in the LPS-induced acute lung injury may be attributed to its local activation by lung microvascular endothelium in the inflamed lungs leading to increased capacity to prevent lung microvascular endothelium and alveolar epithelium from attack by oxidants.
In summary, these studies show potent protective effects of amifostine against LPS-induced lung injury and pulmonary EC barrier dysfunction via attenuation of oxidative stress, inhibition of redox-sensitive MAP kinases, NF-kB inflammatory cascade, attenuation of LPS-induced cytoskeletal remodeling and disruption of endothelial cell adhesions and monolayer integrity (Figure 8). Amifostine is currently used as a cytoprotective compound against the toxic effects of ionizing radiation (53–55). The results of this study suggest a new potential application of amifostine in the treatment of acute lung injury. Ongoing studies in our lab are aimed to elucidate the precise mechanisms underlying regulation of redox-sensitive signaling and lung protection by amifostine in the acute lung pathologies associated with oxidative stress.
Figure 8. Inhibition of LPS-induced inflammation and endothelial barrier dysfunction by amifostine.
Lung exposure to LPS stimulates production of reactive oxygen and nitrogen species (ROS and RNS) by activating NADPH oxidase, NO-synthase, xanthine oxidoreductase and other enzymes, which leads to cellular oxidative stress. As a result, activation of redox-sensitive signaling cascades including NFkB, Erk-1,2 and p38 MAP kinases triggers cellular responses to LPS such as elevated expression of pro-inflammatory cytokines, cytoskeletal remodeling and disruption of endothelial monolayer integrity leading to acute lung injury. Amifostine reduces LPS-induced inflammatory signaling via inactivation of ROS and RNS and blunting the redox-sensitive inflammatory signaling.
Acknowledgments
This work was supported by grants from National Hear, Lung and Blood Institutes (HL075349 and HL076259) for KGB, the ALA Carrier Investigator Award for KGB, and HL058064 for JGN. The authors thank Nurgul Moldobaeva for superior technical assistance.
References
- 1.Schraufstatter IU, Revak SD, Cochrane CG. Proteases and oxidants in experimental pulmonary inflammatory injury. J Clin Invest. 1984;73(4):1175–84. doi: 10.1172/JCI111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin SR, Simon RH, Grum CM, Ketai LH, Boxer LA, Devall LJ. Oxidant activity in expired breath of patients with adult respiratory distress syndrome. Lancet. 1986;1(8471):11–4. doi: 10.1016/s0140-6736(86)91895-7. [DOI] [PubMed] [Google Scholar]
- 3.Antczak A, Nowak D, Bialasiewicz P, Kasielski M. Hydrogen peroxide in expired air condensate correlates positively with early steps of peripheral neutrophil activation in asthmatic patients. Arch Immunol Ther Exp (Warsz) 1999;47(2):119–26. [PubMed] [Google Scholar]
- 4.Terada LS. Oxidative stress and endothelial activation. Crit Care Med. 2002;30(5 Suppl):S186–91. doi: 10.1097/00003246-200205001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Fialkow L, Chan CK, Grinstein S, Downey GP. Regulation of tyrosine phosphorylation in neutrophils by the NADPH oxidase. Role of reactive oxygen intermediates. J Biol Chem. 1993;268(23):17131–7. [PubMed] [Google Scholar]
- 6.Fialkow L, Chan CK, Rotin D, Grinstein S, Downey GP. Activation of the mitogen-activated protein kinase signaling pathway in neutrophils. Role of oxidants. J Biol Chem. 1994;269(49):31234–42. [PubMed] [Google Scholar]
- 7.Gadek JE, Pacht ER. The interdependence of lung antioxidants and antiprotease defense in ARDS. Chest. 1996;110(6 Suppl):273S–277S. doi: 10.1378/chest.110.6_supplement.273s. [DOI] [PubMed] [Google Scholar]
- 8.Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem. 2000;275(28):21130–9. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107(1):3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356(1):1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 11.Chan EL, Murphy JT. Reactive oxygen species mediate endotoxin-induced human dermal endothelial NF-kappaB activation. J Surg Res. 2003;111(1):120–6. doi: 10.1016/s0022-4804(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 12.Sung JY, Hong JH, Kang HS, Choi I, Lim SD, Lee JK, Seok JH, Lee JH, Hur GM. Methotrexate suppresses the interleukin-6 induced generation of reactive oxygen species in the synoviocytes of rheumatoid arthritis. Immunopharmacology. 2000;47(1):35–44. doi: 10.1016/s0162-3109(99)00185-x. [DOI] [PubMed] [Google Scholar]
- 13.Schunemann HJ, Muti P, Freudenheim JL, Armstrong D, Browne R, Klocke RA, Trevisan M. Oxidative stress and lung function. Am J Epidemiol. 1997;146(11):939–48. doi: 10.1093/oxfordjournals.aje.a009220. [DOI] [PubMed] [Google Scholar]
- 14.Kemp G, Rose P, Lurain J, Berman M, Manetta A, Roullet B, Homesley H, Belpomme D, Glick J. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol. 1996;14(7):2101–12. doi: 10.1200/JCO.1996.14.7.2101. [DOI] [PubMed] [Google Scholar]
- 15.Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W, Sauer R. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18(19):3339–45. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 16.Grdina DJ, Kataoka Y, Murley JS, Swedberg K, Lee JY, Hunter N, Weichselbaum RR, Milas L. Antimetastatic effectiveness of amifostine therapy following surgical removal of Sa-NH tumors in mice. Semin Oncol. 2002;29(6 Suppl 19):22–8. doi: 10.1053/sonc.2002.37357. [DOI] [PubMed] [Google Scholar]
- 17.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26(4):453–64. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285(4):L785–97. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 20.Birukov KG, V, Bochkov N, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 21.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169(11):1245–51. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 22.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed cytoprotection after enhancement of Sod2 (MnSOD) gene expression in SA-NH mouse sarcoma cells exposed to WR-1065, the active metabolite of amifostine. Radiat Res. 2002;158(1):101–9. doi: 10.1667/0033-7587(2002)158[0101:dcaeos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Grdina DJ, Murley JS, Kataoka Y, Calvin DP. Differential activation of nuclear transcription factor kappaB, gene expression, and proteins by amifostine’s free thiol in human microvascular endothelial and glioma cells. Semin Radiat Oncol. 2002;12(1 Suppl 1):103–11. doi: 10.1053/srao.2002.31383. [DOI] [PubMed] [Google Scholar]
- 24.Ritter C, da Cunha AA, Echer IC, Andrades M, Reinke A, Lucchiari N, Rocha J, Streck EL, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med. 2006;34(2):471–7. doi: 10.1097/01.ccm.0000199069.19193.89. [DOI] [PubMed] [Google Scholar]
- 25.Kao SJ, Wang D, Lin HI, Chen HI. N-acetylcysteine abrogates acute lung injury induced by endotoxin. Clin Exp Pharmacol Physiol. 2006;33(1–2):33–40. doi: 10.1111/j.1440-1681.2006.04320.x. [DOI] [PubMed] [Google Scholar]
- 26.Koksel O, Ozdulger A, Ercil M, Tamer L, Ercan B, Atik U, Cinel L, Cinel I, Kanik A. Effects of N-acetylcysteine on oxidant-antioxidant balance in oleic acid-induced lung injury. Exp Lung Res. 2004;30(6):431–46. doi: 10.1080/01902140490476319. [DOI] [PubMed] [Google Scholar]
- 27.Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59(1):13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 28.Gaitanaki C, Konstantina S, Chrysa S, Beis I. Oxidative stress stimulates multiple MAPK signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. J Exp Biol. 2003;206(Pt 16):2759–69. doi: 10.1242/jeb.00483. [DOI] [PubMed] [Google Scholar]
- 29.Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997;20(11 Suppl 2):II-11–7. [PubMed] [Google Scholar]
- 30.Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol. 2000;279(1):C21–30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- 31.Nash SP, Heuertz RM. Blockade of p38 map kinase inhibits complement-induced acute lung injury in a murine model. Int Immunopharmacol. 2005;5(13–14):1870–80. doi: 10.1016/j.intimp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima K, Hirade K, Ishisaki A, Matsuno H, Suga H, Kanno Y, Shu E, Kitajima Y, Katagiri Y, Kozawa O. Akt regulates thrombin-induced HSP27 phosphorylation in aortic smooth muscle cells: function at a point downstream from p38 MAP kinase. Life Sci. 2005;77(1):96–107. doi: 10.1016/j.lfs.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Hirade K, Kozawa O, Tanabe K, Niwa M, Matsuno H, Oiso Y, Akamatsu S, Ito H, Kato K, Katagiri Y, Uematsu T. Thrombin stimulates dissociation and induction of HSP27 via p38 MAPK in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;283(3):H941–8. doi: 10.1152/ajpheart.00060.2001. [DOI] [PubMed] [Google Scholar]
- 34.Pichon S, Bryckaert M, Berrou E. Control of actin dynamics by p38 MAP kinase - Hsp27 distribution in the lamellipodium of smooth muscle cells. J Cell Sci. 2004;117(Pt 12):2569–77. doi: 10.1242/jcs.01110. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen A, Chen P, Cai H. Role of CaMKII in hydrogen peroxide activation of ERK1/2, p38 MAPK, HSP27 and actin reorganization in endothelial cells. FEBS Lett. 2004;572(1–3):307–13. doi: 10.1016/j.febslet.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 36.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254(2):210–20. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 37.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273(34):21867–74. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 38.Torres-Rasgado E, Fouret G, Carbonneau MA, Leger CL. Peroxynitrite mild nitration of albumin and LDL-albumin complex naturally present in plasma and tyrosine nitration rate-albumin impairs LDL nitration. Free Radic Res. 2007;41(3):367–75. doi: 10.1080/10715760601064706. [DOI] [PubMed] [Google Scholar]
- 39.Chen K, Keaney J. Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium. 2004;11(2):109–21. doi: 10.1080/10623320490482655. [DOI] [PubMed] [Google Scholar]
- 40.Brisseau GF, Dackiw AP, Cheung PY, Christie N, Rotstein OD. Posttranscriptional regulation of macrophage tissue factor expression by antioxidants. Blood. 1995;85(4):1025–35. [PubMed] [Google Scholar]
- 41.Antonicelli F, Brown D, Parmentier M, Drost EM, Hirani N, Rahman I, Donaldson K, MacNee W. Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant Nacystelyn. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1319–27. doi: 10.1152/ajplung.00329.2003. [DOI] [PubMed] [Google Scholar]
- 42.Rocksen D, Ekstrand-Hammarstrom B, Johansson L, Bucht A. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol. 2003;28(2):199–207. doi: 10.1165/rcmb.4899. [DOI] [PubMed] [Google Scholar]
- 43.Morrow DM, Entezari-Zaher T, Romashko J, 3rd, Azghani AO, Javdan M, Ulloa L, Miller EJ, Mantell LL. Antioxidants preserve macrophage phagocytosis of Pseudomonas aeruginosa during hyperoxia. Free Radic Biol Med. 2007;42(9):1338–49. doi: 10.1016/j.freeradbiomed.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157(4):1630–7. [PubMed] [Google Scholar]
- 45.Ramachandiran S, Huang Q, Dong J, Lau SS, Monks TJ. Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol. 2002;15(12):1635–42. doi: 10.1021/tx0200663. [DOI] [PubMed] [Google Scholar]
- 46.Paliga AJ, Natale DR, Watson AJ. p38 mitogen-activated protein kinase (MAPK) first regulates filamentous actin at the 8–16-cell stage during preimplantation development. Biol Cell. 2005;97(8):629–40. doi: 10.1042/BC20040146. [DOI] [PubMed] [Google Scholar]
- 47.Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med. 1991;91(3C):54S–59S. doi: 10.1016/0002-9343(91)90284-5. [DOI] [PubMed] [Google Scholar]
- 48.Behr J, Degenkolb B, Krombach F, Vogelmeier C. Intracellular glutathione and bronchoalveolar cells in fibrosing alveolitis: effects of N-acetylcysteine. Eur Respir J. 2002;19(5):906–11. doi: 10.1183/09031936.02.00204902. [DOI] [PubMed] [Google Scholar]
- 49.Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, Jonsbo F, Esberg G, Stovring S, Kjartansson S, Stiris T, Lossius K, Virkola K, Fellman V. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr. 2003;143(6):713–9. doi: 10.1067/S0022-3476(03)00419-0. [DOI] [PubMed] [Google Scholar]
- 50.Sandberg K, Fellman V, Stigson L, Thiringer K, Hjalmarson O. N-acetylcysteine administration during the first week of life does not improve lung function in extremely low birth weight infants. Biol Neonate. 2004;86(4):275–9. doi: 10.1159/000080089. [DOI] [PubMed] [Google Scholar]
- 51.Capizzi RL. The preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine (Ethyol) Eur J Cancer. 1996;32A(Suppl 4):S5–16. doi: 10.1016/s0959-8049(96)00333-4. [DOI] [PubMed] [Google Scholar]
- 52.Spencer CM, Goa KL. Amifostine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential as a radioprotector and cytotoxic chemoprotector. Drugs. 1995;50(6):1001–31. doi: 10.2165/00003495-199550060-00008. [DOI] [PubMed] [Google Scholar]
- 53.Khodarev NN, Kataoka Y, Murley JS, Weichselbaum RR, Grdina DJ. Interaction of amifostine and ionizing radiation on transcriptional patterns of apoptotic genes expressed in human microvascular endothelial cells (HMEC) Int J Radiat Oncol Biol Phys. 2004;60(2):553–63. doi: 10.1016/j.ijrobp.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 54.Vujaskovic Z, Feng QF, Rabbani ZN, Samulski TV, Anscher MS, Brizel DM. Assessment of the protective effect of amifostine on radiation-induced pulmonary toxicity. Exp Lung Res. 2002;28(7):577–90. doi: 10.1080/01902140290096791. [DOI] [PubMed] [Google Scholar]
- 55.Guelman LR, Cabana JI, del Lujan Pagotto RM, Zieher LM. Ionizing radiation-induced damage on developing cerebellar granule cells cultures can be prevented by an early amifostine post-treatment. Int J Dev Neurosci. 2005;23(1):1–7. doi: 10.1016/j.ijdevneu.2004.10.001. [DOI] [PubMed] [Google Scholar]