Abstract
Substance dependence disorders are chronic relapsing disorders with immense societal consequences. Twin and family studies have found that there are critical genetic and environmental components in the inheritance of substance use disorders, and modern advances in genetics are making it possible to identify specific variants that may predispose an individual to these disorders. There is no “gene for alcoholism”; rather there are a multitude of genes, each with small effects, that interact with each other (epitasis) as well as with their biologic and external environments (gene-environment interaction) to make an individual more or less susceptible to the development of these complex disorders. The onset of substance dependence is rarely sudden; it is usually preceded by a trajectory of risk-related behavior, with its origins in childhood. Adolescence is a crucial period for the initiation of, and experimentation with, alcohol and other drugs. This experimentation, as well as a degree of other conduct-disordered behavior, is normative; however, adolescent substance use is a known risk factor for the development of later alcohol and substance use problems as well as related externalizing disorders such as antisocial personality disorder. Understanding the early risk factors and trajectories that make these youths vulnerable to substance use disorders is crucial to the development of effective strategies for prevention. This article reviews the genetic origins of adolescent substance use problems, which create one of the largest public health concerns in the United States, and the potential this field of research offers for prevention.
Keywords: Alcohol use, Substance use, Drug use, Adolescence, Genetics, Gene-environment interaction
ADOLESCENT DRUG AND ALCOHOL USE
Adolescent alcohol use is a major public health problem. Youths who drink alcohol are more likely to experience many negative outcomes including academic, social, and legal problems, in addition to unwanted, unplanned, and unprotected sexual activity.1,2 Biologically, adolescence is characterized by strong neuronal plasticity, with sprouting and pruning of synapses, myelinization of nerve fibers, and changes in neurotransmitter concentrations and their receptor levels in brain areas essential for behavioral and cognitive functions.3 Adolescent drinkers are at higher risk for changes in brain development that may have life-long effects.3 Long-term alcohol misuse is associated with liver disease, cancer, cardiovascular disease, and neurologic damage, as well as psychiatric problems such as depression, anxiety, and antisocial personality disorder.4
In addition to the personal risks to the adolescent, the societal and economic costs of alcohol and other substance use disorders in the United States also are substantial. Alcohol is used by more young people in the United States than tobacco or illicit drugs.1 Although it is illegal to drink alcohol before the age of 21 years in all states, in 2007, 26% of high-school students reported episodic heavy or binge drinking and 11% of high-school students reported driving a car or other vehicle during the past 30 days when they had been drinking alcohol. In addition, 29% of students reported riding in a car or other vehicle during the past 30 days driven by someone who had been drinking alcohol.5 In 2005, there were more than 145,000 emergency rooms visits by youths aged 12 to 20 years for injuries and other conditions linked to alcohol.6
COMORBIDITY AND ILLICIT DRUG USE
Cannabis is the most commonly used illicit substance among adolescents and young adults in the United States.7 In 2004, 46% of high-school seniors reported having tried cannabis at some time, 34% reported having used within the past month, and 5.6% reported having smoked cannabis daily.8 Initiation into cannabis use typically begins in adolescence, as youths aged 12 to 17 years constitute about two-thirds of the new cannabis users.9 Approximately 14% of adolescent-onset cannabis users develop cannabis dependence, a rate roughly twice that reported for adult-onset users.10 Further work has focused on the biologic mechanisms involved in the development of cannabis dependence, and this has elucidated some of the underlying pathways from cannabis use to behavior and the potential for the development of problems. For example, the receptor for cannabinoids (CB1) belongs to the Gi/Go protein-coupled receptor family, and, in the mammalian brain, is densely diffused in regions involved in the processing of emotional inputs, rewarding stimuli, habit formation, and higher cognitive functions.11
In recent years there have been reductions in the rates of illicit drug use among adolescents. Current marijuana use decreased from 27% in 1999 to 20% in 2007. Lifetime use of ecstasy among high-school students decreased from 11% in 2003 to 6% in 2007. Hallucinogenic drug use decreased from 13% in 2001 to 8% in 2007.12 Although illicit drug use has declined among youths, rates of nonmedical use of prescription and over-the-counter (OTC) medications, including pain relievers, tranquilizers, stimulants, and depressants, remain high. These easily accessible drugs are falsely believed to be safer than illicit drugs; however, misuse of prescription and OTC medications can cause serious health effects, addiction, and death.7,13,14
EARLY INITIATION
One of the most robust findings in the alcohol literature came out of an influential study by Grant and Dawson15 reporting that individuals who reported that they had first tried alcohol before the age of 15 years were 4 times more likely to have a lifetime diagnosis of alcohol dependence than individuals who reported that they had first tried alcohol after the age of 20 years. This finding has been replicated in many independent samples, using retrospective16–19 and prospective20 data. Alcohol use before 15 years of age is also found to predict other forms of adult disinhibitory psychopathology, including substance use disorders and antisocial personality disorder. Many mechanisms for this association have been proposed.
One theory suggests that early use of alcohol may increase alcoholism risk by altering the course of adolescent development.16 Adolescent alcohol use could alter the course of development by increasing the likelihood that an adolescent is affiliated with deviant peers, thus decreasing the likelihood that an adolescent is affiliated with individuals who reinforce and model prosocial behaviors. A second theory hypothesizes that early use of alcohol may influence biologic development directly, through its effects in the developing adolescent brain.21 Research with rodents22 and humans23 suggests that heavy use of alcohol during adolescence can result in neurocognitive changes that increase the likelihood of subsequent abuse of alcohol in adulthood.
A third hypothesis is that the association of early alcohol use with alcoholism risk arises because early use of alcohol in adolescence and alcoholism in adulthood are manifestations of a general inherited liability to disinhibitory psychopathology.24,25 Support for this idea came initially from a study of nearly 9000 twins that showed that early use of alcohol was heritable and that its association with alcoholism risk was mediated entirely by genetic factors.19 This model also predicts that early use of alcohol should be a nonspecific risk factor for a wide range of behavioral pathologies because it is an indicator of a general disposition toward disinhibited behavior. Consistent with this expectation, alcohol use before 15 years of age is associated with attention deficit/hyperactivity disorder, conduct disorder, personality measures of impulsivity, psychophysiological indicators of disinhibition, academic underachievement, and abuse of substances other than alcohol.18 In their 2008 review of these mechanisms, McGue and Iacono26 point out that none of these mechanisms are mutually exclusive, and that early use of alcohol may be an indicator of inherited risk and disrupt the course of adolescent development.
HERITABILITY
Alcoholism runs in families. This is likely a result of the transmission of genetics as well as the familial environment. Twin methodology has been used to study the measurable contribution of genetic and environmental influences on a particular trait or disease state. Twin studies provide an estimation of a trait’s heritability in a population; that is, what proportion of phenotypic variation is produced by genetic variation underlying the trait. Twin studies accomplish this by comparing phenotypic similarity between monozygotic twins, who share all of their genetic variation, with dizygotic twins, who share (on average) half of their genetic variation. Measures of heritability are a function of the specific population. Heritability of substance use disorders varies among substances (and the measure of substance use), populations, age, and sex. A 2006 meta-analysis of twin studies showed that the heritability of all addictive substances ranges from 40% to 60%.27 Heritabilities in the range of 30% to 60% are also observed for illicit drug dependences.28,29 Variability in the exact estimates is likely a function of the age of the participants (genetic influences often increase across development30), cohort differences, and the exact measure of the phenotype being studied. Current research on the heritability of youth drug and alcohol problems suggests that these disordered behaviors are manifestations of risk to a spectrum of externalizing disorders and that to consider each of these disorders separately may lead us to miss important etiologic clues.
HERITABILITY ACROSS DEVELOPMENT
The use of longitudinal studies of behavior across development has elucidated the dramatic changes that are evident in the importance of genetic and environmental influences throughout the lifespan, observed across multiple behavioral domains including intellectual abilities31 and depression.32 Substance use is another area in which dramatic changes in the relative importance of genetic and environmental effects across development are apparent. This finding has been documented in data from our longitudinal Finnish Twin Studies, in which the authors have found that the importance of genetic effects on drinking patterns increases dramatically from adolescence to young adulthood. At the age of 14 years, genetic influences accounted for only 18% of the variance in drinking initiation, and this was significant only in girls, with no evidence of genetic influence on drinking patterns in boys at this early age.33 However, by 16 years of age, genetic factors accounted for one-third of the variation in drinking patterns in both sexes, and by 18 years of age genetic factors accounted for half of the variation.34 Thus, in a period of slightly more than 4 years, genetic influences changed from having virtually no detectable effect on drinking patterns to accounting for the majority of the variance. Conversely, the importance of common environmental effects decreased significantly from adolescence into adulthood, accounting for more than 70% of the variance at 14 years of age, but only approximately 15% of the variance by 18 years of age. Thus, as drinking patterns develop, differentiate, and stabilize across adolescence, genetic factors assume increasing importance to drinking patterns; however, alcohol use early in adolescence seems to be almost entirely influenced by family, school, and neighborhood.35
In 2008, Kendler and colleagues30 examined these relative changes in the importance of genes and environment in substance use from early adolescence to middle adulthood. The study provides more support for an etiologic model in which initiation and early patterns of use are more strongly influenced by social and familial environmental factors, while later levels of use are more heavily influenced by genetic factors. The importance of environment has been theorized to reflect the important influence of social and familial structure that characterizes development across adolescence. Early on, there is usually less opportunity for adolescents to express their genetic predisposition, as more of their activities and decisions are influenced by figures of authority. As adolescents move into adulthood, and usually out of the social structure of their youth, emerging adults have more opportunity to express their genetic predispositions, choosing more freely their friends and activities. In some studies, it has been observed that some of the environmental influences are still evident after individuals moves out of their parents’ homes. This is believed to reflect personal values that one’s family or community has instilled in the individual, such as religious beliefs.
THE EXTERNALIZING SPECTRUM
Another consideration for understanding genetic influences on pathways of risk for alcohol problems is the robust finding that the overlap between childhood conduct problems and later alcohol problems is largely the result of shared genetic factors. This finding has been shown across multiple twin samples.24,25,36,37 In addition, an offspring of twins study found increased rates of conduct disorder in alcohol-dependent fathers, with transmission patterns supporting the common-genes hypothesis.38 Specific genes that have been associated with adult alcohol dependence have been associated with conduct problems in younger children and adolescents (rather than early adolescent alcohol dependence),39 again suggesting that childhood behavior problems may be an early manifestation of an underlying predisposition to subsequent alcohol problems. The association with behavior problems may emerge earlier in development because genetic factors are apparent in behavior problems (showing up early in childhood) before their effects on patterns of alcohol use (for which genetic influences assume greater importance later in adolescence).
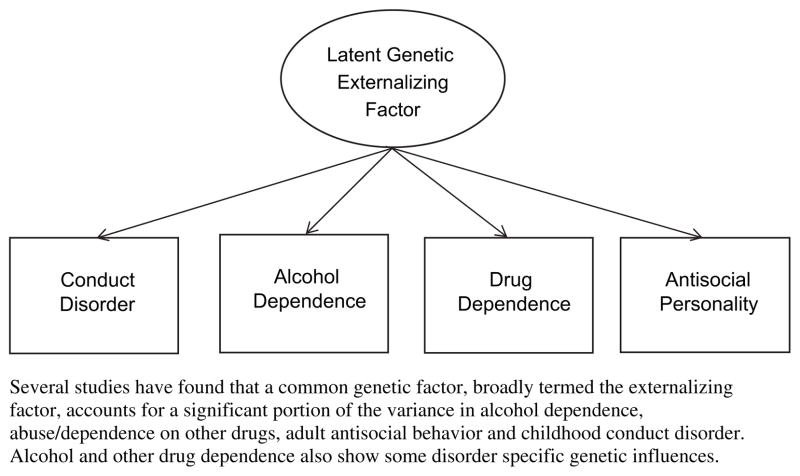
Epidemiologic studies find that individuals rarely abuse a single substance. Instead, polysubstance abuse/dependence is normative, with high rates of comorbidity across various drug classes. In addition, persons with substance use disorders also exhibit higher rates of other psychiatric disorders including mood disorders and antisocial personality disorder. Twin studies suggest that this comorbidity is due at least in part to a shared genetic cause underlying susceptibility to different types of substance use and other psychopathologies. Kendler and colleagues36 used the Virginia Twin Registry sample to identify common genetic factors underlying the major class of psychiatric and substance use disorders, and found that a common genetic factor was shared across alcohol dependence, illicit drug dependence, adult antisocial behavior, and childhood conduct disorder. These results suggest a common genetic factor for substance dependence/abuse and general externalizing psychopathologies (Fig. 1). Alcohol dependence and illicit drug dependence also showed some disorder-specific genetic influences. However, 69% of the total genetic variance on alcohol dependence, and 64% of the genetic variance on illicit drug dependence resulted from the genetic factor shared across externalizing psychopathology. This finding indicates that much of the genetic predisposition to alcohol and other drug dependence is not specific to that disorder. Using only male twins from the same population, Kendler and colleagues40 found that a common genetic factor loaded strongly onto abuse/dependence of all classes of illicit drugs. There has now been much evidence to suggest that youth drug and alcohol problems are a manifestation of risk to a spectrum of externalizing disorders, and that to consider each of these disorders separately may lead us to miss important etiologic clues.
Fig. 1.
The latent genetic externalizing factor.
GENE-ENVIRONMENT INTERACTION
There is an emerging literature documenting how specific environmental factors moderate the importance of genetic effects. A growing number of variables have been shown to moderate the relative importance of genetic effects on substance use and dependence and externalizing behavior. Among the environmental moderators being studied are childhood stressors (emotional, physical, and sexual abuse), availability and access to drugs and alcohol, peer-group antisocial and prosocial behavior, religiosity, parental attitudes toward drugs and alcohol, parental monitoring, and socioregional factors. Religiosity has been shown to moderate genetic influences on alcohol use among females, with genetic factors playing a larger role among individuals without a religious upbringing.41 Social contact and cotwin dependency42 have also been shown to moderate twin similarity, with reduced genetic effects and enhanced environmental influences among more codependent pairs. Genetic influences on adolescent substance use are also enhanced in environments with lower parental monitoring.43 These analyses suggest that when adolescents receive little parental monitoring, it creates an environment that allows for greater opportunity to express genetic predispositions. The moderating effects of peer alcohol use on adolescent drinking has been shown to operate in a similar fashion: among adolescents with a larger number of peers who used alcohol, there was greater expression of genetic predispositions.44 These findings may reflect a situation in which environments characterized by low parental monitoring or high peer substance use create opportunity for adolescents to express genetic predispositions. These results support previous findings from the Finnish Twin Studies, which indicated that in neighborhoods in which there is less stability, presumably engendering less community monitoring, there was greater evidence of genetic influence.45 Conversely, in more supervised and restricted environments, there was less opportunity to express genetic predispositions and greater influence of environmental effects.34,45 Hicks and colleagues46 examined the specificity of each of these environmental risk factors on externalizing spectrum disorders, including substance dependence/abuse. They concluded that, in the context of environmental adversity, broadly defined, genetic factors become more important in the etiology of externalizing disorders. In addition, their results suggest a general mechanism of environmental influence on externalizing disorders, regardless of the specific form of environmental risk.
These analyses illustrate the importance of incorporating measured aspects of the environment into genetically informative twin models to understand how specific environments act and interact with genetic predispositions. They may also have implications for studying the risk associated with specific genes. For example, a recent study47 aimed to characterize the pathway of risk associated with GABRA2 (for each known human gene the Human Genome Organization [HUGO] Gene Nomenclature Committee approves a gene name and symbol [short-form abbreviation]. Details on this process can be found on the HUGO Web site at http://www.hugo-international.org/comm_genenomenclaturecommittee.php), a gene previously associated with adult alcohol dependence,48 in a community sample of children followed longitudinally from childhood to young adulthood. Association between GABRA2 and trajectories of externalizing behavior was tested from adolescence to young adulthood and moderation of genetic effects by parental monitoring was also tested. Two classes of externalizing behavior emerged: a stable, high externalizing class and a moderate, decreasing externalizing-behavior class. The GABRA2 gene was associated with class membership, with subjects who showed persistent increased trajectories of externalizing behavior more likely to carry the genotype previously associated with increased risk of adult alcohol dependence. A significant interaction with parental monitoring emerged; the association of GABRA2 with externalizing trajectories diminished with high levels of parental monitoring.43
GENE IDENTIFICATION EFFORTS
Candidate Gene Approach
Evidence for significant heritability for all of the addictive disorders has led to considerable efforts to identify the specific genes involved. The gene search has been complicated by addictive behaviors being complex genetic traits that are phenotypically and genetically heterogeneous. It is expected that there are multiple genetic loci influencing manifestation and variation in these behaviors, and that these loci vary in the direction and magnitude of their effects. Beyond the multiple loci involved, it is likely that there are also many layers of intricate interactions between loci (epistasis), in addition to gene-environment interactions.
Because of the challenges associated with gene identification efforts for multifactorial traits (traits that are influenced by multiple genes and environments) such as substance dependence, several strategies have been used to identify genes involved in addictive disorders. One approach is the candidate gene approach. This strategy involves investigation of a gene that is believed to be involved in the cause of a specific disorder for known or hypothesized biologic reasons. A classic example of the candidate gene approach is the association of genes involved in alcohol metabolism with susceptibility to alcohol dependence. Multiple alcohol-metabolism genes have been repeatedly implicated in the development of alcohol addiction and susceptibility to dependence on other drugs. These genes were initially suspected as having a role in dependence, as they are known to be involved in the metabolism of ethanol. For example, alcohol dehydrogenase 1B (ADH1B) is involved in the conversion of ethanol to acetaldehyde; acetaldehyde is toxic and its accumulation leads to an unpleasant physiologic reaction involving headache, nausea, and heart palpitations. Polymorphisms in alcohol metabolism genes that affect acetaldehyde levels can have a profound effect on drinking behavior.49,50 For example, the ADH1B*2 allele rapidly oxidizes ethanol, and is protective against alcoholism; this effect is most evident among East Asian and Ashkenazi Jewish populations, in whom the frequency of this allele is high. Individuals carrying at least 1 of these alleles are far less likely to develop alcoholism. The advantage of the candidate gene approach is that it provides targeted hypothesis testing, but this approach is limited by knowledge of human biology.
Linkage and Association
With the increasing availability of genetic markers localized across the genome, more systematic approaches to gene identification have gained favor. Such studies are hypothesis free in their design, scanning large portions of the genome to identify regions that are significantly associated with the phenotype of interest. Linkage mapping was an early technique used to systematically scan the genome, in which approximately 400 to 1000 highly polymorphic markers were genotyped to test for genomic regions showing increased allele sharing among affected family members. Many large-scale alcohol-dependence gene identification projects have used genetic linkage mapping; the largest of these is the Collaborative Study of the Genetics of Alcoholism (COGA). COGA is a family-based study that has collected detailed phenotypic data on individuals in families with multiple alcoholic members. The strongest linkage regions with alcohol dependence to emerge from this project were on chromosomes 4 and 7. Follow-up association studies of candidate genes in the linkage regions have identified association with several genes in each region, including GABRA2,48 NFKB1,51 ADH4, ADH1A, ADH1B,49 CHRM2,52 and TAS2R16.53 Although these studies illustrate the potential of genome-wide linkage approaches (followed by targeted association tests in the linked regions) to identify novel genetic variants, linkage studies are largely underpowered to detect genes of small effect, such as those hypothesized to be involved in addiction. Although linkage was successful in the identification of genes involved in monogenic diseases, mapping polygenic disease genes requires a large number of family members to ensure sufficient power to detect genetic variants.54 In addition, linkage mapping lacks the precision other methods provide, because the linkage peaks resulting from this strategy do not precisely localize the associated genes, and imprecise linkage peaks make it difficult to know the exact region to target for follow-up with association studies.
For these reasons, genome-wide association studies (GWAS) has gained favor. GWAS theoretically combines the advantages of linkage mapping (a systematic genome-wide scan that has the capability of identifying novel genetic variants) and the advantages of association (higher resolution that provides more power to detect genes of subtle effect). Currently, there is only 1 published GWAS for alcohol dependence.55 Treutlein and colleagues55 found 2 genome-wide significant results in closely linked intergenic single nucleotide polymorphisms (SNPs), located on chromosome region 2q35, and 9 SNPs located in genes (CDH13 and ADH1C), all of which have previously been implicated in the alcohol dependence literature. This study is the first published GWAS to identify a genome-wide significant association in alcohol dependence. In addition to this study, many GWAS are currently underway for alcohol-related phenotypes including efforts from large gene identification projects, the COGA, the Irish Affected Sib-Pair Substance and Alcohol Dependence (IASPSAD), and the Study of Addiction: Genetics and Environment (SAGE), with results expected imminently. Although GWAS provides several advantages, including the ability to more precisely localize associated variants, it also provides many challenges including the need for large samples to detect genes of small effect, many genetic markers, and multiple testing concerns.56 There are strengths and weaknesses to all gene-finding techniques, and converging evidence of association across techniques will be key to validating specific genetic variants involved in complex traits.
GENE IDENTIFICATION IN ADOLESCENT POPULATIONS
Many of these gene identification efforts focus on adult alcohol and substance dependence. This focus is in part so that subjects will have passed through the age of onset for common addictive substance disorders and to ensure that there is no ambiguity associated with whether an unaffected individual simply has not yet manifested problems. However, some projects have focused specifically on adolescent samples. Many of these studies have come from the Colorado Center for the Genetics of Antisocial Drug Dependence (CADD), which is an ongoing multicomponent, collaborative study at the University of Colorado57 consisting of more than 5000 youths. The CADD is using several research designs and strategies in its study of the genetic basis for antisocial drug dependence in adolescents. Stallings and colleagues57 reported a genome-wide linkage study for the average number of substance dependence symptoms (a quantitative index of substance use liability) that implicated several genomic regions, including chromosomes 3q24-25 (near markers D3S1279 and D3S1614) and 9q34 (near markers D9S1826 and D9S1838). A 2008 study58 reported SNP association results from a targeted gene assay designed to test 50 candidate genes with previous associations with substance use disorders and conduct disorder, in a sample of male probands in treatment of antisocial drug dependence and a matched set of community controls. After gene-based permutation tests, 2 genes probed with multiple SNPs (OPRM1 and CHRNA2) emerged as plausible candidates for a role in antisocial drug dependence. Other studies have focused on candidate genes, such as the neuronal nicotinic acetylcholine receptors and tobacco and alcohol phenotypes. Ehringer and colleagues59 examined 2 neuronal nicotinic receptor subunit candidate genes, and found that CHRNA4 was associated with past 6-month use of alcohol in White, and CHRNB2 was associated with the initial subjective response to alcohol and tobacco.
Another strategy that has been used to identify genes involved in adolescent substance dependence is to initially search for genetic variants associated with adult substance dependence, and then to study the risk associated with these variants in adolescent samples. One example of this strategy in use is the identification of the gene GABRA2, first found in association with alcohol dependence,48,60 and subsequently with illicit drug dependence, antisocial personality disorder, and conduct disorder, suggesting that this gene may be involved in addictions through general externalizing pathways.61 In 2006, Dick and colleagues39 extended the work on GABRA2 by using children and adolescents recruited from alcohol-dependent COGA families and control families obtained from community sources. Results suggested that individuals carrying at least 1 A allele of the SNP rs279871, which was significantly overtransmitted to alcohol-dependent subjects in the adult COGA sample, were twice as likely to meet the 3-symptom threshold of DSM-III-R conduct disorder. This effect has subsequently been replicated in an independent sample.62 These findings suggest that this gene may be involved in addictions through general externalizing pathways that manifest first as conduct problems in adolescence, and later in adulthood as a spectrum of related disorders, including alcohol and drug dependence and antisocial personality disorder.
APPLICATIONS FOR PREVENTION
When disseminating this research to the public, it is crucial to convey how to appropriately interpret this information. Substance dependences are complex disorders, phenotypically and etiologically. There is no gene that makes an individual an alcoholic or a cocaine user; instead, there are multiple genes of subtle effects that interact to make the individual more vulnerable to a host of behaviors and disorders, many of which are characterized by behavioral disinhibition, including alcoholism and illicit drug use. These genetic vulnerabilities work in tandem with the individual’s dynamic environment to protect or promote the development of externalizing spectrum problems. Although knowledge of the specific genes involved in addiction is not sufficiently advanced for this information to be used in clinical settings at the present time, the hope is that eventually information about specific genes that alter susceptibility for addictive disorders can be used to provide more individual-specific risk assessments. This information could be used to create more tailored programs for prevention and intervention. Recognizing this potential for genetic information will necessitate our understanding the pathways of risk and environmental factors that moderate risk associated with specific genetic profiles.
References
- 1.US Department of Health and Human Services. The surgeon general’s call to action to prevent and reduce underage drinking. Bethesda (MD): US Department of Health and Human Services, Office of the Surgeon General; 2007. [PubMed] [Google Scholar]
- 2.Hedlund JH, Ulmer RG, Preusser DF. Determine why there are fewer young alcohol impaired drivers. Washington, DC: National Highway Traffic Safety Administration; 2001. DOT HS 809 348. [Google Scholar]
- 3.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect. 2000;108(3 Suppl):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Alcohol-attributable deaths and years of potential life lost–United States, 2001. MMWR Morb Mortal Wkly Rep. 2004;53(37):866–70. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Youth Risk Behavior Surveillance—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(4 Suppl):S1–131. [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2005: national estimates of drug-related emergency department visits. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2005. [Accessed August 24, 2009]. Available at: http://dawninfo.samhsa.gov/pubs/edpubs/default.asp. [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: national findings. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2006. [Accessed August 24, 2009]. Available at: http://dawninfo.samhsa.gov/pubs/edpubs/default.asp. [Google Scholar]
- 8.Merline AC, O’Malley PM, Schulenberg JE, et al. Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. Am J Public Health. 2004;94(1):96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration. 2004 National Survey on Drug Use and Health: detailed tables. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2004. [Accessed August 24, 2009]. Available at: http://www.oas.samhsa.gov/NSDUH/2k6NSDUH/tabs/LOTSect2pe.htm#AlcAge. [Google Scholar]
- 10.Chen K, Kandel DB, Davies M. Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug Alcohol Depend. 1997;46(1):53–67. doi: 10.1016/s0376-8716(97)00047-1. [DOI] [PubMed] [Google Scholar]
- 11.Howlett AC, Bidaut-Russell M, Devane WA, et al. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13(10):420–3. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- 12.Johnston LD, O’Malley PM, Bachman JG, et al. [Accessed August 24, 2009];Overall, illicit drug use by American teens continues gradual decline in 2007. Available at: www.monitoringthefuture.org.
- 13.Substance Abuse and Mental Health Services Administration. Misuse of over-the-counter cough and cold medications among persons aged 12 to 25. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2008. [Accessed August 24, 2009]. Available at: http://dawninfo.samhsa.gov/pubs/edpubs/default.asp. [Google Scholar]
- 14.National Institute on Drug Abuse. NIH publication no 01-4881. Bethesda (MD): US Department of Health and Human Services, National Institutes of Health; 2001. [Accessed August 24, 2009]. Research report series: prescription drugs: abuse and addiction. Revised August 2005. Available at: http://www.cdc.gov/healthyYouth/alcoholdrug/index.htm. [Google Scholar]
- 15.Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J Subst Abuse. 1998;10(2):163–73. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- 16.Dewit DJ, Adlaf EM, Offord DR, et al. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157(7):45–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 17.Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160(7):739–46. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- 18.McGue M, Iacono WG, Legrand LN, et al. Origins and consequences of age at first drink: associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25(8):1156–65. [PubMed] [Google Scholar]
- 19.Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23(1):101–7. [PubMed] [Google Scholar]
- 20.Keyes MA, Iacono WG, McGue M. Early onset problem behavior, young adult psychopathology, and contextual risk. Twin Res Hum Genet. 2007;10(1):45–53. doi: 10.1375/twin.10.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Tapert SF, Caldwell L, Burke C. Alcohol and the adolescent brain: human studies. Alcohol Res Health. 2004;28(4):205–12. [Google Scholar]
- 22.Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol. 2002;14(Suppl):71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- 23.Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Ann N Y Acad Sci. 2004;10(21):234–44. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- 24.Krueger RF, Hicks BM, Patrick CJ, et al. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111(3):411–24. [PubMed] [Google Scholar]
- 25.Young SE, Stallings MC, Corley RP, et al. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet. 2000;96(5):684–95. [PubMed] [Google Scholar]
- 26.McGue M, Iacono WG. The adolescent origins of substance use disorders. Int J Methods Psychiatr Res. 2008;17(Suppl 1):S30–8. doi: 10.1002/mpr.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 28.Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155(8):1016–22. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- 29.Tsuang MT, Lyons MJ, Meyer JM, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55(11):967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Schmitt E, Aggen SH, et al. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65(6):674–82. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouchard TJ, Jr, McGue M. Genetic and environmental influences on human psychological differences. J Neurobiol. 2003;54(1):4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- 32.Boomsma DI, van Beijsterveldt CE, Hudziak JJ. Genetic and environmental influences on Anxious/Depression during childhood: a study from the Netherlands twin register. Genes Brain Behav. 2005;4(8):466–81. doi: 10.1111/j.1601-183X.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 33.Rose RJ, Dick DM, Viken RJ, et al. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol Clin Exp Res. 2001;25(11):1594–604. [PubMed] [Google Scholar]
- 34.Rose RJ, Dick DM, Viken RJ, et al. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001;25(5):637–43. [PubMed] [Google Scholar]
- 35.Rose RJ, Viken RJ, Dick DM, et al. It does take a village: nonfamilial environments and children’s behavior. Psychol Sci. 2003;14(3):273–7. doi: 10.1111/1529-1006.03434. [DOI] [PubMed] [Google Scholar]
- 36.Kendler KS, Prescott C, Myers J, et al. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 37.Slutske WS, Heath AC, Dinwiddie SH, et al. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998;107(3):363–74. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- 38.Haber JR, Jacob T, Heath AC. Paternal alcoholism and offspring conduct disorder: evidence for the ‘common genes’ hypothesis. Twin Res Hum Genet. 2005;8(2):120–31. doi: 10.1375/1832427053738782. [DOI] [PubMed] [Google Scholar]
- 39.Dick DM, Bierut L, Hinrichs A, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577–90. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 40.Kendler KS, Jacobson KC, Prescott CA, et al. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160(4):687–95. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 41.Koopmans JR, Slutske WS, Van Baal GC, et al. The influence of religion on alcohol use initiation: evidence for genotype X environment interaction. Behav Genet. 1999;29(6):445–53. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- 42.Rose RJ, Kaprio J, Williams CJ, et al. Social contact and sibling similarity: facts, issues, and red herrings. Behav Genet. 1990;20(6):763–78. doi: 10.1007/BF01065919. [DOI] [PubMed] [Google Scholar]
- 43.Dick DM, Viken R, Purcell S, et al. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. J Abnorm Psychol. 2007;116(1):213–8. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshal MP, Chassin L. Peer influence on adolescent alcohol use: the moderating role of parental support and discipline. Appl Dev Sci. 2000;4:80–8. [Google Scholar]
- 45.Dick DM, Rose RJ, Viken RJ, et al. Exploring gene-environment interactions: so-cioregional moderation of alcohol use. J Abnorm Psychol. 2001;110(4):625–32. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- 46.Hicks BM, South SC, Dirago AC, et al. Environmental adversity and increasing genetic risk for externalizing disorders. Arch Gen Psychiatry. 2009;66(6):640–8. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dick DM, Latendresse SJ, Lansford JE, et al. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Arch Gen Psychiatry. 2009;66(6):649–57. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705–14. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edenberg HJ, Xuei X, Chen HJ, et al. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum Mol Genet. 2006;15(9):1539–49. doi: 10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- 50.Kuo PH, Kalsi G, Prescott CA, et al. Association of ADH and ALDH genes with alcohol dependence in the Irish Affected Sib Pair Study of Alcohol Dependence (IASPSAD) sample. Alcohol Clin Exp Res. 2008;32(5):785–95. doi: 10.1111/j.1530-0277.2008.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edenberg HJ, Xuei X, Wetherill LF, et al. Association of NFKB1, which encodes a subunit of the transcription factor NF-kappaB, with alcohol dependence. Hum Mol Genet. 2008;17(7):963–70. doi: 10.1093/hmg/ddm368. [DOI] [PubMed] [Google Scholar]
- 52.Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903–11. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 53.Wang JC, Hinrichs AL, Bertelsen S, et al. Functional variants in TAS2R38 and TAS2R16 influence alcohol consumption in high-risk families of African-American origin. Alcohol Clin Exp Res. 2007;31(2):209–15. doi: 10.1111/j.1530-0277.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 54.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–7. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 55.Treutlein J, Cichon S, Ridinger M, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66(7):773–84. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sebastiani P, Timofeev N, Dworkis DA, et al. Genome-wide association studies and the genetic dissection of complex traits. Am J Hematol. 2009;84(8):504–15. doi: 10.1002/ajh.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stallings MC, Corley RP, Hewitt JK, et al. A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug Alcohol Depend. 2003;70(3):295–307. doi: 10.1016/s0376-8716(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 58.Corley RP, Zeiger JS, Crowley T, et al. Association of candidate genes with antisocial drug dependence in adolescents. Drug Alcohol Depend. 2008;96(1–2):90–8. doi: 10.1016/j.drugalcdep.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehringer MA, Clegg HV, Collins AC, et al. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B Neuropsychiatr Genet. 2007;144(5):596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 60.Dick DM, Plunkett J, Wetherill LF, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin Exp Res. 2006;30(7):1101–10. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 61.Dick DM. Identification of genes influencing a spectrum of externalizing psychopathology. Curr Dir Psychol Sci. 2007;16(6):331–5. [Google Scholar]
- 62.Sakai JT, Stallings MC, Crowley TJ, et al. Test of association between GABRA2 (SNP rs279871) and adolescent conduct/alcohol use disorders utilizing a sample of clinic referred youth with serious substance and conduct problems, controls and available first degree relatives. Drug Alcohol Depend. 2010;106(2–3):199–203. doi: 10.1016/j.drugalcdep.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]