Abstract
Emerging evidence points to a critical role for the skeleton in several homeostatic processes including energy balance. The connection between fuel utilization and skeletal remodeling begins in the bone marrow with lineage allocation of mesenchymal stromal cells into adipocytes or osteoblasts. Mature bone cells secrete factors that influence insulin sensitivity and fat cells synthesize cytokines that regulate osteoblast differentiation. The emerging importance of the bone-fat interaction suggests that novel molecules could be used as targets to enhance bone formation and possibly prevent fractures. In this review, we discuss three pathways that could favor pharmacologic intervention with the ultimate goal of enhancing bone mass and reducing osteoporotic fracture risk. Not surprisingly, because of the complex interactions across homeostatic networks, other pathways will likely be activated by this targeting and these could prove to be beneficial or detrimental for the organism. Hence a more complete picture of energy utilization and skeletal remodeling will be required to bring these potential agents into any future clinical armamentarium.
Introduction
In the past decade there have been significant advances in our understanding of skeletal acquisition and maintenance. There has also been a growing awareness that bone remodeling requires an energy source and is intimately tied to other homeostatic pathways. Several lines of evidence support this recent emphasis. First, osteoblasts and adipocytes arise from the same mesenchymal stromal cell (MSC) in the bone marrow milieu. Recent work has further clarified the process of MSC lineage allocation although more studies are needed to understand whether plasticity between these two cell types exists at various developmental stages (see Figure 1). Second, changes in glucose and fat metabolism in a host of conditions including diabetes mellitus, Cushings’ syndrome, and anorexia nervosa significantly impact skeletal health. Similarly, bone specific proteins secreted from osteoblasts have been shown to regulate glucose metabolism. Third, central nervous system processing in the hypothalamus from efferent fat depots regulates skeletal turnover via the sympathetic nervous system. Fourth, obesity in childhood has been associated with a greater fracture risk, even though body mass index has been shown to directly correlate with bone mineral density. The paradox between the positive effects of adipose ‘insulation’ on the cortical skeleton and the inherent capacity of adipose tissue to function as an endocrine organ secreting inflammatory cytokines and adipokines that are detrimental to the trabecular skeleton still requires more comprehensive studies. But, it also raises the provocative possibility that future therapeutics could directly target fat cells in order to positively impact the skeleton. This review will focus on strategies using three targets in the bone-fat network: leptin, Pparγ (peroxisome proliferator activated receptor gamma), and osteocalcin. These molecules were chosen in part because of very recent progress and a growing understanding of their integration into homeostatic processes of the skeleton.
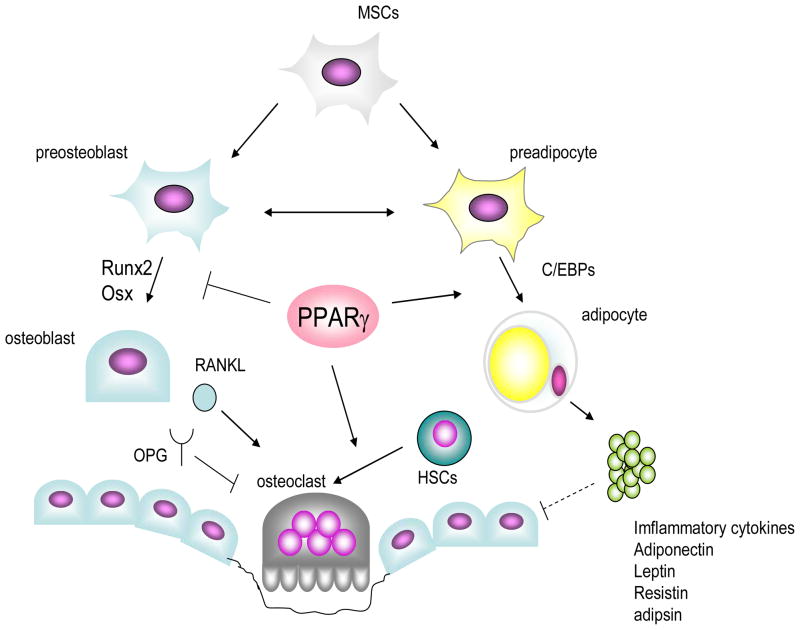
Figure 1.
PPARγ regulates bone mass in bone marrow milieu. First step of regulation resides in the determination of lineage allocation of mesenchymal stem cells. Several transcription factors including PPARγ and C/EBPs govern adipogenesis, while transcription factors such as Runx2 and Osx are necessary for osteoblastogenesis. PPARγ favors adipogenesis, and suppresses osteoblastogenesis partly through inhibiting Runx2 function, resulting in the reduction of osteoblast pool in bone marrow. Second, PPARγ stimulates osteoclastogenesis by enhancing c-fos expression in osteoclast precursor cell. Third, secretory factors including inflammatory, leptin, adipsin, adiponectin and resistin are also produced by marrow adipocytes. These cytokines are possibly acting on osteoblasts in a paracrine manner and suppressing osteoblast function and/or differentiation in pathogenic conditions. PPAR: peroxisome proliferative activated receptors, C/EBP: CCAAT/enhancer binding proteins; RUNX: runt-related transcription factor, Osx: osterix, RANKL: receptor activator of nuclear transcription factor κB ligand, OPG: osteoprotegerin, MSC: mesenchymal stem cell, HSC: hematopoietic stem cell.
Leptin
Leptin is an adipokine produced by fat cells that mediates energy homeostasis, appetite levels and reproductive capacity1. Since its discovery 15 years ago, there have been tremendous expectations about its possible therapeutic use in the treatment of obesity. Unfortunately, most of the clinical studies in obese patients, barring a few case reports in subjects with loss- of-function leptin or leptin receptor mutations, have failed to meet those expectations. However, there is emerging evidence that leptin treatment might be an important adjunct to acquired lipodystrophic disorders including chronic HIV infections and insulin resistant diabetes mellitus. Similarly, although its role in skeletal physiology is complex, augmentation of leptin may have therapeutic implications for bone, particularly in disordered states of fat metabolism associated with marrow adiposity.
The major paradox concerning leptin relates to the extent to which leptin acts via the hypothalamus and skeletal innervation to regulate bone mass, vs. its putative direct actions on osteoblasts 2–11 (see Figure 2). In humans, the relationship between leptin and bone mass varies with age, gender, energy status, and skeletal site, limiting its clinical utility as a marker of skeletal status. Leptin receptor (LEPR) polymorphisms affect circulating leptin levels and/or bone mass, but the relationship between serum leptin and bone mineral density (BMD) is unclear, with studies reporting both positive and negative associations, particularly after body composition adjustment12–25. Skeletal effects of leptin via a central hypothalamic pathway were first reported by Ducy et al. in 20005. Intracerebroventricular (ICV) leptin infusion in mice stimulates ventromedial hypothalamic Lepr, triggering norepinephrine release by sympathetic nervous system (SNS) fibers and activation of beta2-adrenergic receptors (βadr2) in osteoblasts. βadr2 upregulation decreases osteoblast activity and bone formation and increases bone resorption via Receptor Activator of NF-κB Ligand (RANKL) production, leading to vertebral trabecular bone loss3–7,26,27. Treatment with a β-adrenergic agonist (isoproterenol) replicates the phenotype of low bone mass6,26–28. However, parabiosis of ICV-treated with untreated mice demonstrates that the latter do not lose bone; since ICV leptin does not cross into the bloodstream, these data confirmed that leptin caused bone loss through increased sympathetic tone, rather than through the circulation27.
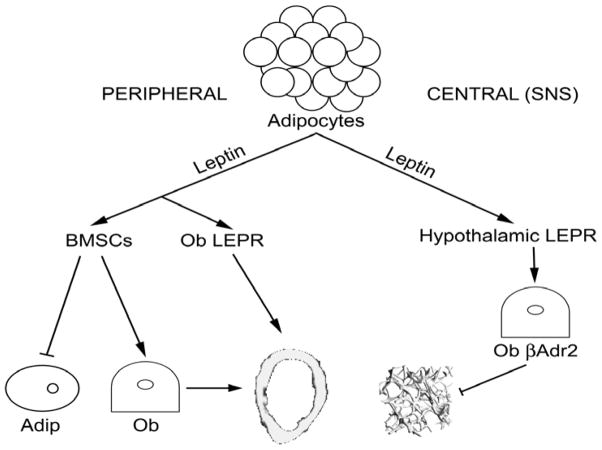
Figure 2.
Central leptin signaling via hypothalamic leptin receptors (LEPR) and sympathetic nervous system (SNS) to osteoblast b-adrenergic (Ob b-adr) receptors decreases trabecular bone volume (BV/TV). Peripheral leptin reportedly increases cortical bone growth and bone marrow stromal cell (BMSC) differentiation to osteoblast (Ob) vs. adipocyte (Adip) lineage.
Gain of function and loss of function mouse models in this pathway provide additional insight, although they may complicate therapeutic considerations. For example, the skeletal phenotype of ob/ob (leptin-deficient) and db/db (Lepr null) mice includes high vertebral trabecular bone volume, but low femoral cortical and trabecular bone volume 4,5,11,29–32. Interestingly, hypoleptinemia secondary to caloric restriction produces a similar phenotype with normal whole body BMD and improved vertebral trabecular bone, but decreased femoral length, areal BMD and strength33–42. Similarly, the db/db mouse does not lose bone under caloric restriction suggesting Lepr involvement in hypoleptinemia-induced bone loss36. Finally, mice null for βadr2 exhibit increased trabecular bone mass in vertebrae and distal femur as well as increased cortical bone volume at the femoral midshaft, despite normal body mass and a normal endocrine profile 26,6.
The effects of changes in the beta1-adrenergic receptor are also complex. Although the beta1-adrenergic receptor (βadr1) is expressed at low levels (if at all) in bone, mice null for both βadr1 and βadr2 show decreased periosteal bone formation, cortical bone mass and BMD, while mice lacking all three β-adrenergic receptors (β-less) have increased body mass, leptin levels, cortical and trabecular bone mass vs. controls26,43. Thus it has been suggested that the three beta-adrenergic receptors exert complementary effects in bone although the role of leptin in such a mechanism is unclear 26. Moreover, although prevailing data from mice are compelling, the relationship of changes in adrenergic receptors in humans to skeletal mass remains unclear.
There is also conflicting evidence regarding the direct anabolic actions of leptin in bone. In a recent study, Shi et al. compared mice with conditional Lepr deletion in neurons vs. osteoblasts 44. Lepr(neuron)−/− mice exhibit increased vertebral trabecular bone properties, similar to the db/db global Lepr knockout. In stark contrast, Lepr deletion in osteoblasts (Lepr(osteoblast)−/−) produces no skeletal phenotype in vertebral or distal femoral trabecular bone. However, leptin gain-of-function (l/l) mice have decreased bone mass and trabecular bone volume44. While these results imply that leptin has no direct effect on osteoblasts, previous data suggest leptin has an osteogenic function in cortical bone in some circumstances, either through inhibition of osteoclasts or stimulation of osteoblasts11,45. In addition, leptin treatment can increase periosteal bone formation and commitment of mesenchymal stem cells (MSCs) to bone vs. fat lineages, as well as inhibiting ovariectomy-induced bone loss8,10,46,47, and reversing both cortical and trabecular decrements in the limbs of ob/ob mice9. In hindlimb unloading in which there is profound suppression of bone formation and increases in bone resorption, treatment with either leptin or a non-selective beta-blocker (propranolol) reduced bone loss with equal efficacy48,49.
There are several possible reasons for these contradictory results. First, antiosteogenic leptin effects are often seen in axial elements, e.g. vertebral trabecular bone, while anabolic effects are observed in appendicular components, e.g. limbs11. One might speculate that decreased leptin levels enhance vertebral trabecular bone formation while inhibiting limb bone acquisition as an adaptation to starvation50. Although the lack of a trabecular bone phenotype in the Lepr(osteoblast)−/− mice44 is persuasive, there are no data on bone density in a purely cortical bone site, e.g. the midshaft femur. Second, it has been suggested that leptin has a biphasic effect in cortical bone, with bone formation enhanced at lower doses but suppressed at higher doses51. It is conceivable that replacement with leptin in a deficient state is anabolic to bone, whereas the elevated sympathetic tone of hyperleptinemia triggers bone loss. Third the effect of leptin resistance must be considered in any state of high circulating leptin. Finally, studies of leptin action vary in dosing, route of administration (i.e. ICV, intraperitoneal, subcutaneous), and duration, contributing to divergent outcomes.
Currently, the clinical potential of leptin supplementation, which may reverse bone loss in some scenarios and which may be beneficial to trabecular bone, is too preliminary to draw any conclusions 51,52. In respect to β–blockers and osteoporosis, the epidemiologic studies of individuals treated with β–blockers for hypertension or angina fail to show a convincing effect of these agents on bone density or fractures, in part because of the various types of β–blockers being studied, and the heterogeneity within cohorts 53–56. Furthermore, there are no long term randomized placebo controlled studies of β–blockers for fracture efficacy 56. In recent studies of women with hypothalamic amenorrhea, modest dose escalation of daily recombinant leptin restores the gonadotropin reproductive axis and stimulates lipolysis 52. It is conceivable, although not proven, that recombinant leptin could either alone or in combination with restoration of estrogen, reduce marrow adiposity, enhance osteoblast differentiation, and strengthen bone mass. But, once again, there are no large scale studies to determine efficacy. Similarly, the fat redistribution that characterizes chronic HIV infection has been associated with low bone mass. Unpublished studies using recombinant leptin have shown promise in reversing the lipodystrophy due to HIV and/ or the drugs used to combat this virus. It remains to be established whether these changes are accompanied by any beneficial effect on the skeleton. Very recently efforts have begun to focus on leptin-mimetics that could recapitulate the positive benefits of leptin on body composition, while sparing the potential adverse skeletal effects.
Peroxisome Proliferator-Receptor Activated Gamma (Pparγ)
Peroxisome Proliferator-Receptor Activated Gamma (Pparγ) is a member of the nuclear receptor super family and a critical transcription factor in adipogenesis. There are four isoforms of the PPARG protein, but only PPARG2 is specific for adipose tissue. In the adipogenic lineage scheme, PPARG heterodimerizes with RXRα. Activation of PPARG requires the binding of a ligand and recruitment of specific co-activators, to allow the PPARG-RXRα heterodimer to induce gene transcription of insulin sensitizing targets such as adiponectin and lipoprotein lipase57. Ligands for PPARG include the naturally occurring PGJ2 and 9(S)-HODE compounds as well as the thiazolidinedione (TZD) class of synthetic compounds58, 59. In 1999, two TZDs, rosiglitazone and pioglitazone, were approved by the U.S. FDA for the treatment of type 2 diabetes mellitus based on their inherent property to enhance insulin sensitivity. However, as will be discussed, there are differences within this class of agents not only in respect to their lipid lowering properties, but also in their propensity to increase the risk of cardiovascular disease, and most intriguingly, their capacity to cause bone loss and marrow adipogenesis.
During the process of adipogenesis, induction of Pparγ is necessary to convert adipocyte precursors to fully differentiated adipose cells (See Figure 1). CCAAT/enhancer-binding protein β (Cebp-β) strongly induces the expression of Pparγ2 and in turn, PPARG protein can stimulate the expression of CCAAT/enhancer-binding protein α (Cebpa). Early B-Cell Factor-1 (Ebf1), a Helix-Loop-Helix DNA-binding protein, has been shown to be critical for hematopoietic B-cell development60. Interestingly, expression of this nuclear factor has been found in a variety of tissue sites, including white adipose tissue, and recent studies suggest that EBF1 binds directly to the Pparγ promoter and may act between CEBPβ and CEBPα/PPARG in the adipocyte differentiation cascade 60,61.
Elbrecht et al. first showed that Pparγ was expressed in bone marrow mesenchymal stromal cells62. Subsequently, it was demonstrated that treatment of marrow stromal cells with the TZDs resulted in the differentiation of these cells into adipocytes63. When UAMS-33 cells, a pluripotent cell line is transfected with Pparγ 2 and treated with rosiglitazone, adipocyte-like cells appear and these cells cannot form a mineralized matrix64. This has led some to conclude that Pparγ activation precludes osteogenesis. Certainly, this would seem to be the case when analyzing various micro array studies of gene expression; however, in vivo these networks are counter-balanced by compensatory changes and other variables such as age and gender. Thus predictions of gene action based solely on expression may be misleading. For example, when UAMS-33 cells are transfected with Pparγ 2 and treated with rosiglitazone, significant suppression of RANKL and m-CSF mRNA is noted 65. But, in vivo, cre-lox P inactivation of Pparγ 2 using the Tie2 promoter in hematopoietic stem cells results in inhibition of bone resorption and impaired osteoclastogenesis 66.
Mouse models are critical in defining in vivo targeting effects from changes in Pparγ 2. For example, homozygous Pparg knockout animals (strain Ppargtm1Tka) are not viable, but heterozygous Pparγ +/− mice have a pronounced bone phenotype of increased bone density and decreased marrow adiposity67. Similarly, Pparγ hyp/hyp mice with a partial loss of function mutation in the Pparγ gene have high bone mass and little marrow fat 68. And, deletion of Ppargamma;2 in adipose tissue using the aP2 promoter results in a high bone mass phenotype (Lecka-Czernik, personal communication). Another approach is to directly treat various animal models with the TZDs. Such studies have demonstrated these agents can affect bone mass and marrow adiposity. Tornvig et al. first demonstrated that troglitazone increased marrow adiposity in the Apoe−/− strain, although no changes in bone mass were observed69. Darglitazone, which is twenty times more potent than rosiglitazone and a hundred times more potent than pioglitazone causes a reduction in both trabecular and cortical bone and increased marrow fat70,71. In contrast, Netoglitazone, a relatively weak TZD, increased marrow adiposity, but did not affect trabecular bone volume or whole body areal (a)BMD in C57BL/6 mice72. Rosiglitazone treatment, particularly in older B6 mice, causes a significant decrease in trabecular bone density and a substantial increase in marrow adiposity, but these changes are genotype and gender specific 72,73. Thus ligand specificity and potency, co-activator and repressor recruitment, and background strain are all variables that predict murine responsiveness of the skeleton to TZDs.
In humans, variable skeletal responses to TZDs have also been reported. There are now several large randomized trials of rosiglitazone and pioglitazone for the treatment of T2D demonstrating improved glycemic control, albeit with an associated increased risk of peripheral fractures, particularly in women74–81. Smaller studies have also shown a reduction in markers of bone formation markers and rapid bone loss from the axial skeleton in pre- and postmenopausal women78–81. Although there is significant inter-subject variability in these trials, uncoupling of formation from resorption after treatment with the TZDs appears to be comparable to the early changes observed in glucocorticoid induced osteoporosis. Interestingly, reversibility of TZD induced bone loss has not been demonstrated.
Based on these studies the Pparγ gene could become a drug target to enhance bone mass, since it can regulate lineage allocation within the marrow compartment. However, attempts to design drugs that suppress Pparγ expression in marrow stromal cells, but preserve its ability to enhance insulin sensitivity have met with significant difficulties. An alternative approach would be to manipulate a downstream target of Pparγ in the bone marrow that would not affect glucose metabolism but at the same time would stimulate bone formation by redirecting marrow stromal cells. Currently, this strategy is being investigated by targeting one or more of the peripheral ‘clock’ genes that are expressed in bone as well as fat and are immediately downstream of Pparγ 46,82.
Osteocalcin
Clinical findings previously suggested there was a strong connection between energy status of the organism and skeletal turnover82. For example, patients with impaired glucose disposal, e.g. Type I or Type II diabetes, have a greater risk of osteoporotic fractures even in the face of normal bone mass83,84. Young women with anorexia nervosa exhibit very low bone mass, increased adiponectin production and insulin sensitivity, and enhanced skeletal fragility85,86. In contrast, glucocorticoid excess, whether endogenous or exogenously-induced, causes an adipose redistribution syndrome associated with low bone mass and insulin resistance87,88. Despite these observations, the network linking fat deposition to insulin sensitivity and skeletal remodeling was obscure for many years. The discovery that leptin worked indirectly via the hypothalamus to regulate osteoblastic activity set off a search for novel signals that connected adipose tissue to bone. Lee et al. provided the first concrete evidence that the skeleton could function as an endocrine organ regulating glucose metabolism through the secretion of osteocalcin (OC) 89. These investigators showed that OC increased adiponectin and insulin expression in adipocytes and β-cells respectively, and that osteoblasts isolated from osteocalcin −/− (null) mice were incapable of producing this effect. Consistent with this finding, OC −/− mice were found to be obese and have higher glucose and lower insulin levels than littermate controls even though they had no demonstrable skeletal phenotype. To prove that OC was involved in the interaction between bone and energy, these same authors identified a phosphatase, Esp, which was expressed only in bone and testes. Interestingly, Esp−/− mice are born with profound hypoglycemia and increased insulin sensitivity. When one copy of OC was deleted in Esp−/− mice, the metabolic phenotypes were reversed, indicating that Esp and OC were in the same regulatory pathway for glucose metabolism.
OC is an osteoblast-specific protein and a major non-collagenous protein in the extracellular matrix. Glutamic-acid residues in OC undergo post-translational γ-carboxylation into γ-carboxyglutamic acid (Gla); this enhances OC’s affinity for extracellular matrices, especially hydroxyapatite90,91. Esp is thought to be involved in γ-carboxylation of OC because mice lacking the Esp gene have increased serum levels of uncarboxylated OC. Karsenty and colleagues recently demonstrated that uncarboxylated OC, acting as a pro-hormone, can increase β-cell proliferation, insulin secretion, insulin sensitivity, and adiponectin expression89,92. Thus, osteoblasts may be able to regulate glucose metabolism by modulating the bioactivity of OC, possibly through Esp. However, several questions remain unanswered. First, the receptor for un- or under carboxylated OC has not been described. Second, Esp is a mouse gene not expressed in humans; hence the significance of this pathway in humans will require more work. Finally, it is not clear how cells sense the varying ratios of un- or under-carboxylated osteocalcin to the fully carboxylated molecule.
Notwithstanding these important issues, several recent studies have illustrated progress in this area. For example, Hinoi et al. noted that OC bioactivity is modulated by enhanced sympathetic tone driven by leptin93. Remarkably, leptin has been shown to suppress insulin secretion by β-cells94, 95. Since leptin also negatively regulates osteoblast function, questions arose as to whether the inhibitory effect of leptin on insulin secretion was partially mediated by sympathetic tone. To answer that question, osteoblast-specific Adr β2 knockout mice were generated and exhibited hyperinsulinemia compared to controls, a finding similar to the ob/ob mice. In addition, sympathetic tone increased Esp expression in osteoblasts thereby enhancing γ carboxylation but reducing insulin secretion. To conclusively prove that leptin regulated insulin synthesis through OC, Esp−/− mice were crossed with ob/ob mice. Ob/ob/Esp−/− mice had increased insulin levels compared to controls and had improved glucose tolerance. Thus, a novel picture has emerged linking glucose metabolism, adipose stores and skeletal activity. This network is initiated by leptin, which when secreted by adipocytes, stimulates sympathetic tone through the hypothalamus; sympathetic discharges in turn may increase Esp expression in osteoblasts resulting in decreased OC bioactivity; impaired OC subsequently alters insulin secretion from β-cells in the pancreatic islet cells.
This pathway is both challenging and fascinating in terms of potential clinical implications. First, the Esp-OC network could be a suitable pharmacologic target to improve insulin sensitivity in adipocytes.. Three recent studies have demonstrated an inverse correlation between serum OC and plasma glucose levels, supporting a role for this pathway in humans96–98. Conceivably, administration of an agent that enhances uncarboxylated osteocalcin, or recombinant osteocalcin itself, might enhance insulin secretion. However, several caveats must be considered. First, as noted, Esp is not expressed in humans, hence any studies targeting this pathway will first have to delineate the mechanisms of osteocalcin induced insulin sensitivity. Notwithstanding, warfarin, which blocks γ-carboxylation of several molecules including osteocalcin, is widely used as an anti-coagulant, and has been reported to rarely cause hypoglycemia. Surprisingly, analysis of glucose metabolism in patients using this drug is lacking. Second, increased uncarboxylated OC seems to be beneficial for glucose metabolism, but the consequence of increases in this prohormone on the skeleton has not been firmly established. In vitro and in vivo, high OC levels are positively correlated with increased bone turnover and formation but neither OC-deficient nor OC transgenic mice have a skeletal phenotype99,100. Theoretically inhibition of γ-carboxylation of OC could have an impact on bone formation and bone quality. Finally, we do not fully understand the evolutionary implications of this network. Pharmacologic manipulation of osteocalcin synthesis or carboxylation may alter the homeostatic balance between the other two pathways that connect bone and fat.
Summary
In summary novel studies targeting three distinct networks linking bone and fat provide us with new insight into energy metabolism and its relationship to skeletal turnover. Moreover, progress in this nascent field is accelerating at a rapid pace. For example, Yadav and colleagues recently reported that circulating serotonin, principally synthesized from enterochromaffin cells in the gut, inhibits bone formation. Moreover, this group showed that LRP 5 in the intestine tonically suppresses serotonin generation by regulating the enzyme Tph1101. These surprising findings provide another link between energy metabolism and the skeleton, this time through the gut. Similarly, our understanding of the bone-fat ‘neighborhood’ within the marrow has grown significantly102. There is evidence to suggest that adipocytes are some of the earliest cells to appear during osteogenesis103. Their precise role is not known, but changes in oxygen tension and vascular recruitment are clearly important downstream events in this cascade after the appearance of adipocytes.
Thus, it is imperative to consider the array of cell-cell communications that regulate osteoblast differentiative function and MSC fate. Signals connecting peripheral adipocytes, with β-cells and bone cells implies that stem cells in various adipose depots might become a target for the treatment of osteoporosis. However, more work is necessary to fully understand the consequences of pharmacologically manipulating these networks in order to enhance bone mass and prevent osteoporotic fractures.
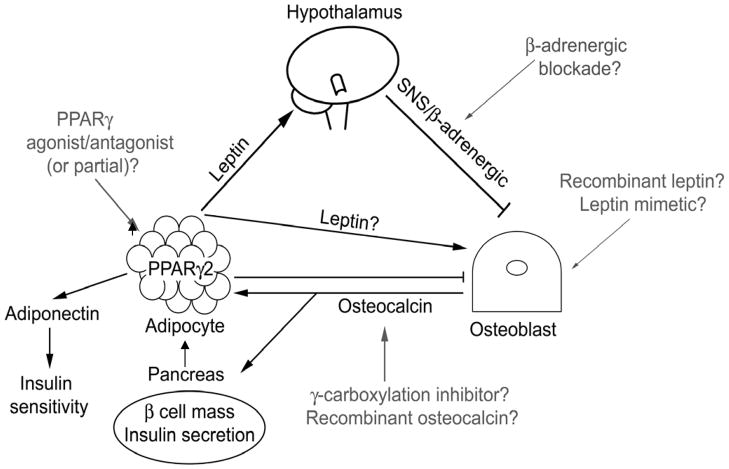
Figure 3.
Leptin signaling via the hypothalamus to the SNS/b-adrenergic receptors in osteoblasts triggers bone loss, but putative direct anabolic leptin effects on osteoblasts remain unresolved. Osteocalcin produced by osteoblasts decreases fat mass, promotes adiponectin production and insulin sensitivity, and increases pancreating b-cell mass and insulin secretion. Adipose-derived PPARg2 promotes marrow adiposity and decreases bone mass. Potential therapeutic targets include b-adrenergic blockade to reduce leptin-induced bone loss, recombinant leptin or leptin mimetic to increase bone mass, PPARg agonism/antagonism to inhibit marrow adiposity and increase osteoblast differentiation, and recombinant osteocalcin or g-carboxylation inhibitors to inhibit adipose deposition and improve bone mass.
KEY POINTS.
Bone and fat arise from the same mesenchymal stem cell in the bone marrow.
Osteoblasts secrete factors that regulate insulin production and adipocyte sensitivity to insulin.
Leptin is an adipokine that acts through the hypothalamus to regulate appetite and bone remodeling.
Peripheral fat stores are hormonally active and may regulate bone turnover.
REVIEW CRITERIA.
We searched for original articles focusing on SUBJECT in MEDLINE and PubMed published between 1980 and 2009. The search terms we used were bone, fat, adipose tissue, osteocalcin, Pparg, leptin and bone remodelling. All papers identified were English-language full text papers. We also searched the reference lists of identified articles for further papers.
Acknowledgments
This work was supported by NIH NIAMS grants AR 45433, 54604 to CJR.
Biographies
Clifford Rosen, MD, is a senior scientist at the Maine Medical Center Research Institute and Professor of Nutrition at the University of Maine. He is past president of the American Society for Bone and Mineral Research, and past chairperson of the FDA Endocrinologic and Metabolic Advisory Committee. Currently, Dr Rosen is the author or coauthor of more than 310 original research papers and reviews. His scientific interest lies in the interaction between bone and fat cells, and the regulation of IGF-I.
Maureen Devlin, PhD is a postdoctoral fellow in the laboratory of Mary Bouxsein, studying the effects of diet on skeletal acquisition during growth.
Masanobu Kawai MD, PhD is a postdoctoral fellow in the Rosen Laboratory studying the role of nocturnin, a circadian clock gene on fat metabolism.
Footnotes
COMPETING INTERESTS
None of the authors have competing interests or grants related to the compounds mentioned in this manuscript.
References
- 1.Flier JS. Clinical review 94: What’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 2.Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 3.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 4.Patel MS, Elefteriou F. The new field of neuroskeletal biology. Calcif Tissue Int. 2007;80:337–347. doi: 10.1007/s00223-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 5.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 6.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 7.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 9.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 10.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 11.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Kim SM, Kim SH, Lee JR, Jee BC, Ku SY, Suh CS, Choi YM, Kim JG, Moon SY. Association of leptin receptor polymorphisms Lys109Arg and Gln223Arg with serum leptin profile and bone mineral density in Korean women. Am J Obstet Gynecol. 2008;98:421, e1–8. doi: 10.1016/j.ajog.2007.10.799. [DOI] [PubMed] [Google Scholar]
- 13.Koh JM, Kim DJ, Hong JS, Park JY, Lee KU, Kim SY, Kim GS. Estrogen receptor alpha gene polymorphisms Pvu II and Xba I influence association between leptin receptor gene polymorphism (Gln223Arg) and bone mineral density in young men. Eur J Endocrinol. 2002;147:777–783. doi: 10.1530/eje.0.1470777. [DOI] [PubMed] [Google Scholar]
- 14.Richert L, Chevalley T, Manen D, Bonjour JP, Rizzoli R, Ferrari S. Bone mass in prepubertal boys is associated with a Gln223Arg amino acid substitution in the leptin receptor. J Clin Endocrinol Metab. 2007;92:4380–4386. doi: 10.1210/jc.2007-0932. [DOI] [PubMed] [Google Scholar]
- 15.Crabbe P, Goemaere S, Zmierczak H, Van Pottelbergh I, De Bacquer D, Kaufman JM. Are serum leptin and the Gln223Arg polymorphism of the leptin receptor determinants of bone homeostasis in elderly men? Eur J Endocrinol. 2006;154:707–714. doi: 10.1530/eje.1.02130. [DOI] [PubMed] [Google Scholar]
- 16.Fairbrother UL, Tankó LB, Walley AJ, Christiansen C, Froguel P, Blakemore AI. Leptin receptor genotype at Gln223Arg is associated with body composition, BMD, and vertebral fracture in postmenopausal Danish women. J Bone Miner Res. 2007;22:544–550. doi: 10.1359/jbmr.070114. [DOI] [PubMed] [Google Scholar]
- 17.Weiss LA, Barrett-Connor E, von Muhlen D, Clark P. Leptin predicts BMD and bone resorption in older women but not older men: the Rancho Bernardo study. J Bone Miner Res. 2006;21:758–764. doi: 10.1359/jbmr.060206. [DOI] [PubMed] [Google Scholar]
- 18.Thomas T, Burguera B, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Riggs BL, Khosla S. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29:114–120. doi: 10.1016/s8756-3282(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf) 2001;55:341–347. doi: 10.1046/j.1365-2265.2001.01361.x. [DOI] [PubMed] [Google Scholar]
- 20.Blain H, Vuillemin A, Guillemin F, Durant R, Hanesse B, de Talance N, Doucet B, Jeandel C. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2002;87:1030–1035. doi: 10.1210/jcem.87.3.8313. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Takeda N, Sarui H, Takami R, Takami K, Hayashi M, Sasaki A, Kawachi S, Yoshino K, Yasuda K. Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metab. 2001;86:5273–5276. doi: 10.1210/jcem.86.11.8020. [DOI] [PubMed] [Google Scholar]
- 22.Lorentzon M, Landin K, Mellstrom D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006;21:1871–1878. doi: 10.1359/jbmr.060814. [DOI] [PubMed] [Google Scholar]
- 23.Filip R, Raszewski G. Bone mineral density and bone turnover in relation to serum leptin, alpha-ketoglutarate and sex steroids in overweight and obese postmenopausal women. Clin Endocrinol (Oxf) 2009;70:214–220. doi: 10.1111/j.1365-2265.2008.03313.x. [DOI] [PubMed] [Google Scholar]
- 24.Jurimae J, Jurimae T. Influence of insulin-like growth factor-1 and leptin on bone mineral content in healthy premenopausal women. Exp Biol Med (Maywood) 2006;231:1673–1677. doi: 10.1177/153537020623101013. [DOI] [PubMed] [Google Scholar]
- 25.Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26:618–623. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact. 2008;8:94–104. [PubMed] [Google Scholar]
- 27.Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42:837–840. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Elefteriou F. Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci. 2005;62:2339–2349. doi: 10.1007/s00018-005-5175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gat-Yablonski G, Ben-Ari T, Shtaif B, Potievsky O, Moran O, Eshet R, Maor G, Segev Y, Phillip M. Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology. 2004;145:343–350. doi: 10.1210/en.2003-0910. [DOI] [PubMed] [Google Scholar]
- 30.Kishida Y, Hirao M, Tamai N, Nampei A, Fujimoto T, Nakase T, Shimizu N, Yoshikawa H, Myoui A. Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone. 2005;37:607–621. doi: 10.1016/j.bone.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Lorentzon R, Alehagen U, Boquist L. Osteopenia in mice with genetic diabetes. Diabetes Res Clin Pract. 1986;2:157–163. doi: 10.1016/s0168-8227(86)80017-1. [DOI] [PubMed] [Google Scholar]
- 32.Ealey KN, Fonseca D, Archer MC, Ward WE. Bone abnormalities in adolescent leptin-deficient mice. Regul Pept. 2006;136:9–13. doi: 10.1016/j.regpep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23:870–878. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- 34.Engelbregt MJ, van Weissenbruch MM, Lips P, van Lingen A, Roos JC, Delemarre-van de Waal HA. Body composition and bone measurements in intra-uterine growth retarded and early postnatally undernourished male and female rats at the age of 6 months: comparison with puberty. Bone. 2004;34:180–186. doi: 10.1016/j.bone.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Friedman SM, Gamba CA, Boyer PM, Guglielmotti MB, Vacas MI, Rodriguez PN, Guerrero C, Lifshitz F. Growth deceleration and bone metabolism in nutritional dwarfing rats. Int J Food Sci Nutr. 2001;52:225–233. doi: 10.1080/09637480120044129. [DOI] [PubMed] [Google Scholar]
- 36.Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149:634–641. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- 37.Boyer PM, Compagnucci GE, Olivera MI, Bozzini C, Roig MC, Compagnucci CV, Alippi RM. Bone status in an animal model of chronic sub-optimal nutrition: a morphometric, densitometric and mechanical study. Br J Nutr. 2005;93:663–669. doi: 10.1079/bjn20041331. [DOI] [PubMed] [Google Scholar]
- 38.Handler P, Baylin GJ, Follis RH., Jr The Effects of Caloric Restriction on Skeletal Growth: Four Figures. J Nutr. 1947;34:677–689. doi: 10.1093/jn/34.6.677. [DOI] [PubMed] [Google Scholar]
- 39.LaMothe JM, Hepple RT, Zernicke RF. Selected contribution: Bone adaptation with aging and long-term caloric restriction in Fischer 344 x Brown-Norway F1-hybrid rats. J Appl Physiol. 2003;95:1739–1745. doi: 10.1152/japplphysiol.00079.2003. [DOI] [PubMed] [Google Scholar]
- 40.Lambert J, Lamothe JM, Zernicke RF, Auer RN, Reimer RA. Dietary restriction does not adversely affect bone geometry and mechanics in rapidly growing male wistar rats. Pediatr Res. 2005;57:227–231. doi: 10.1203/01.PDR.0000148715.61869.4E. [DOI] [PubMed] [Google Scholar]
- 41.Berrigan D, Lavigne JA, Perkins SN, Nagy TR, Barrett JC, Hursting SD. Phenotypic effects of calorie restriction and insulin-like growth factor-1 treatment on body composition and bone mineral density of C57BL/6 mice: implications for cancer prevention. In Vivo. 2005;19:667–674. [PubMed] [Google Scholar]
- 42.Ferguson VL, Greenberg AR, Bateman TA, Ayers RA, Simske SJ. The effects of age and dietary restriction without nutritional supplementation on whole bone structural properties in C57BL/6J mice. Biomed Sci Instrum. 1999;35:85–91. [PubMed] [Google Scholar]
- 43.Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, Ferrari SL. Mice lacking beta-adrenergic receptors have increased bone mass, but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology (2008) Endocrinology. 2009;150:144–152. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG, Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 46.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 47.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19:905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 48.Martin A, de Vittoris R, David V, Moraes R, Bégeot M, Lafage-Proust MH, Alexandre C, Vico L, Thomas T. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology. 2005;146:3652–3659. doi: 10.1210/en.2004-1509. [DOI] [PubMed] [Google Scholar]
- 49.Baek K, Bloomfield SA. Beta-Adrenergic Blockade and Leptin Replacement Effectively Mitigate Disuse Bone Loss. J Bone Miner Res. 2008 Dec 29; doi: 10.1359/jbmr.081241. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res. 2004;19:1607–1611. doi: 10.1359/JBMR.040712. [DOI] [PubMed] [Google Scholar]
- 51.Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology. 2007;148:3419–3425. doi: 10.1210/en.2006-1541. [DOI] [PubMed] [Google Scholar]
- 52.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 53.Meisinger C, Heier M, Lang O, Doring A. Beta-blocker use and risk of fractures in men and women from the general population: the MONICA/KORA Augsburg cohort study. Osteoporos Int. 2007;18:1189–1195. doi: 10.1007/s00198-007-0354-8. [DOI] [PubMed] [Google Scholar]
- 54.Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC Geelong Osteoporosis Study. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:19–24. doi: 10.1359/JBMR.0301214. [DOI] [PubMed] [Google Scholar]
- 55.Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, Benhamou CL. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone. 2007;40:1209–1216. doi: 10.1016/j.bone.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB. Effects of a beta-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:5212–5216. doi: 10.1210/jc.2005-0573. [DOI] [PubMed] [Google Scholar]
- 57.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential Expression and Activation of a Family of Murine Peroxisome Proliferator-Activated Receptors. Proceedings of the National Academy of Sciences. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 59.Cock T-A, Houten SM, Auwerx J. Peroxisome proliferator-activated receptorgamma: too much of a good thing causes harm. EMBO reports. 2004;5:142–147. doi: 10.1038/sj.embor.7400082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagman J, Belanger C, Travis A, Turck CW, Grosschedl R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 1993;7:760–773. doi: 10.1101/gad.7.5.760. [DOI] [PubMed] [Google Scholar]
- 61.Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical Role for Ebf1and Ebf2 in the Adipogenic Transcriptional Cascade. Mol Cell Biol. 2007;27:743–757. doi: 10.1128/MCB.01557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elbrecht A, Chen Y, Cullinan C, Hayes N, Leibowitz M, Moller D, Berger J. Molecular cloning, expression and characterization of human peroxisome proliferators activated receptors gamma 1 and gamma 2. Biochem Biophys Res Commun. 1996;224:431–437. doi: 10.1006/bbrc.1996.1044. [DOI] [PubMed] [Google Scholar]
- 63.Gimble JM, Robinson CE, Wu X, Kelly K, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- 64.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 65.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik BJ. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. Cell Biochem. 2009 Feb 1;106(2):232–46. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung U-i, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007 Dec;13(12):1496–503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 68.Cock TA, Back J, Elefteriou F, Karsenty G, Kastner P, Chan S, Auwerx J. Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep. 2004 Oct;5(10):1007–12. doi: 10.1038/sj.embor.7400254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tornvig L, Mosekilde L, Justesen J, Falk E, Kassem M. Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int. 2001;69:46–50. doi: 10.1007/s002230020018. [DOI] [PubMed] [Google Scholar]
- 70.Aleo MD, Lundeen GR, Blackwell DK, Smith WM, Coleman GL, Stadnicki SW, Kluwe WM. Mechanism and Implications of Brown Adipose Tissue Proliferation in Rats and Monkeys Treated with the Thiazolidinedione Darglitazone, a Potent Peroxisome Proliferator-Activated Receptor-{gamma} Agonist. J Pharmacol Exp Ther. 2003;305:1173–1182. doi: 10.1124/jpet.102.042648. [DOI] [PubMed] [Google Scholar]
- 71.Li M, Pan LC, Simmons HA, Li Y, Healy DR, Robinson BS, Ke HZ, Brown TA. Surface-specific effects of a PPAR agonist, darglitazone, on bone in mice. Bone. 2006;39:796–806. doi: 10.1016/j.bone.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Lazarenko O, Rzonca S, Suva L, Lecka-Czernik B. Netoglitazone is a PPAR gamma ligand with selective effects on bone and fat. Bone. 2006;38:74–84. doi: 10.1016/j.bone.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain specific effects of Rosiglitazone on bone mass, body composition and serum IGF-I. Endocrinology. 2008;150:1330–1340. doi: 10.1210/en.2008-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerstein H, Yusuf S, Bosch J, Pogue J, Sheridan J, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman R. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 75.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 76.Krentz A, Bailey C. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR. Thiazolidinedione (TZD) use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor- agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 79.Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G Diabetes Outcome Progression Trial (ADOPT) Study Group. Rosiglitazone-associated fractures in type 2 diabetes: an analysis from ADOPT. Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 80.Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168:820–825. doi: 10.1001/archinte.168.8.820. [DOI] [PubMed] [Google Scholar]
- 81.Schwartz AV. TZDs and Bone: A Review of the Recent Clinical Evidence. PPAR Res. 2008;2008:297893. doi: 10.1155/2008/297893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosen CJ. Bone Remodeling, Energy Metabolism, and the Molecular. Clock Cell Metab. 2008;7:7–10. doi: 10.1016/j.cmet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91:3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 84.Melton LJ, 3rd, Riggs BL, Leibson CL, Achenbach SJ, Camp JJ, Bouxsein ML, Atkinson EJ, Robb RA, Khosla S. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab. 2008;93:4804–4809. doi: 10.1210/jc.2008-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lawson EA, Klibanski A. abnormalilies in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407–414. doi: 10.1038/ncpendmet0872. [DOI] [PubMed] [Google Scholar]
- 86.Rosen CJ, Klibanski A. Bone, Fat and Body Composition: Evolving Concepts in the Pathogenesis of Osteoporosis. Am J Med In press. 2009 doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 87.Cui Q, Wang GJ, Balian G. Pluripotential marrow cells produce adipocytes when transplanted into steroid-treated mice. Connect Tissue Res. 2000;41:45–56. doi: 10.3109/03008200009005641. [DOI] [PubMed] [Google Scholar]
- 88.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 89.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matriz Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 91.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr, Chua SC, Jr, Kim JK, Kaestner KH, Karsenty G. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 2006;4:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2753–2756. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2008 Dec 16; doi: 10.1210/jc.2008-1422. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:45–49. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- 98.Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellström D. Plasma Osteocalcin is Inversely Related to Fat Mass and Plasma Glucose in Elderly Swedish Men. J Bone Miner Res. 2008 Dec 8; doi: 10.1359/jbmr.081234. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 99.Delmas PD. Biochemical markers of bone turnover for the clinical investigation of osteoporosis. Osteoporos Int. 1993;3(Suppl 1):81–86. doi: 10.1007/BF01621873. [DOI] [PubMed] [Google Scholar]
- 100.Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- 101.Rosen CJ. Serotonin rising--the bone, brain, bowel connection. N Engl J Med. 2009 Mar 5;360(10):957–9. doi: 10.1056/NEJMp0810058. [DOI] [PubMed] [Google Scholar]
- 102.Rosen CJ, Ackert-Bicknell C, Rodriguez JP. Marrow Fat and the Bone Micro-environment: Developmental, Functional, and Pathological Implications. Critical Reviews in Eukaryotic Gene Expression. 2009 doi: 10.1615/critreveukargeneexpr.v19.i2.20. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. Hypoxic adipocytes pattern early heterotopic bone formation. AmJ Path. 2007 Feb;170(2):620–32. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]