Abstract
Restless Legs Syndrome (RLS) is a prevalent sleep-associated movement disorder greatly affecting patients’ quality of life (QoL). Several drugs can be used to control this condition although the first-line dopamine agents often cause adverse effects. Non-dopaminergic drugs such as oral gabapentin (GBP) have been more recently advocated. Despite ameliorating RLS symptoms, GBP’s pharmacokinetic limitations restrict its overall effectiveness. A novel specifically designed prodrug, gabapentin enacarbil (GE), has demonstrated successful RLS alleviation with a superior pharmacokinetic profile. This review aims to examine the efficacy and tolerability of both GBP and GE as pharmacotherapy for RLS. Despite some heterogeneity and limitations across research methodologies, GE appears to be a potential RLS therapy superior to GBP and other dopaminergic agents.
Keywords: restless legs syndrome, pharmacotherapy, gabapentin, gabapentin enacarbil
Introduction
Restless Legs Syndrome (RLS) is a prevalent, disabling sleep-associated movement disorder requiring long-term drug therapy. Approximately 2%–3% of the US population encounters symptoms clinically troublesome enough to require pharmacotherapy. It is recognized by a desire to move the legs during immobility to alleviate leg uneasiness. RLS typically reduces during motion and worsens throughout the evening. The arms (although less frequently) may also be involved. Overall quality of sleep, life and everyday activity can noticeably be affected as a result of leg uneasiness. Various pharmacotherapies are utilized, including dopaminergic drugs (levodopa), dopamine agonists (ropinirole) and non- dopaminergic medications such as Gabapentin (GBP) Somnolence, tiredness, sickness and dizziness are the most frequent typically encountered adverse-effects. DA agonists are the first-line treatments however often induce augmentation. Consequently symptoms are more severe and commence earlier. They have also been associated with leg swelling, sickness and dizziness. Alternative medications are therefore required. GBP has been effective for RLS sufferers however its use remains questionable due to an unknown underlying mechanism of action and several pharmacokinetic limitations, including an unpredictable bioavailability and limited half-life. The specifically manufactured prodrug Gabapentin Enacarbil (GE) possesses enhanced pharmacokinetics which overcome GBP’s limitations, possibly enabling more efficient RLS treatment.1 Research evaluating the efficacy alongside tolerability of GBP and GE will be presented, methodological quality examined and a conclusion established regarding GE’s place in practice.
Current Pharmacotherapy: Dopaminergic Agents
Dopaminergic agents, such as ropinirole and pramipexole, have demonstrated good efficacy in treating RLS. However, these drugs fail to enhance sleep architecture and approximately 30% of sufferers complain of symptom exacerbation. They are also associated with the unpleasant side-effect of augmentation. As a result, symptoms can be more severe, present earlier during the daytime and throughout upper limbs. This side-effect has been found to occur in up to 80% of sufferers given levodopa or carbidopa and approximately 30%–50% of individuals receiving DA agonists. Vomiting, reduced impulse monitoring, pathological gambling, hallucinations, tiredness, postural hypotension, dyskinesia, dizziness alongside Parkinsonism exacerbation are additional adverse effects.2 Due to these complications alternative nondopaminergic pharmacotherapies such as GBP and GE have been investigated. Table 1 shows the comparision of the side-effect profiles of DA agents, GBP and GE.
Table 1.
Comparision of the side-effect profiles of dopamine agonist (DA) agents, gabapentin (GBP) and gabapentin enacarbil (GE).
| DA agents | GBP | GE | |
|---|---|---|---|
| Side-effects |
|
|
|
Gabapentin
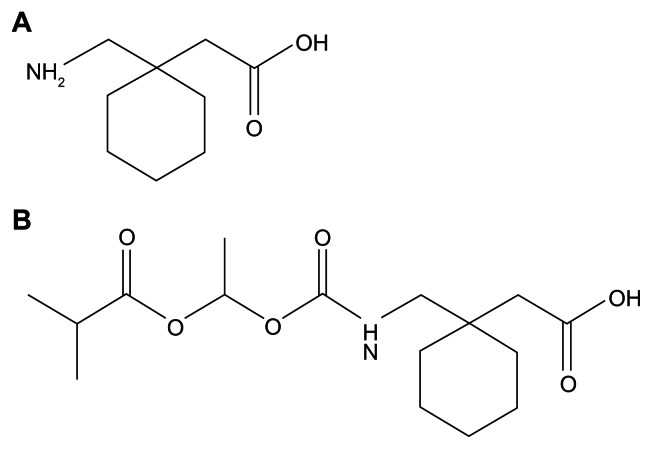
GBP is structurally analogous to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) (Fig. 1A). It is an effective anticonvulsant and analgesic however only minimally ameliorates RLS due to several pharmacokinetic limitations. Absorption occurs via a low-capacity solute transporter (most likely an L-type amino acid transporter) localized to the upper smaller intestine.1 This saturates as therapeutic quantities are delivered. Consequently dose-proportionality does not occur. Drug bioavailability is reduced and continues to lessen as doses are increased. GBP is quickly excreted unaltered by the kidneys within the urine possessing a half-life ranging between five and seven hours.1 A limited half-life alongside an unpredictable bioavailability restricts use.3 Regular dosing is thus required to maintain beneficial concentrations. This can result in patient noncompliance and missing dosages reducing clinical efficiency. Despite slightly alleviating RLS symptoms, GBP is not a Food and Drug Administration (FDA) accepted pharmacotherapy however is utilized off-label for this condition.1
Figure 1.
Chemical structure of Gabapentin (A) and Gabapentin Enacarbil (B).
Adler4 administered open-label GBP (average 1163 mg daily) to eight idiopathic RLS sufferers for seven days. Four responded favourably and three others experienced nearly full symptom eradication continuing for six months. Dizziness, sickness and sedation were predominantly encountered resulting in two patients withdrawing from the trial. GBP was thus a tolerated pharmacotherapy illustrating a lasting efficacy.
A one-month open-label pilot study5 investigated GBP efficacy and tolerance amongst nine idiopathic RLS sufferers. Patients initially received GBP (300 mg) which was titrated upwards until symptoms alleviated. Eight sufferers experienced noticeable subjective amelioration of sensorimotor features, sleep quality alongside daytime somnolence. Significant improvement in Periodic Leg Movement during Sleep (PLMS) also occurred. One sufferer who did not report symptom relief experienced depression. For this individual GBP was raised to 3600 mg without effect. Treatment effects on sleep efficiency, overall sleep duration, latency alongside length of slow wave sleep were not significant. Despite RLS impairing Quality of Life (QoL), patients’ initial sleep dysfunction was minor. Significant effects upon sleep could therefore be hard to uncover. No severe adverse events were recorded. The open design and restricted sample size could explain the lack of significant drug effect.
Thorp et al6 performed a double-blind, randomized, placebo-controlled crossover trial administering GBP (200–300 mg) or placebo to 16 diagnosed RLS sufferers receiving haemodialysis. A seven day washout interval was included. Withdrawal occurred in two cases due to tiredness considered to be GBP-related. 11 sufferers were responders to GBP over placebo. One individual reacted to both drugs and another only to placebo.
Garcia-Borreguero et al7 conducted a 6-week randomized, double-blind crossover trial exploring GBP efficacy amongst 22 idiopathic RLS sufferers and two patients with secondary RLS from iron deficiency. GBP or a placebo was administered for six weeks followed by a seven day washout interval. Participants then received the other condition for a further six weeks. GBP was found to ameliorate RLS. Treatment effectiveness was stronger for individuals experiencing greater symptom intensity. Those generating raised Pain Analogue Scale (PAS) ratings received the greatest advantage from GBP. This cohort could therefore represent a population of more likely responders. Effects were not limited to pain alleviation as those who did not experience pain also improved. PLMS, overall sleep duration, sleep quality alongside slow wave sleep improved. Stage 1 sleep also reduced. Sleep latency did not vary between GBP and placebo implying results on sleep could arise from PLMS alleviation compared to sleep-initiating effects. Sedation and headache relating to pharmacotherapy were the predominant side-effects. Groups did not significantly differ in side-effect prevalence and none resulted in medication being stopped. Approximately 50 to 82% of RLS sufferers receiving levodopa pharmacotherapy encounter augmentation,8 ie, escalation and anticipation of symptoms.2 Augmentation was unobserved with GBP, thus suggesting a selective link with dopamine therapy. However this phenomenon may require weeks or months to become evident therefore could have been overlooked in this short trial. Doses were mainly administered at night possibly explaining lack of daytime somnolence.7
Happe et al9 explored the efficacy alongside tolerability of open-GBP (300 mg) compared to ropinirole (0.5 mg) for one month in 16 idiopathic RLS sufferers. Drug quantities were up-titrated until RLS alleviation became apparent. Both agents produced significant improvements in International Restless Legs Syndrome (IRLS) Study Group questionnaire ratings and Epworth Sleepiness Scale (ESS) measures stayed stable. Polysomnography illustrated PLMS decreased with both drugs. Furthermore, for the majority, RLS amelioration continued for six to 10 months. Ropinirole-treated individuals experienced lighter sleep, reduced deep sleep, less REM sleep, diminished sleep efficiency and reduced overall sleep. At 6 to 10 month follow-up three ropinirole-treated individuals had remained on this drug whereas every GBP-treated patient had maintained GBP. This implies GBP may be better tolerated and more effective in the long-term. Minor and temporary side-effects occurred for both drugs. Despite the open-labeled design and restricted sample size, this study was the first to directly compare the effectiveness of ropinirole and GBP in treating RLS. The two medications demonstrated similar efficacy and tolerability in treating idiopathic RLS symptomatology.
RLS frequently occurs amongst individuals receiving haemodialysis leading to higher rates of morbidity and mortality. Micozkadioglu et al10 compared the therapeutic efficacy of levodopa (125 mg daily) and GBP (200 mg daily) amongst 15 haemodialysis patients identified as suffering RLS in an open-label trial. A 14 day washout interval was included. Significant improvements in overall health, pain and social functioning occurred for GBP. It demonstrated significant superiority over levodopa in sleep quality, latency alongside disruption. Sufferers were also more satisfied with GBP in relation to symptom amelioration. Despite levodopa increasing patient satisfaction, complaints resurfaced within hours of treatment. Drugs did not significantly differ in adverse-effects. Both were effective in improving sleep quality, latency and length although levodopa was viewed by sufferers to have a shorter duration of action. RLS features reappeared once pharmacotherapy ceased.
GBP possesses several drawbacks limiting its clinical effectiveness. Firstly, it has a short half-life requiring regular dosing. Additionally, for some patients, the therapeutic benefits might not continue during night time. Due to an absence of colonic absorption, extended-release formulations cannot be manufactured. Absorption through a restricted dispersal of lower capacity transporters produces a bioavailability which is reliant upon dose. Consequently, GBP generates more unpredictable serum plasma levels. Finally, he availability of the transporter involved in GBP absorption significantly differs amongst patients, resulting in between-patient variations in plasma GBP levels and clinical responsiveness.2 These limitations stimulated a focus on a prodrug of GBP, GE.
Gabapentin Enacarbil
GE is a specifically designed prodrug of GBP intended to overcome GBP’s pharmacokinetic limitations (Fig. 1B). This acyloxyalkylcarbamate analog is effectively transformed by non-specific esterases within tissue to GBP. Although the underlying mechanism of action remains unknown GE can be considered a prodrug of GBP. It is an identified substrate of two higher-capacity nutrient transporters: monocarboxylate transporter type I (MCT-1) and sodium-dependent multivitamin transporter (SMVT). Both are widely distributed along the entire human intestinal tract thus increase bioavailability.1 This greater transportation potential enables larger quantities of GE to be distributed within efficient dose proportions preventing saturation from occurring at therapeutic levels. It is administered orally either as an immediate-release (IR) or extended-release (XR) preparation. Hydrolysis generates one GBP molecule, one carbon dioxide molecule, acetaldehyde alongside isobutyric acid, considered secure Generally Recognized As Safe (GRAS) compounds by the FDA.1
The pharmacokinetics of GE has been investigated through several in vitro, in vivo alongside phase I, II and III trials. Cundy et al11 explored in vitro GE metabolism and distribution compared to GBP using numerous tissues and buffers. GE was confirmed chemically secure at physiological pH undergoing prompt hydrolysis to GBP by unspecific esterases. GE did not significantly interact (either as a substrate or inhibitor) with leading CYP450 isoforms. A significant risk of drug to drug interaction after administration within practice is therefore minimal. As GE targets higher-capacity transporters, drug interaction arising from active transport competition is improbable. Two high-capacity transporters (MCT-1 and SMVT) located across the span of the intestine were shown to actively distribute GE. Additionally, GE demonstrated pH-reliant passive permeability. It is argued that in vivo absorption therefore incorporates passive absorption alongside active transportation enabling effective oral bioavailability and avoiding saturation. Oral quantities of GE could enable more proportionate dosaging and foreseeable GBP availability. An expansive supply of high-capacity transporters assists absorption within the colon. This suggests an XR format could provide maintained plasma GBP concentrations resulting in reduced dosaging, better drug compliance and a greater pharmacotherapy.
Cundy et al12 explored the pharmacokinetics, safety alongside tolerability of GE in comparison to GBP in four phase 1 trials using 136 healthy volunteers. Two studies administered immediate release (IR) GE and two gave XR GE. The first placebo-controlled DB-RCT (double-blind, randomized control trial) administered five solitary doses of IR GE (350, 700, 1400, 2100 and 2800 mg) or a placebo to participants. Seven days afterwards equivalent GBP dosages (200, 400, 800, 1200 and 1400 mg) or placebo were given. GE illustrated a shorter Tmax rate (2.06–2.63 hours) compared to GBP (2.8–5.3 hours). GE also demonstrated a shorter half-life (4.38–5.47 hours) over GBP (5.40–9.26 hours). GE provided dose-proportionality and increased bioavailability.
In the second placebo-controlled DB-RCT four cohorts each containing 10 participants were administered IR GE (350, 700, 1400 or 2100 mg) or placebo twice per day for one week. GBP bioavailability was consistently high (74%) for all GE doses.
A randomized crossover trial compared solitary dosages of XR GE (1200 mg) given with or without food, to GBP (600 mg) without food including a one week washout interval. Every treatment regimen was received by participants. GE produced longer Tmax rates (8.4 alongside 5.08 hours with or without food respectively) compared to GBP (2.73 hours). GE provided greater GBP bioavailability (74.5% alongside 46.5%) over GBP (36.6%).
A further randomized crossover trial administered solitary dosages of XR GE (300, 600 and 1200 mg) to 36 participants with or without food including a one-week washout interval. Dose-proportionality occurred for all drug quantities. Receiving GE alongside food postponed maximum plasma concentrations and increased GBP bioavailability.
IR alongside XR preparations were tolerated across all four trials and no severe side-effects were observed. Sedation, dizziness alongside headaches were most often encountered. Adverse effects frequency associated with treatment dose. IR GE (2100 mg and 2800 mg twice per day) was effectively absorbed, quickly metabolized and produced dose-proportional availability in contrast to GBP where absorption decreased as dosage increased. XR GE generated sustained GBP concentrations and greater bioavailability in comparison to GBP (600 mg). For extended GE preparations, food within the large intestine also significantly increased GBP bioavailability. GE possibly enables greater predictability of GBP availability leading to reduced dosaging. Saturation was unobserved with GE at clinically effective levels in comparison to GBP. Larger GBP availability could thus enable better therapy responsiveness. Despite manifesting comparable side-effects to GBP, daily GE doses as great as 2800 mg were endured and generated greater GBP plasma concentrations. Reduced between-patient variability in GBP availability occurred with IR GE. Overall, GE enabled greater absorption, predictable GBP concentrations, lower between-patient variability and reduced dosaging frequency. It could therefore be a more efficient RLS pharmacotherapy.
Lal et al13 administered solitary quantities of XR GE (2400, 3600, 4800 or 6000 mg) or placebo to 32 healthy volunteers in a single-site, placebo-controlled, double-blind crossover trial. A seven day washout interval was included. GE was quickly metabolized following effective absorption. The available GBP plasma concentration was equivalent to GE dose quantity. Sickness and dizziness were the most frequently experienced side-effects. Two participants encountered serious medication-induced side-effects. However all adverse-effects ceased and none resulted in treatment cessation or withdrawal. Reduced between-participant variability in GBP availability occurred with GE (11%–26%) than GBP (47%–57%). Despite the rate of absorption within the intestine being higher for GE, this was not quicker than the elimination rate of GBP. Side-effect prevalence was greater than prior trials possibly as a result of using larger un-titrated GE quantities. Plasma concentrations of unaltered GE were minimal for every dose.
Kushida et al14 conducted a two-week multi- center, placebo-controlled, double-blind randomized crossover study investigating XR GE (1800 mg) efficacy alongside tolerability. Research was conducted across nine US centers using 38 treatment-naive moderate-to- severe RLS sufferers. A one week washout was included. Overall IRLS ratings were significantly lower for GE than placebo at trial completion. GE provided significant RLS amelioration as early as week one which continued to therapy completion. Polysomnography demonstrated significant improvements in sleep structure with GE. The percentage of GE-treated patients (85.3%) stating their symptoms were ‘much improved’ or ‘very much improved’ was greater than placebo (14.7%). Clinical Global Impression-Improvement (CGI-I) ratings, quality of sleep, duration RLS features were apparent, RLS seriousness, time awake each night and arousal frequency was also improved with GE. Improvement in subjective assessments, objective measures alongside participant and investigator ratings was demonstrated. Regularly encountered side-effects included sedation and dizziness. Doses were reduced for four participants as a result although withdrawal was unobserved.
Walters et al15 conducted a multi-center, two-week phase 2b, double-blind randomized parallel trial investigating XR GE efficacy alongside tolerability. Research was undertaken across 14 US centers. 95 drug-naïve patients suffering marked-to-severe primary RLS randomly received GE (600 mg or 1200 mg per day) or placebo at 5:00 PM alongside food. Average differences in IRLS ratings were significantly higher for GE (1200 mg) than placebo providing greater RLS improvement. Effects were evident by week one. CGI-I ratings (investigator and participant-scored) both significantly improved. Patients who received 1200 mg of GE reported greater sleep quality, more nights without RLS features, reduced arousal from symptoms and experienced less sleeplessness than placebo. Mood observations, average duration to RLS commencement and RLS length alongside intensity also improved with GE. Daily diary documentation demonstrated benefits continued from 8:00 PM to 4:00 AM, an interval where symptoms are typically worse. Findings for GE at a daily dose of 600 mg were comparable to placebo. Despite both GE dosages being tolerated, greater symptom amelioration was proven with 1200 mg. Frequently experienced medication-induced side effects included minor sedation and dizziness. Withdrawal occurred in two cases receiving GE (1200 mg) due to side-effects.
Kushida et al16 explored the efficacy and tolerability of XR GE in a 12-week, randomized, multi-site, double-blind, placebo-controlled parallel study. 22 US sites were included. 222 primary moderate- to-severe RLS sufferers were administered GE (1200 mg per day) or placebo alongside food at 5:00 PM. 68.3% of participants were drug-naïve. GE significantly alleviated RLS symptomatology over placebo. Average differences in IRLS ratings compared to baseline were larger for GE than placebo. Covariance analyses with adjustments for baseline measures across all sites produced average treatment differences of 4.0 (confidence intervals 6.2–1.9). A higher percentage of GE-treated subjects (76.1%) were viewed as responders by researchers on the CGI-I scale over placebo (38.9%). Significant improvement in sleep and RLS-related pain was experienced with GE. GE demonstrated superiority for all measures compared to placebo as early as day seven which continued to trial completion. RLS amelioration was comparable to that previously found with dopamine pharmacotherapy. GE demonstrated comparable tolerability to prior findings of GBP. Daytime somnolence did not worsen and spontaneous sleep episodes were unreported. Daily diary recordings showed GE delayed symptom commencement from six to 23.5 hours in comparison to placebo (6–11.5 hours). Approximately 50% of treated individuals in contrast to placebo (17.7%) were absent of symptoms within one day. GE-treated participants alongside placebo experienced side-effects including predominantly minor sedation and dizziness. Withdrawal occurred in one case due to sedation before initial observation. Nine additional individuals withdrew from side-effects. Adverse effects were medication-associated, presented during the initial 14 days and typically subsided. Clinically important alterations in laboratory measurements, vital signs alongside echocardiograms were not observed.
Bogan et al17 evaluated long-term XR GE efficacy alongside tolerance amongst 327 primary moderate-to-severe RLS sufferers. An initial 24-week single-blind therapy interval administered GE (1200 mg per day) or placebo to participants at 5:00 PM alongside food. Those viewed as responders during the initial single- blind interval (88%) subsequently commenced a 12-week, double-blind, randomized parallel trial. Research was conducted across 27 US sites. Sufferers were given GE (1200 mg per day) for 3 months, GBP (600 mg per day) for 14 days and placebo for 10 weeks. GE significantly postponed symptom commencement. RLS features demonstrated a significantly greater prevalence for placebo over GE across all measures (overall IRLS ratings, researcher and subject-rated CGI-I scores, Medical Outcomes Study (MOS) sleep scale alongside Post-Sleep Questionnaire (PSQ) outcome). Above 50% of GE-treated subjects were absent of symptoms throughout a one day observation interval. RLS amelioration from GE continued across nine months with sleep disruption and efficiency improving significantly. Subjective sleep assessments also improved significantly. Patients experienced reduced arousal from symptoms and more evenings without symptoms. The percentage of patients experiencing relapsing RLS symptoms was significantly less for GE (9%) over placebo (23%). Side-effects consisted mainly of sedation and dizziness. Clinically important alterations in laboratory measures, vital signs alongside echocardiograms were unobserved for all participants. Furthermore GE improved QoL probably as a result of RLS alleviation and through improved sleep.
The pharmacological profiles of GBP and GE are summarized in Table 2.
Table 2.
Comparison of gabapentin (GBP) and gabapentin enacarbil (GE).
| GBP | GE | |
|---|---|---|
| Chemical structure | Structural analog of inhibitory neurotransmitter GABA | Prodrug of GBP, acyloxyalkylcarbamate analog |
| Pharmacokinetics |
|
|
| Pharmacodynamics |
|
|
| Administration | 3–4 doses/day | once daily |
| Efficacy |
|
|
| Safety and tolerability |
|
|
Both GBP and GE have demonstrated good tolerability and effectiveness in different studies. Trials investigating GBP since 1996 have not reported augmentation occurrence as a side-effect. GE has comparable efficacy to dopaminergic agents and also does not produce augmentation. This reinforces the advantages of GBP and GE over the dopaminergic agents levidopa or carbidopa, where augmentation has been found to present in up to 80% of patients. Approximately 30%–50% of patients receiving DA agonists also report augmentation as a side-effect. Specifically, GE has shown several pharmacokinetic and pharmacodynamic advantages over GBP. GE overcomes GBP’s dose-reliant bioavailability necessitating regular dosing. Between-patient variation is reduced with GE and GBP bioavailability is increased. Overall sleep duration and efficiency is significantly greater with GE. Slow-wave sleep is improved and arousal following sleep commencement lessened. GE also demonstrates comparable adverse-effects to GBP. It is suggested that trials directly comparing these two drugs, including administering equimolar dosages, should be conducted in order to confirm GE’s superiority in clinical practice.2
Methodological Evaluation
Heterogeneity exists across studies causing difficulty when comparing findings. The majority of studies used selected samples which restricts generalization to the entire RLS population. Four trials obtained patients suffering moderate-to-severe primary RLS.14–17 Only focusing on such individuals overlooks drug efficacy in cases of mild and very severe RLS. GBP and GE might produce different outcomes according to RLS severity in these instances therefore findings cannot be generalized to these populations. Two trials utilized healthy volunteers12,13 which introduce the risk of volunteer bias. Three other studies included patients encountering secondary RLS.6,7,10 Thorp et al6 and Micozkadioglu et al10 specifically focused on patients receiving haemodialysis. This cohort could be impaired in their drug elimination ability compared to RLS patients without renal impairment and healthy volunteers. The pharmacokinetics of any RLS pharmacotherapy could thus differ for these patients altering therapeutic outcome. Garcia-Borreguero et al7 incorporated two individuals suffering iron deficiency within their sample. These cases are representative of secondary RLS and drug action could differ accordingly. Two trials used treatment-naive participants.14,15 Drug effectiveness for these individuals cannot be compared to those who have previously received RLS pharmacotherapy. Treatment-naive patients could respond more favourably to the drug or encounter worse side-effects compared to previously-treated individuals where tolerance could have developed.
Patient selection
Several studies obtained clinician-identified patients from haemodialysis centers6,10 and US centers.14–17 This introduces a selection bias and overlooks sufferers within the general population or those unregistered with general practitioners. This prevents extrapolation of findings to affected individuals unknown to the healthcare system. Between-group comparisons of drug efficacy and tolerability are therefore difficult. GBP and GE efficacy could differ between these specified samples restricting generalization to all RLS sufferers. Doses demonstrated to be effective in these particular populations may not apply to all RLS patients.
Type of pharmacotherapy
Some trials compared GE to GBP12,17 whereas others used a placebo6,7,12–17 or alternative medications9,10 as comparative conditions. Although this establishes conclusions regarding GE efficacy and tolerability in comparison to a range of other pharmacotherapies, studies cannot be directly compared. Furthermore, some trials administered GE with food12,15–17 whereas others gave the drug independently.12 Food could affect drug pharmacodynamics (sustain its action) leading to greater therapeutic effectiveness. Comparisons subsequently made between studies administering the drug with food and those that give GE in isolation are less reliable.
Study design
The crossover studies6,7,12–14 introduce the risk of carryover effects. Despite including a washout period of at least one week prior to initiating the subsequent drug, a longer washout interval would be more desirable. Order effects are also possible. However participants operate as their own control which improves power to identify drug effects and lessens variability alongside sample size.
The parallel trials15–17 avoid these risks although between-participant differences in drug responsiveness are increased. RCTs determine drug efficacy and safety under controlled conditions and enable direct comparisons. Drug effects can be directly assessed and causal relationships inferred. Being double-blind avoids the experimenter bias and expectancy effects possible in single-blind investigations.
Most of the studies presented used small samples increasing the possibility of Type I and II error and limiting valid conclusions regarding drug efficacy. Larger samples would allow more precise conclusions. The open-label trials4,5,9,10 generate information regarding drug efficacy, safety, dosing and tolerability. However being retrospective, uncontrolled and non-blind reduces their reliability. Experimenter bias is possible and causal relationships cannot be inferred. Some studies did not include placebo or comparison conditions4,5 which restricts assessment of drug effects. RLS severity whilst not receiving GBP or GE cannot be ascertained. RLS could show natural progression thus worsen or improve regardless of receiving medication. This could itself worsen patient QoL obscuring symptom assessment. Whether improvements or exacerbations therefore are a result of the drug cannot be established.
Most trials excluded individuals experiencing secondary RLS, those who were pregnant along with patients encountering and being treated for marked-to- serious depression. Exclusion criteria also included alternative primary sleep dysfunction, neurological conditions, movement disorder or previous experience of augmentation and rebound from dopaminergic pharmacotherapy. The majority of studies established RLS diagnosis using operationalized IRLS Study Group diagnostic criteria. This reduces the risk of misdiagnosis and increases reliability enabling accurate comparisons between studies. Recognized rating scales with proven validity and reliability were also utilized. However, Adler4 included a diagnostic scale measuring RLS symptoms unfounded from IRLS Study Group criterion. Findings were only generated from subjective measures and uncorroborated by objective polysomnography sleep measurement and periodic leg movement observation. The diagnostic screening scale used by Thorp et al6 was invalidated reducing the reliability of their findings. Most investigations performed Intention To Treat (ITT) analyses preventing attrition bias and confounding effects from crossover designs. Power calculations were also performed for most trials. All except two trials9,17 measured short-term drug effectiveness. Longitudinal research is required to assess whether effects are maintained. GE is currently a novel therapy therefore it is imperative to evaluate the long-term drug outcome in RLS sufferers. Certain findings are limited to particular geographical areas the research was undertaken in therefore preventing cross-cultural generalization.
GE dosages differed between studies causing difficulty when establishing a generalized clinically effective dose. GE cannot unequivocally be compared to GBP as variations exist regarding molecular weight and pharmacokinetic features. Some studies administered IR GE12 whereas others gave XR GE12–17 which could vary in their effects. GBP and GE dosages remained constant in some trials yet were titrated in others.5,9 The drug profiles of GBP and GE could therefore depend upon what they are compared against, whether they are administered independently or with food, time of dosing and the type of sample receiving the pharmacotherapy.
Between-participant differences could confound findings. Some individuals may differ in transporter concentration, absorption rate, metabolism and excretion. Patients may also vary in their initial RLS severity. The pharmacodynamics of GE could therefore differ for each participant producing individual variations in drug outcome. Kushida et al14 permitted continuation of alternative pharmacotherapies additionally to GBP and GE which could mask direct drug effects. Polypharmacy itself could affect RLS presentation. All other medication should be discontinued to observe the effects of GBP and GE alone.
Despite their methodological limitations, previous studies have produced converging findings with regards to GBP and GE effectiveness and tolerability, suggesting good validity. Moreover, these studies have consistently demonstrated significant advantages of non-dopaminergic pharmacotherapies over first-line dopaminergic treatments.
Efficacy and Tolerability Issues
GE seemed a promising drug which could potentially have been approved by the FDA. It has demonstrated effectiveness in both animal and human experiments suggesting it is safe amongst an expansive population. No serious side-effects were found amongst the trials and any adverse events experienced were comparable to predictable and well-tolerated responses already identified with GBP. In February 2010, GE was denied approval by the FDA. From preclinical trials XR GE (Horizant) was associated with a risk of acinar cell tumors amongst rats. GSK were aware of comparable findings for GBP during its approval for epilepsy however the benefits were seen to outweigh the risks. GE is therefore currently awaiting approval from the FDA. Further studies should also be conducted amongst less specified samples and the general population to observe whether drug response generalizes beyond the particular samples previously used.
Response variation due to genetic and population differences must not be overlooked. A therapeutically effective dose could depend upon individual differences in drug absorption, metabolism, and drug history alongside other physiological, psychological and emotional factors. RLS patients could be prescribed more than one pharmacotherapy therefore the potential for drug-drug interaction should not be ignored. A number of drugs including naproxen and cimetidine could interact with GE as they are substrates for the same transporters. GE is an MCT-1 substrate and GBP is an OCT2 substrate. Lal et al18 investigated the interaction of XR GE with naproxen (MCT-1 substrate) and cimetidine (OCT2 substrate) amongst healthy adults in an open-label, single-center trial. XR GE (1200 mg) was administered daily and subsequently by naproxen alone, cimetidine alone or a combination. GE availability did increase minimally during co-administration with naproxen or cimetidine, however naproxen and cimetidine availability remained unchanged. Moreover, co-administration did not produce adverse effects which were not already observed with these drugs independently. Minor tiredness and dizziness were the most often encountered adverse effects. GE was tolerated independently and when co-administered with naproxen or cimetidine. Saturation was not observed, therefore GE dose alteration was not required when administered alongside naproxen or cimetidine. However only healthy individuals were enrolled, therefore further studies are needed to assess whether drug interactions occur amongst RLS sufferers.
Conclusions
Despite heterogeneity amongst research methodologies and certain methodological limitations, GE has demonstrated consistent efficacy from in vitro, in vivo, phase I, II and III trials. This reinforces the therapeutic effectiveness provided from this drug suggesting it should be considered a valid RLS treatment. It has shown increased bioavailability, absorption alongside rapid metabolism in comparison to GBP implicating it as a superior pharmacotherapy. These improvements enable reduced dosages, therefore preventing noncompliance/missed doses and increasing patient satisfaction and convenience. GE is generally well-tolerated demonstrating no additional side-effects to those already encountered with GBP. It also avoids unpleasant side-effects encountered from the first-line dopaminergic agonists such as augmentation and symptom rebound. The encouraging findings from both objective and subjective measures strengthen GEs effectiveness. RLS is a long-term, often disabling condition therefore a well-tolerated, effective drug such as GE would prove a successful pharmacotherapy.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Agarwal P, Griffith A, Costantino HR, Vaish N. Gabapentin enacarbil— clinical efficacy in restless legs syndrome. Neuropsychiatric Disease and Treatment. 2010;6:151–8. doi: 10.2147/ndt.s5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imamura S, Kushida K. Gabapentin enacarbil (XP13512/GSK1838262) as an alternative treatment to dopaminergic agents for restless legs syndrome. Expert Opinion on Pharmacotherapy. 2010;11:1925–32. doi: 10.1517/14656566.2010.494598. [DOI] [PubMed] [Google Scholar]
- 3.Yaltho TC, Ondo WG. The use of gabapentin enacarbil in the treatment of restless legs syndrome. Therapeutic Advances in Neurological Disorders. 2010:1–7. doi: 10.1177/1756285610378059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler CH. Treatment of restless legs syndrome with gabapentin. Clinical Neuropharmacology. 1997;20:148–51. doi: 10.1097/00002826-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Happe S, Klösch G, Saletu B, Zeitlhofer J. Treatment of idiopathic restless legs syndrome (RLS) with gabapentin. Neurology. 2001;57:1717–9. doi: 10.1212/wnl.57.9.1717. [DOI] [PubMed] [Google Scholar]
- 6.Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. American Journal of Kidney Diseases. 2001;38:104–8. doi: 10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin. Neurology. 2002;59:1573–9. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 8.Allen RP, Earley CJ. Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep. 1996;19:205–13. doi: 10.1093/sleep/19.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Happe S, Sauter C, Klösch G, Saletu B, Zeitlhofer J. Gabapentin versus ropinirole in the treatment of idiopathic restsless legs syndrome. Neuropsychobiology. 2003;48:82–6. doi: 10.1159/000072882. [DOI] [PubMed] [Google Scholar]
- 10.Micozkadioglu H, Ozdemir FN, Kut A, Sezer S, Saatchi U, Haberal M. Gabapentin versus levodopa for the treatment of restless legs syndrome in hemodialysis patients: an open-label study. Renal Failure. 2004;26:393–7. doi: 10.1081/jdi-120039823. [DOI] [PubMed] [Google Scholar]
- 11.Cundy KC, Branch R, Chernov-Rogan T, et al. XP1352, a novel gabapentin prodrug: 1. design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. The Journal of Pharmacology and Experimental Therapeutics. 2004;311:315–23. doi: 10.1124/jpet.104.067934. [DOI] [PubMed] [Google Scholar]
- 12.Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM. Clinial pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. The Journal of Clinical Pharmacology. 2008;48:1378–88. doi: 10.1177/0091270008322909. [DOI] [PubMed] [Google Scholar]
- 13.Lal R, Sukbuntherng J, Luo W, et al. Pharmacokinetics and tolerability of single escalating doses of gabapentin enacarbil: a randomized-sequence, double-blind, placebo-controlled crossover study in healthy volunteers. Clinical Therapeutics. 2009;31:1776–86. doi: 10.1016/j.clinthera.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Kushida CA, Walters AS, Becker P, et al. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep. 2009;32:159–68. doi: 10.1093/sleep/32.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walters AS, Ondo WG, Kushida CA, et al. The XP045 Study Group. Gabapentin enacarbil in restless legs syndrome: a phase 2b, 2-week, randomized, double-blind, placebo-controlled trial. Clinical Neuropharmacology. 2009;32:311–20. doi: 10.1097/WNF.0b013e3181b3ab16. [DOI] [PubMed] [Google Scholar]
- 16.Kushida CA, Becker PM, Ellenbogen AL, Canafax DM, Barrett RW The XP052 Study Group. Randomized, double-blind, placebo-controlled study of XP13512/GSK1838262 in patients with RLS. Neurology. 2009;72:439–46. doi: 10.1212/01.wnl.0000341770.91926.cc. [DOI] [PubMed] [Google Scholar]
- 17.Bogan RK, Cramer Bornemann MA, Kushida CA, Trân PV, Barrett RW The XP060 Study Group. Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. Mayo Clinic Proceedings. 2010;85:512–21. doi: 10.4065/mcp.2009.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lal R, Sukbuntherng J, Luo W, et al. Clinical pharmacokinetic drug interaction studies of gabapentin enacarbil, a novel transported prodrug of gabapentin, with naproxen and cimetidine. British Journal of Pharmacology. 2010;69:498–507. doi: 10.1111/j.1365-2125.2010.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]