Abstract
Purpose
The common change in corneal morphology that is seen in keratoconus (KC) suggests the presence of common high- order aberration (HOA) structures, and the potential for HOA corrections that apply to more than one eye. The purpose of this investigation is to classify a sample of KC eyes into sub-groups based on similar HOA characteristics and simulate optical performance of common HOA corrections on members of each sub-group.
Methods
HOA was recorded over a 5mm pupil on 111 KC eyes. The eyes were divided into 5 sub-groups based on observed commonality in HOA structure. From each sub-group, two eyes were removed for later evaluation of corrections. Principal components analysis was performed on the remaining eyes of each sub-group. The first principal component was multiplied by −1.00, and scaled in steps equivalent to 0.25D of sphere to form a set of corrections. Simulated optical correction of the test eyes was performed by identifying the magnitude of the inner-group correction providing the lowest level of residual higher order RMS wavefront error (HORMS). Residual uncorrected HORMS was compared to levels found in uncorrected normal eyes and KC eyes wearing rigid gas permeable (GP) corrections.
Results
Ninety of the 111 eyes (81%) were included in 1 of the 5 sub-groups. All 10 test eyes experienced a reduction in HORMS with a template correction compared to their uncorrected levels. Median HORMS reduced from uncorrected levels of 2.14μm to 0.97μm. On average, the 10 subjects experienced a 51% reduction in HORMS (min: 16%, max: 81%, p<0.01). When scaled to a 4mm pupil, 5 of the 10 eyes experienced residual uncorrected HORMS within limits associated with GP wear.
Conclusions
Overlap exists across these templates due to the dominance of vertical coma in the HOA structure. All eyes evaluated received reduced HORMS with a template-based correction.
Keywords: common optical correction, high-order aberration, keratoconus, Zernike
“Keratoconus is a clinical term used to describe a condition in which the cornea assumes a conical shape because of thinning and protrusion. The process is non-inflammatory. Cellular infiltration and vascularization do not occur. It is usually bilateral and, although it involves the central two-thirds of the cornea, the apex of the cornea is usually centered just below the visual axis. This disease process results in mild to marked impairment of visual function”1
The quotation above by Feder and Gan is found in ‘Cornea Fundamentals, Diagnosis and Management. Vol 1’, and is representative of descriptions of keratoconus (KC) found in the literature: notably that it is a disease leading to changes in corneal morphology (shape). The corneal first surface is responsible for approximately 2/3 of the refracting power of the eye2 and changes in corneal morphology result in changes in optical performance. The downward displacement of the corneal apex in KC described above is particularly devastating to retinal image quality, as it induces high-order aberration (HOA) that cannot be compensated with standard sphero-cylindrical corrections such as spectacles and soft contact lenses.
Several studies have examined uncorrected and corrected levels of HOA in KC. In the uncorrected eye, it has been shown that KC HOA was approximately 5.5 times higher than levels found in a normal population and that 53% of the HOA variance in the KC population can be accounted for by vertical coma (term ) alone.3 Even in the presence of the gold standard rigid gas permeable corrections (GPs) used by clinicians to correct optical error in KC, HOA levels remain elevated compared to normal, demonstrating that available methods of correction do not always provide the individual KC subject with normal levels of retinal image quality.4–6
Scarring accompanies disease progression in KC7 and correction of HOA will not compensate for visual deficits associated with scarring. So, to what degree is scarring visually significant in this population? Reporting scarring data by patient, Barr showed that 727 of 1086 KC patients (or 67%) in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study do not exhibit definite corneal scarring in the central 6mm of either eye.8 An additional 226 patients met the criteria for scarring in only 1 eye. Therefore, 87.8% of subjects in the CLEK cohort have at least one eye that is free of corneal scarring. When the same patients are examined by eye, Zadnik reported that 77.3% of all eyes in the CLEK study are free of corneal scarring.9 Scarring will reduce visual performance by inducing forward scatter, backscatter and absorption, all of which will alter the image forming properties of the eye. This fact, taken with the finding above that residual HOA remains elevated and VA reduced, strongly suggests that uncorrected optical aberration is a limiting factor in achieving normal retinal image quality in the majority of KC eyes. This is reflected in the visual performance results reported by the CLEK study, where best corrected high contrast logMAR VA reached 20/20 in only 34.6% of the unscarred eyes studied,9 whereas it has been shown that distance corrected VA in normal eyes is 20/16 well into the 6th decade of life.10
Evidence for commonality in HOA structure in the KC population exists in the literature. The field of disease detection is replete with examples of algorithms that differentiate normal from KC eyes based on common features related to corneal shape. Examples of these algorithms include anterior and posterior corneal elevation, anterior chamber depth, cone location and magnitude index (CLMI), corneal diameter, Fourier-incorporated keratoconus detection Index (FKI), inferior-superior (I-S) value, keratoconus severity index (KSI), keratometric astigmatism index (AST), keratometry (K) value, keratoconus percentage index KISA% index, mean curvature mapping, minimal corneal thickness, modified Rabinowitz-McDonnell test, placement of the apex, relative skewing of the steepest radial axes (SRAX), Sim K, steepest corneal curvature, vertical coma, vertical D and Z3 (combination of coma and trefoil).11–29 These algorithms seek to identify KC based on some common aspect of corneal shape or the presence of a specific optical signature.
In an effort to translate laboratory science into clinical practice, the authors hypothesize that aberration data may be used to define sub-groups within the KC population itself, and that the common HOA features of these sub-groups can be used to define common optical corrections that apply to the members of the group. In essence, this is the same concept that is used in prescribing sphero-cylindrical lenses to correct lower order aberration in normal eyes.
As a whole, the literature cited above demonstrates that 1) HOA is elevated and visual performance reduced in KC even when eyes are corrected with gold standard methods; 2) scarring is not the source of this visual deficit in the vast majority of KC eyes, rather uncorrected optical aberrations are a likely cause; and 3) common corneal morphology exists in this population. Based on these observations, it is hypothesized that a set of common HOA structures may exist in KC, and that these structures can be utilized to define common HOA corrections applicable to more than one individual. The purpose of this investigation is to classify a sample of KC eyes into KC sub-groups and simulate optical performance of common, scalable corrections.
METHODS
The study adhered to the tenets of the Declaration of Helsinki and received approval of the ethical committee of the Antwerp University Hospital and the institutional review board of the University of Houston. Signed informed consent was obtained from participating subjects prior to aberration measurement.
Wavefront Aberration Dataset for Keratoconic Eyes
All patients were referred to the Antwerp University Hospital by peripheral ophthalmologists for treatment of a clinically evident keratoconus. The diagnosis was confirmed by observing the typical changes obtained by both the Pentacam and a placido-based device: increased float of the posterior surface and localized thinning of the cornea; steepening of the cornea on the keratometric map of the anterior surface with skewing of the axes of astigmatism or with a localized area of steepening or with a crab claw pattern of peripheral keratoconus. Only clear cases of keratoconus were included in this analysis; keratoconus suspect cases were removed, as the diagnosis was not yet confirmed. Wavefront aberration data of 111 eyes of 60 keratoconus subjects were recorded over a 5mm pupil with a Tracey iTrace aberrometer (Tracey Technologies, Houston, Texas). One measure of wavefront error was included for each eye and reported over 8 orders of the Zernike polynomial. All right eye Zernike data were transformed to represent left eye Zernike data.31 Lower order aberration data was not included in this analysis. Fifty-one of the individuals had both eyes represented in the dataset and 9 of the individuals had a single eye represented. Distribution of the severity of eyes represented was 24 mild (21.6%), 55 moderate (49.5%) and 31 severe (27.9%), with 1 eye having unknown severity, as determined from the CLEK topographic criteria for keratoconus severity.30 Table 1 reports the severity of keratoconus for the eyes reported in this study.
Table 1.
Severity of disease for 110 eyes in the dataset used in this experiment (1 eye did not have data on disease severity). The Collaborative Longitudinal Evaluation of Keratoconus (CLEK) criteria for mild (steep K < 45D) moderate (45D <= Steep K < 52D) and severe (steep K <= 52D) disease was used to classify the severity of eyes.
| Count (%) | Severity | Average Steep K (D) |
|---|---|---|
| 24 (22) | Mild | 43.41 |
| 55 (50) | Moderate | 48.14 |
| 31 (28) | Severe | 57.29 |
Grouping Keratoconic Aberration Structures
A high-order aberration map representing Zernike orders 3 – 8 for each eye in the data set was generated with Visual Optics Laboratory 6.89 (Sarver and Associates, Carbondale, IL) and printed in color on 2″×3″ pieces of paper, forming a set of KC aberration cards shown in Figure1. An observer grouped the KC aberration cards by inspection, based on observed similarity in the cards. No a priori constraints (e.g., group size, number of groups) were placed on the grouping process. Table 2 reports the observed features of aberration cards that were identified by the observer and used to identify sub-groups. Notably, coma is a prominent feature of all sub-groups identified by this observer. Figure 2 shows a representative member of each subgroup, displaying the characteristics described in Table 2.
Figure 1.

Aberration cards used to define sub-groups: (A) Wavefront aberration cards are shown depicting the high-order aberration structures through the 8th radial order for a sample of 111 eyes with keratoconus. (B) A magnified section of Figure 1A, showing 7 of the 111 aberration cards.
Table 2.
Distinguishing characteristics of higher order aberration maps used by the observer to form 5 sub-groups. Coma is a prominent feature of all sub-groups, which is expected due to the fact that vertical coma is the dominant higher order aberration in keratoconus.3
| Sub-group | Qualitative grouping characteristics of the 5 sub-groups |
|---|---|
| 1 | coma structure, superior coma lobe blending into the temporal pupil border |
| 2 | coma with triangular, peaked superior lobe |
| 3 | pure coma, rotation allowed |
| 4 | superior coma lobe enveloping inferior coma lobe |
| 5 | horizontally striped coma-like pattern, from nasal to temporal pupil border |
Figure 2.

One representative member of each of the 5 sub-groups: The members of the sub-groups (SG) in Figure 2 provide an example of the characteristics used in the grouping process. These characteristics are reported in Table 1.
Twenty one (19%) of the eyes were placed into sub-groups that were small (< 5% of the total sample). When considered in a clinical context, the authors deemed these groups to be too small to justify the development of an entire off-the-shelf template correction set. Instead, the eyes in these small sub-groups were classified as unique and would require correction with an alternate (non-template based) method. Therefore, these 21 eyes were excluded from further analysis. The remaining 90 eyes (81%) grouped into 5 sub-groups (see ordered grouping numbers above) containing 26, 23, 11, 17, and 13 members. HOA structures for one sub-group (sub-group 3) are shown in Figure 3. The 21 excluded eyes originated from 20 subjects. The 90 eyes in the sub-groups originated from 60 subjects. Of the 60 individuals contributing to the subgroups, 13 had both eyes grouped into the same sub-group.
Figure 3.
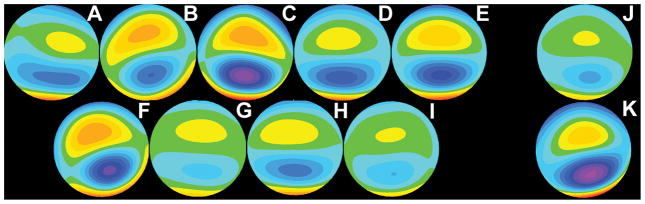
One sub-group of aberration structures: The 11 high-order aberration structures included in sub-group 3. Eyes A – I participated in the definition of the common aberration template. Eyes J & K were held in reserve for testing the common corrections under development. In Figure 3, all aberration maps have been placed on a common scale, such that each color step represents 0.1μm increment steps. Note that the aberration map in Figure 3G is the map representing group 3 in Figure 2.
Selecting Eyes from the Sample for Test of Template-based Corrections
Symmetry may exist in the fellow eyes of a single subject, and for this reason both eyes of a single subject were included when defining the templates. However, to eliminate potential bias in the application of the templates associated with symmetry within a subject, none of the test eyes had fellow eyes in the template-defining dataset.
Two eyes were removed from each of the 5 sub-groups (10 total) and held in reserve for later evaluation of the correcting templates. Six of the 10 test eyes were chosen from the individuals in the data set where data was only recorded on a single eye (meaning the eye chosen did not have a fellow eye in the data set). Three of the 10 test eyes were chosen from individuals where the fellow eye was one of the 21 eyes grouped into sub-groups with less than 5% and excluded from further analysis. The final test eye was chosen from an individual where both eyes fell into 1 of the 5 sub-groups. The fellow eye from this individual was excluded from further analysis. The process for identifying test eyes described above resulted in:
removal of single eyes from the dataset used to calculate correcting template corrections and
assurance that none of the fellow eyes of the 10 test eyes was included in the definition of the template corrections.
Principal Components Analysis - Definition of Scalable Template-based Corrections
The procedure described here was used to define a correcting template for each of the 5 subgroups defined above. Principal components analysis (PCA) was performed on the members of the sub-group (excluding eyes removed for later evaluation of corrections) using custom software written in Matlab R2009b (The Mathworks, Natick MA). PCA seeks to reduce the dimensionality of variables describing a dataset. The new, more efficient variables defined through PCA capture and capitalize on covariance of terms in the original dataset, making the new variables (principal components) more efficient at describing the underlying data. Here, the 1st principal component identified by the algorithm was chosen as a representative aberration template, reducing the number of variables describing the HOA to 1. To assure the template was a correcting template (opposite in sign from the members of the group), the sign of the vertical coma term of the template resulting from PCA was examined, and the entire template scaled by −1.00 if the sign was negative (mimicking the eyes). The remaining principal components were discarded. The correcting templates identified by this process are shown in Figure 4 below. The prominent positive vertical coma in all templates denotes that they are all designed to correct the negative vertical coma that has been reported in this disease.
Figure 4.
Scalable template-based corrections: Wavefront aberration maps are shown for the five scalable correcting templates. Each correcting template is transformed into a set of corrections by scaling the entire aberration structure in discrete magnitude steps. Template 3 is the correcting template for the eyes pictured in Figure 3.
Defining a Set of Corrections Based on the Correction Templates
Each correcting template shown in Figure 4 was used to define a set of corrections by applying a scaling factor ranging from 0.000 to 9.040 (The 0.000 condition is included as a reference, representing the uncorrected state). The scaling factor was increased in steps of 0.226. This level of scaling factor was chosen because the clinical increment used in scaling spectacle corrections is 0.25D of defocus ( ). Over a 5mm pupil, 0.25D of defocus is equivalent to 0.226μm of defocus. Therefore, the factor 0.226 was chosen as the unitless scaling factor applied to all terms defining the template. This scaling operation resulted in each template correction having up to 41 unique magnitudes. Figure 5 shows 5 scaled corrections for the correcting template calculated for sub-group 1.
Figure 5.

Scaled template corrections for template 1: Note the similarity in the HOA structure exhibited in the aberration structures in Figures 5A – 5E. The only difference across these 5 aberration structures is the overall magnitude, relative amounts of individual Zernike terms remains constant.
Simulating Correction of Test Eyes with Template-based Corrections
Test eyes were removed from the sub-groups after the sub-grouping process was complete. Therefore, each test eye was associated with a specific sub-group. Optical correction of each test eye was simulated by mathematically adding the series of correcting templates defined for the sub-group to the test eye, which resulted in a set of residual aberrations not corrected by the template. The scaled correcting template resulting in the lowest level of HORMS was then identified for each of the 10 test eyes. To further assess the efficacy of the template corrections, the test eyes were also corrected with the remaining four template series, and a template defined by vertical coma. The lowest achievable HORMS level for these two conditions was compared to the level achieved with the template defined for the subset.
RESULTS
HORMS Reduction with Template-based Correction for 10 Test Eyes
Figure 6 shows the reduction in HORMS achieved for the two test eyes in each of the 5 subgroups. In all cases, HORMS is reduced from uncorrected levels. Median HORMS reduced from uncorrected levels of 2.14μm to 0.97μm. On average, the 10 subjects experienced a 51% ± 22% reduction in HORMS (p < 0.01).
Figure 6.
(A–E) Reduction of HORMS for the 10 test eyes as a function of template magnitude: simulation of HORMS reduction as a function of template scaling magnitude was performed for ten eyes (two per template). A scaling factor of 0.00 represents zero amplitude to the correction, or no correction, and reflects the uncorrected HORMS for the test eye. The scaling factor was increased in unitless steps of 0.226.
Comparing Resultant HORMS to Clinical Benchmarks
The 5mm data were rescaled to 4mm data using ZerShifter v1.2 (Sarver and Associates, Carbondale IL). Here 4mm data was chosen for comparison because comparative data is available in the literature for both GP-corrected KC eyes and normal eyes and is a reasonable estimate of mean pupil diameter under photopic conditions. Figure 7 displays an example of the application of a scaled template correction to test eyes. None of the 10 test eyes achieved levels of HORMS within normal limits. However, 5 of the 10 eyes experienced residual uncorrected HORMS at levels within limits associated with gold standard GP wear (0.31μm ± 0.14μm). These results are shown in Figure 8.
Figure 7.
Examples of the application of a scaled template correction to a test eye: Figure 7A – 7C shows the results of a template correction using template 4 scaled by a factor of 2.712, resulting in a 53% reduction in HORMS from 3.08μm to 1.45μm (one of the highest levels of residual HORMS experienced). Figure 7D – 7F shows a template correction using template 5 scaled by a factor of 1.356, resulting in a 67% reduction in HORMS from 1.35μm to 0.45μm (one of the lowest levels of residual HORMS experienced).
Figure 8.
Residual HORMS (4mm) for 10 test eyes compared to 2 clinical benchmarks: Five of the 10 test eyes achieve HORMS levels consistent with levels seen in normal (mean_2SD) and KC GP wear (mean+2SD).4–6 Inset percentages reflect the amount of HORMS reduction achieved. Numbers above each bar represent the assigned subgroup. [note that this is the only data reported at 4mm pupil size]
The above analysis was conducted for a set of 5 sub-groups. However, as discussed above, commonality is observed across the correcting templates, which can be observed in Figure 4. Depending on the underlying aberration structure of a given eye, it is possible that an eye may fit equally well into more than one sub-group, which suggests that eyes may be well treated with corrections defined from other sub-groups. Table 3 reports the lowest HORMS for each test eye after correction with the remaining four templates and with a template that completely removes vertical coma over a 5mm pupil. In 6 of the 10 cases, maximal HORMS reduction occurs for a correction from outside the assigned sub-group. Examination of Table 3 shows that maximal HORMS reduction could be achieved in 9 of the 10 test eyes with 3 correction templates (sub-groups 1,3 and 5).
Table 3.
5mm Residual HORMS achieved for all templates applied to all test eyes. Residual HORMS is reported for each of the 10 test eyes (2 per sub-group) studied in this experiment. The grey diagonal highlights the residual HORMS achieved for the assigned template (test eye assigned to sub-group being corrected by sub-group 1 template). In 6 of the 10, maximal HORMS reduction occurs for a correction from outside the assigned sub-group. Bold numbers identify the lowest level of HORMS achieved for each test eye.
| Assigned sub-group | Test Eye | Residual HORMS (μm) Correction Defined From…
|
|||||
|---|---|---|---|---|---|---|---|
| Group 1 template | Group 2 template | Group 3 template | Group 4 template | Group 5 template | Vertical coma | ||
| 1 | 11 | 1.675 | 1.606 | 0.968 | 1.383 | 1.576 | 1.138 |
| 45 | 0.737 | 1.309 | 1.652 | 1.435 | 0.542 | 1.360 | |
|
| |||||||
| 2 | 23 | 1.556 | 1.359 | 0.956 | 1.316 | 1.579 | 1.218 |
| 67 | 1.036 | 0.938 | 0.799 | 0.964 | 1.039 | 0.917 | |
|
| |||||||
| 3 | 91 | 0.512 | 0.516 | 0.427 | 0.522 | 0.430 | 0.453 |
| 108 | 1.391 | 1.358 | 1.122 | 1.777 | 1.595 | 1.378 | |
|
| |||||||
| 4 | 1 | 0.763 | 0.953 | 0.997 | 1.008 | 1.039 | 0.951 |
| 48 | 0.878 | 1.339 | 1.506 | 1.453 | 1.262 | 1.449 | |
|
| |||||||
| 5 | 35 | 0.551 | 0.756 | 0.987 | 0.844 | 0.445 | 0.859 |
| 56 | 1.029 | 1.654 | 1.882 | 1.324 | 0.597 | 1.362 | |
DISCUSSION
Methods for Improving Definition Of Sub-groups
In this initial experiment examining common correction strategies for HOA in KC eyes, subgroups were defined by inspection, which is qualitative, subjective and grader-dependent. This can be seen in Table 4, where additional statistics are presented for 7 additional graders asked to perform the grouping task. Graders provided between 3 and 7 sub-groups for the 111 eyes in the dataset, demonstrating that different graders are defining groups based on different qualities of the HOA maps. This also supports the observation that some eyes may fit well into several groups, based on commonality in the aberration structure.
Table 4.
Sub-groups defined subjectively by several graders. Eight different sub-groupings by 8 different groupers are reported. On average, 4 out of 5 eyes KC eyes is included in a sub-group. Sub-groupings used in the analysis presented in the results section of this experiment are indicated with an asterisk.
| Grader | Sub-groups | # eyes in each sub-group (by descending size) | % of eyes in a sub-group |
|---|---|---|---|
| 1 | 3 | 30, 29, 24 | 74.7 |
| 2 | 3 | 38, 36, 18 | 82.9 |
| 3 | 3 | 48, 26, 21 | 85.6 |
| 4* | 5 | 26, 23, 17, 13, 11 | 81.1 |
| 5 | 6 | 21, 18, 15, 14, 13, 13 | 84.7 |
| 6 | 6 | 24, 18, 17, 14, 12, 11 | 86.5 |
| 7 | 7 | 21, 19, 14, 8, 7, 6, 6 | 72.9 |
| 8 | 7 | 23, 16, 15, 14, 9, 7, 6 | 81.1 |
The number of groups remains relatively small across all graders and the number of eyes included in the groups encompasses between 73 and 87% of the eyes in the sample. On average, 81% of eyes in the sample (or 4 out of 5 eyes) fell into one of the sub-groups, regardless of the grader forming the groups.
Objective methods for the definition of templates based on cluster analysis are now under investigation. These objectively defined groups will be compared to the subjective groupings in Table 4 to assess the qualities of the KC HOA maps that are used in group definition under both subjective and objective division.
Uniqueness of the Sub-groups
The fact that maximal HORMS reduction occurs for sub-group 3 in 5 of the eyes, which is a group defined largely by the presence of coma, suggests that coma in-and-of itself could be a viable correction template. However, the fact that none of the test eyes achieved maximal HORMS reduction when vertical coma was the sole aberration corrected suggests that the other, non- vertical coma components of sub-group 3 may be beneficial in reducing HOA. A scaled coma template that is allowed to rotate (combining both vertical and horizontal coma) may be an efficacious template. Table 4 shows that maximal HORMS reduction could be achieved in 9 of the 10 test eyes with 3 correction templates (sub-groups 1,3 and 5). This does not necessarily imply that sub-groups 2 and 4 are not efficacious groupings, simply that the test eyes for these groups are equally well classified in one of the other existing sub-groups.
Use of Principal Components Analysis to Define the Template
Here PCA is used to define the template corrections under study. The choice of PCA was predicated on the use of this method by other vision scientists studying the efficiency of the Zernike polynomial for describing aberration in the eye of normal subjects.32 Using PCA, Porter et. al. reported that the Zernike coefficients were an efficient method for describing a sample of data collected on normal eyes. In the present study, we employed the additional step of subjectively grouping the eyes before applying the PCA. In doing so, the templates defined for the sub-groups actually represent common aberrations for the group, not across the entire KC population. Additional methods for defining templates may prove equally or more efficacious, including use of more than 1 principal component and simple averaging of the aberration terms across subjects in the sub-groups.
Visual Performance vs. Optical Quality
Here, RMS is used as a performance metric in these initial investigations of template-based correction, even though it is not the metric best correlated with resulting visual performance at low levels. One demonstration of this fact was reported by Applegate et. al., who constructed four sets of two aberration terms, keeping the overall RMS level constant at 0.25μm.33 The results showed that while all conditions led to reduced visual performance compared to perfect correction, different combinations led to different levels of visual acuity loss. In the current experiment, no attempt is made to establish resulting visual performance with the template in the eyes studied. As a subject would attempt to use lower order sphere and cylinder corrections to provide best retinal image quality, and since the data here represent only higher order aberrations, this data is not amenable to predicting resultant visual performance or change in visual performance in the presence of the corrections. Future studies will examine HOA compensating templates integrated with lower order corrections. Certainly, integration of HOA templates with lower order corrections and evaluation of the resultant visual performance is a requisite if the corrections are to obtain clinical relevance.
Challenges Associated with Clinical Deployment of the Template Method
The investigators hypothesize that it may be possible to deliver correction with this template method in the modality of a soft contact lens. However, challenges exist in terms of delivery of the template method in this manner. Notably, mis-registration of the geometric center of the lens relative to the pupil center, rotation of the lens, temporal instability of the correction as the lens moves on the eye and flexure of the correction with regard to the pupil will further reduce the efficacy of the individual on-eye corrections reported here. It may be useful to define a pupil offset for use in shifting the correcting optics placement within a sub-group. Table 4 shows that roughly 1 in 5 KC eyes would be excluded from this correction strategy. These limitations assure that clinical deployment of a template method would, at best, complement existing forms of correction.
While it is true that the template method will, by design, never provide a full HOA correction to any given individual, the method has the distinct advantage that it is already familiar to the clinician. Prescription of sphere and sphero-cylindrical corrections in the clinic today operate on a template-based method, where discrete steps of defocus and astigmatism are applied in a lens. Therefore, the template application of HOA compensation is an extension of what the contact lens practitioner already knows and does well. The method may extend the clinicians capability to mitigate HOA without the need to learn a wholly new correction philosophy. However, clinical deployment would require development of methods for prescribing the correct template to an individual, which was not discussed here.
The authors hypothesize that there are two main benefits of the scalable correction. First, a single lens is common to more than one eye, where the ‘measure and correct’ strategy proposed for HOA compensation in custom wavefront guided soft contact lenses can only be effectively applied to a single individual eye. It is true that that the scaled template will not correct any eye perfectly, but the authors feel this disadvantage is outweighed by the potential to provide the scalable corrections off-the-shelf. Second, if this method could be integrated into soft contact lenses, they may provide longer wear time and improved comfort than current rigid corrections. These two benefits may, given individual patient preference, serve to offset any loss in visual performance associated with a general template correction. The proposed method provides another tool at the disposal of clinicians in providing a solution to the patient with KC.
How many groups are needed to translate this method to the clinic? This will be based on individual patient satisfaction with the available template lenses. It could be that practitioners using the template method find that 2 templates are sufficient, or that additional templates to those reported here are needed. Some patients may not accept the degraded image quality associated with templates, and would require a fully custom lens. In the end, the number of templates truly depends on the level of correction provided and the patient’s satisfaction with the correction. For this reason, the authors have reported quantitative levels of optical performance, and do not report a definitive set of templates ready for industry adoption.
The dataset employed here was composed of single measures on 111 eyes over a 5mm pupil, which is an excellent dataset for use in evaluating the feasibility of template-based correction. In order to integrate optical corrections defined from templates into testable laboratory corrections, multiple measures over larger, dilated pupils are planned to account for inter-measure variation and larger patient pupil sizes.
Future directions for this work include evaluating the efficacy of the template-based correction for improving visual performance in the laboratory. This can be done by implementing phase plates and using these plates to correct HORMS in KC eyes, through the implementation of a psychophysical AO system, through aberrated chart experiments or through actual implementation of template-based soft contact lenses.
Acknowledgments
Funding: NEI-NIH R01 EY019105 – PI: Raymond A. Applegate, NEI-NIH P30 EY007551 – Core Grant PI: Laura J. Frishman PhD, and IWT-110684 – PI: Jos Rozema.
The authors thank the members of the VOI (James Elswick, Ayeswarya Ravikumar, Chi Nguyen, Anita Ticak and Shi Yue) for input on the project and Ed and Charlene Sarver at Sarver and Associates for use of Visual Optics Laboratory and Zer Shifter programs.
Footnotes
Commercial Interest: patent application on template correction concept: JDM and RAA
Presentations: Association for Research in Vision and Ophthalmology, May 8th 2012, Ft. Lauderdale, FL.
References
- 1.Feder RS, Gan TJ. Noninflammatory ectatic disorders. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. 3. Vol. 1. St. Louis: Mosby/Elsevier; 2011. pp. 865–78. [Google Scholar]
- 2.Smith G, Atchison DA. The Eye and Visual Optical Instruments. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 3.Pantanelli S, MacRae S, Jeong TM, Yoon G. Characterizing the wave aberration in eyes with keratoconus or penetrating keratoplasty using a high-dynamic range wavefront sensor. Ophthalmology. 2007;114:2013–21. doi: 10.1016/j.ophtha.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Kosaki R, Maeda N, Bessho K, Hori Y, Nishida K, Suzaki A, Hirohara Y, Mihashi T, Fujikado T, Tano Y. Magnitude and orientation of Zernike terms in patients with keratoconus. Invest Ophthalmol Vis Sci. 2007;48:3062–8. doi: 10.1167/iovs.06-1285. [DOI] [PubMed] [Google Scholar]
- 5.Marsack JD, Parker KE, Pesudovs K, Donnelly WJ, 3rd, Applegate RA. Uncorrected wavefront error and visual performance during RGP wear in keratoconus. Optom Vis Sci. 2007;84:463–70. doi: 10.1097/OPX.0b013e31802e64f0. [DOI] [PubMed] [Google Scholar]
- 6.Negishi K, Kumanomido T, Utsumi Y, Tsubota K. Effect of higher-order aberrations on visual function in keratoconic eyes with a rigid gas permeable contact lens. Am J Ophthalmol. 2007;144:924–9. doi: 10.1016/j.ajo.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Zadnik K, Barr JT, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea. 1996;15:139–46. doi: 10.1097/00003226-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Barr JT, Zadnik K, Wilson BS, Edrington TB, Everett DF, Fink BA, Shovlin JP, Weissman BA, Siegmund K, Gordon MO. Factors associated with corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2000;19:501–7. doi: 10.1097/00003226-200007000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Zadnik K, Barr JT, Edrington TB, Nichols JJ, Wilson BS, Siegmund K, Gordon MO. Corneal scarring and vision in keratoconus: a baseline report from the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2000;19:804–12. doi: 10.1097/00003226-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Elliott DB, Yang KC, Whitaker D. Visual acuity changes throughout adulthood in normal, healthy eyes: seeing beyond 6/6. Optom Vis Sci. 1995;72:186–91. doi: 10.1097/00006324-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Rabinowitz YS, McDonnell PJ. Computer-assisted corneal topography in keratoconus. Refract Corneal Surg. 1989;5:400–8. [PubMed] [Google Scholar]
- 12.Maeda N, Klyce SD, Smolek MK. Comparison of methods for detecting keratoconus using videokeratography. Arch Ophthalmol. 1995;113:870–4. doi: 10.1001/archopht.1995.01100070044023. [DOI] [PubMed] [Google Scholar]
- 13.Schwiegerling J, Greivenkamp JE. Keratoconus detection based on videokeratoscopic height data. Optom Vis Sci. 1996;73:721–8. doi: 10.1097/00006324-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Smolek MK, Klyce SD. Current keratoconus detection methods compared with a neural network approach. Invest Ophthalmol Vis Sci. 1997;38:2290–9. [PubMed] [Google Scholar]
- 15.Dastjerdi MH, Hashemi H. A quantitative corneal topography index for detection of keratoconus. J Refract Surg. 1998;14:427–36. doi: 10.3928/1081-597X-19980701-09. [DOI] [PubMed] [Google Scholar]
- 16.Accardo PA, Pensiero S. Neural network-based system for early keratoconus detection from corneal topography. J Biomed Inform. 2002;35:151–9. doi: 10.1016/s1532-0464(02)00513-0. [DOI] [PubMed] [Google Scholar]
- 17.Arntz A, Duran JA, Pijoan JI. Subclinical keratoconus diagnosis by elevation topography. Arch Soc Esp Oftalmol. 2003;78:659–64. [PubMed] [Google Scholar]
- 18.Gobbe M, Guillon M. Corneal wavefront aberration measurements to detect keratoconus patients. Cont Lens Anterior Eye. 2005;28:57–66. doi: 10.1016/j.clae.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Tang M, Shekhar R, Huang D. Mean curvature mapping for detection of corneal shape abnormality. IEEE Trans Med Imaging. 2005;24:424–8. doi: 10.1109/tmi.2004.843192. [DOI] [PubMed] [Google Scholar]
- 20.Twa MD, Parthasarathy S, Roberts C, Mahmoud AM, Raasch TW, Bullimore MA. Automated decision tree classification of corneal shape. Optom Vis Sci. 2005;82:1038–46. doi: 10.1097/01.opx.0000192350.01045.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessho K, Maeda N, Kuroda T, Fujikado T, Tano Y, Oshika T. Automated keratoconus detection using height data of anterior and posterior corneal surfaces. Jpn J Ophthalmol. 2006;50:409–16. doi: 10.1007/s10384-006-0349-6. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud AM, Roberts C, Lembach R, Herderick EE, McMahon TT. Simulation of machine-specific topographic indices for use across platforms. Optom Vis Sci. 2006;83:682–93. doi: 10.1097/01.opx.0000232944.91587.02. [DOI] [PubMed] [Google Scholar]
- 23.Alió JL, Shabayek MH. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006;22:539–45. doi: 10.3928/1081-597X-20060601-05. [DOI] [PubMed] [Google Scholar]
- 24.Abad JC, Rubinfeld RS, Del Valle M, Belin MW, Kurstin JM. Vertical D: a novel topographic pattern in some keratoconus suspects. Ophthalmology. 2007;114:1020–6. doi: 10.1016/j.ophtha.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoud AM, Roberts CJ, Lembach RG, Twa MD, Herderick EE, McMahon TT CLEK Study Group. CLMI: the cone location and magnitude index. Cornea. 2008;27:480–7. doi: 10.1097/ICO.0b013e31816485d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holladay JT. Keratoconus detection using corneal topography. J Refract Surg. 2009;25:S958–62. doi: 10.3928/1081597X-20090915-11. [DOI] [PubMed] [Google Scholar]
- 27.Souza MB, Medeiros FW, Souza DB, Garcia R, Alves MR. Evaluation of machine learning classifiers in keratoconus detection from Orbscan II examinations. Clinics (Sao Paulo) 2010;65:1223–8. doi: 10.1590/S1807-59322010001200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bühren J, Kook D, Yoon G, Kohnen T. Detection of subclinical keratoconus by using corneal anterior and posterior surface aberrations and thickness spatial profiles. Invest Ophthalmol Vis Sci. 2010;51:3424–32. doi: 10.1167/iovs.09-4960. [DOI] [PubMed] [Google Scholar]
- 29.Bessho K, Maeda N, Kuroda T, Fujikado T, Tano Y, Oshika T. Automated keratoconus detection using height data of anterior and posterior corneal surfaces. Jpn J Ophthalmol. 2006;50:409–16. doi: 10.1007/s10384-006-0349-6. [DOI] [PubMed] [Google Scholar]
- 30.Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, Shin JA, Sterling JL, Wagner H, Gordon MO. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. [PubMed] [Google Scholar]
- 31.Thibos LN, Applegate RA, Schwiegerling JT, Webb R. Standards for reporting the optical aberrations of eyes. J Refract Surg. 2002;18:S652–60. doi: 10.3928/1081-597X-20020901-30. [DOI] [PubMed] [Google Scholar]
- 32.Porter J, Guirao A, Cox IG, Williams DR. Monochromatic aberrations of the human eye in a large population. J Opt Soc Am (A) 2001;18:1793–803. doi: 10.1364/josaa.18.001793. [DOI] [PubMed] [Google Scholar]
- 33.Applegate RA, Marsack JD, Ramos R, Sarver EJ. Interaction between aberrations to improve or reduce visual performance. J Cataract Refract Surg. 2003;29:1487–95. doi: 10.1016/s0886-3350(03)00334-1. [DOI] [PubMed] [Google Scholar]






