Abstract
Fibromyalgia (FM) is a chronic disorder characterized by widespread pain and other associated symptoms including fatigue, insomnia, cognitive/memory problems, and even psychological distress. Duloxetine is one of three FDA approved medications (the other two being milnacipran and pregabalin) for the treatment of FM. It has been demonstrated that FM patients possess low central nervous system levels of serotonin and norepinephrine. Duloxetine, which is classified pharmacologically as a serotonin-norepinephrine reuptake inhibitor (SNRI), may be beneficial for FM patients by increasing these levels. This review will touch briefly upon the pathophysiology of FM, diagnostic tools, currently available therapeutic options (both pharmacologic and non-pharmacologic), as well as the pharmacokinetic/pharmacodynamic properties of duloxetine. In addition, the efficacy and safety/tolerability of duloxetine exclusively in FM will be assessed through examination of 5 randomized controlled trials, as well as pooled analyses of current data. Suggestions for a therapeutic niche for duloxetine in FM are discussed based on a presentation of the characteristics of duloxetine.
Keywords: duloxetine, fibromyalgia, safety, efficacy, treatment, pain
Introduction
Fibromyalgia (FM) is a central pain disorder that is somewhat controversial and seems to involve altered afferent processing, resulting in augmentation of peripheral stimuli, especially the nociceptive types. The “core” symptoms seen in FM and many other central sensitization disorders include multifocal pain, fatigue, insomnia, cognitive/memory problems, and psychological distress. However, FM patients may experience a multitude of other symptoms, including dysesthesias, stiffness, poor balance, oral/ocular symptoms (eg, keratoconjunctivitis sicca), headaches, sexual dysfunction, and impaired physical function (see Fig. 1).
Figure 1.
Fibromyalgia domains.
Currently there are three agents approved by the US Federal Drug Administration (FDA) for the treatment of FM: pregabalin, milnacipran, and duloxetine. Duloxetine is among the class of drugs known as serotonin–norepinephrine reuptake inhibitors (SNRIs), and represents an important pharmacologic therapeutic option for FM.
Fibromyalgia Syndrome
It appears that 2%–4% of the population suffers from FM, with the disorder being 2 times more prevalent among women than men.1,2 This latter statement may be attributed to the fact that women tend to be more tender than men. The disorder is predominantly diagnosed in patients aged 20–60 years (mean age, 49 years).1,2 FM negatively impacts the physical functioning of its patients, as evidenced by difficulties with multiple daily activities.3 62% of patients have difficulty climbing stairs, 55% have difficulty walking two blocks, and 35% have difficulty with activities of daily life (ADLs).3 The pain disorder, however, seems to have the most significant impact on emotional health and social functioning.4 The EPISER study demonstrated that patients with FM had similar physical impairment but worse psychological impairment than patients with rheumatoid arthritis.4 Women with FM have lower quality of life (QOL) measures than women with other chronic disorders, including rheumatoid arthritis, chronic obstructive pulmonary disease, and diabetes mellitus.2 Finally, FM appears to negatively affect personal relationships, career, and mental health.5
Pathophysiology of fibromyalgia
The precise mechanisms responsible for FM are unknown, but most likely involve alterations in pain and sensory processing systems. In particular, it is thought that patients with FM have inefficient descending inhibitory pathways, which normally function as endogenous analgesic systems to ameliorate pain in healthy subjects. These pathways are mediated in part by the neurotransmitters serotonin and norepinephrine (see Fig. 2).
Figure 2.
Neural influences on pain and sensory processing.
Studies demonstrate that patients with FM have lower cerebrospinal fluid (CSF) levels of metabolites of biogenic amines (eg, serotonin and norepinephrine).6 Further evidence comes from treatment studies which reveal that any agent that simultaneously raises both serotonin and norepinephrine (eg, tricyclic antidepressants, duloxetine, milnacipran, tramadol) has been shown to be efficacious in treating FM.
Another mechanism thought to play a role in the pathophysiology of FM is the presence of augmented pain pathways in these patients. These pathways are mediated in part by substance P and the excitatory amino acid glutamate (see Fig. 2).7 Studies demonstrate that patients with FM have significantly higher concentrations of substance P in CSF compared with healthy subjects.8–12 CSF levels of glutamate are also twice as high in patients with FM compared with healthy controls.13 Brain imaging studies also support the existence of central pain augmentation in patients with FM.14
Gracely et al performed a study utilizing functional MRI (fMRI) in patients with FM in 2002.14 When stimuli of equal magnitude were administered to both FM and healthy subjects, there was increased regional blood flow in FM patients compared with controls.14 The regions exhibiting increased activity included the primary and secondary somatosensory cortex, the insula, and the anterior cingulate cortex, all areas which exhibit increased blood flow when normal subjects experience pain.14
Assessment of fibromyalgia
The American College of Rheumatology (ACR) criteria15 require that an individual possess both a history of chronic widespread pain and ≥11 of 18 possible tender points on physical examination. The Manchester criteria16 utilize a whole body diagram to indicate areas of pain, thereby obviating the necessity of tender points. However, both of these criteria are used predominantly for research/epidemiologic purposes. The use of tender points as diagnostic criteria is beginning to fade as it fails to recognize the presence of other symptoms that need to be addressed to optimally manage FM patients.17
In 2003 Wolfe et al conducted a study in which they mailed surveys to 12,799 patients with either rheumatoid arthritis, osteoarthritis, or FM.18 They found that pain present in 19 primarily non-articular sites differentiated FM from the other two disorders.18,19 This study led to the proposal of diagnostic criteria that assessed chronic widespread pain without the use of trigger points. It expanded the definition of FM to include symptoms other than pain, such as fatigue, sleep disturbance, and cognitive dysfunction.20 The criteria also include a separate measure of symptom-related severity, which is an important component in the adequate evaluation and management of FM patients.21
The Fibromyalgia Impact Questionnaire (FIQ) is a validated, disease-specific composite measure that was developed to determine the range of symptoms experienced by FM patients and responses to therapy.22 It was updated in 1997 and 2002 to reflect experience with using the instrument and to clarify the scoring system.23 It includes 20 questions that assess functionality with ADLs, work difficulty, general feelings of well-being, sleep quality and the severity of symptoms including pain, fatigue, depression, anxiety, and stiffness.24 Bennett et al performed an analysis which demonstrated that a 14% change in the FIQ total score represented a statistically and clinically meaningful difference for the patient. The results of this analysis should enhance the utility of the FIQ for clinical and research purposes.25
The Revised Fibromyalgia Impact Questionnaire (FIQR) is an updated version of the FIQ that has good psychometric properties, is easy to score, and can be completed in less than 2 minutes.23 It has the same 3 domains as the FIQ: function, overall impact, and symptoms. It differs from the FIQ in that it has modified function questions and includes questions pertaining to memory, tenderness, balance, and environmental sensitivity. All questions are graded on a 0–10 numerical scale.23 Each of the three domains of the FIQR correlated well with the related domains of the FIQ (r = 0.69 to 0.88, P < 0.01). The total scores of the FIQR and the FIQ were also closely correlated (r = 0.88, P < 0.001).
Pharmacologic treatment of fibromyalgia
The majority of clinical trials evaluating FM therapy have included antidepressants of one class or another, especially the older, tricyclic antidepressants (TCAs). Uceyler et al performed a meta-analysis on the efficacy of antidepressants for treating FM. The authors found amitriptyline, studied in 13 randomized controlled trials (RCTs), to provide a moderate magnitude of relief to FM patients (pain reduction by mean of 26%, improvement in QOL by 30%).26 Other RCTs demonstrate the effectiveness of amitriptyline (a TCA) and cyclobenzaprine (structurally similar to amitriptyline) in reducing the symptoms of pain, poor sleep, and fatigue.27 Cyclobenzaprine, a centrally acting muscle relaxant, has been used to treat the musculoskeletal component and improve sleep in FM patients.28
Most of the SNRIs clinically available for the treatment of FM have more of a significant impact on serotonin compared with norepinephrine activity (see Fig. 3).
Figure 3.
Relative activity on serotonin and norepinephrine reuptake among antidepressants.
Abbreviations: S, serotonin; N, norepinephrine.
SNRIs tend to be better tolerated than older TCAs. Venlafaxine, the first SNRI available in the US, tends to have clinically significant effects on norepinephrine reuptake only when used at higher doses.29 Thus, venlafaxine could potentially be beneficial in FM patients when used at these doses.29 Duloxetine and milnacipran are two SNRIs that are approved for the treatment of FM in the US (in 2008 and 2009, respectively) and have been shown to be efficacious in this disorder.30,31 The efficacy and tolerability of duloxetine will be discussed later in this paper. Milnacipran is one of the few SNRIs that inhibits norepinephrine reuptake more than serotonin reuptake. The standard dosing is 100 mg/day, which in selected patients can be increased to 200 mg/day based on responsiveness and tolerability. The usual half-life of milnacipran is 6–8 hours for the parent compound and 8–10 hours for d-milnacipran, the active isomer; thus twice-daily dosing is recommended.32 Milnacipran has shown benefit in the treatment of FM, improving the symptoms of fatigue, reduced physical functioning, and discomfort.33–35
Pregabalin, approved for the treatment of FM in the US in 2007, is a gamma-aminobutyric acid (GABA) analog which binds to the alpha-2-delta subunit of calcium ion channels. The half-life of pregabalin is 5.5–6.7 hours in the presence of a normal CrCl.32 The dosing for this agent, however, is dependent upon the patient’s CrCl because elimination is a function of renal clearance. Decremental dosing changes are recommended in patients with impaired renal function. Dosing secondary to side effects is based on 1-week intervals focusing on patient responsiveness and tolerability.32 Häuser et al conducted a systematic review evaluating pregabalin that included 5 studies.36 There was strong evidence demonstrating reduction of pain, improvement in sleep, and improved health-related quality of life (HRQOL), but not depressed mood.36 These studies potentially lack external validity in that patients with severe co-morbid depression and disability were excluded from participation.37 The FREEDOM (Fibromyalgia Relapse Evaluation and Efficacy for Durability of Meaningful Relief) double-blinded trial38 evaluated the durability of pregabalin in 1,051 FM patients in whom the drug initially worked. By the end of the double-blinded phase, 61% of patients in the placebo group had stopped responding compared with 32% in the pregabalin treatment group.38
Gabapentin is another alpha-2-delta ligand and antiepileptic drug structurally similar to pregabalin, but not approved for the treatment of FM. However, the agent has shown potential benefit in clinical trials. Arnold et al found gabapentin (1,200 −2,400 mg/day) to be effective and safe in FM.39
Other centrally acting agents may show benefit in FM patients with a predominant symptom-type. For example, gamma-hydroxybutyrate, with its strong sedative qualities, may be clinically useful for FM patients with insomnia/sleep disturbance.40 Pramipexole, a dopamine agonist used for Parkinson’s disease, could be potentially useful for FM patients with concomitant restless leg syndrome.41 Tramadol, which possesses some analgesic activity, may be utilized for FM patients with a significant pain component to their disease.42,43 Finally, tizanidine, an alpha-2-adrenergic agonist muscle relaxant, could be potentially used for FM patients with spasticity.44
Non-pharmacologic treatment of fibromyalgia
Non-pharmacologic approaches such as exercise, education, and cognitive behavioral therapy have a positive impact in FM, but it is felt that these treatments appear to be underutilized in usual clinical practice.45
Several studies have shown that exercise is beneficial in FM patients, especially with respect to reducing physical symptoms and improving functional capacity.46 Exercise modalities studied included land and water aerobics, strength training, flexibility training, and various combinations of these. The strongest evidence demonstrating benefit in FM is for aerobic and mixed-type exercises, with growing evidence for positive effects from strength training.47–50 Patients were more likely to adhere to regimens that were low-impact, low-intensity and individualized to meet their specific needs.46 Furthermore, high-intensity exercise seemed to provoke pain compared with low-intensity exercise.46
Studies evaluating flexibility training, such as yoga, in FM patients are yielding positive results.50,51 The paucity of evidence, however, does not support the use of flexibility training as a single modality.50,51 Emerging evidence surrounding the benefits of movement-based therapies in FM, such as Qi Gong and T’ai Chi, are positive but more research needs to be performed.52,53
Patient education has also been analyzed as a therapeutic option for FM patients. Rooks et al54 completed a RCT with 207 participants with FM who were randomized to four groups: 1) aerobic and flexibility training group; 2) strength, aerobic, and flexibility training group; 3) the Fibromyalgia Self-Help Course; or 4) a combination of the previous three. The combination group was found to provide the most benefit. Thus, education may be useful for FM patients when utilized with other multi-modal interventions.
Cognitive-behavioral therapy (CBT) combines aspects of both cognitive and behavioral interventions. Catastrophic thoughts, which are beliefs that the worst possible outcome is going to occur, is associated with increased pain severity, reduced functional capacity, and affective distress in FM patients.55 Cognitive therapy focuses on taking catastrophic thoughts and reframing them into more positive beliefs.56 Behavioral therapy, in contrast, stresses the importance of operant behavioral change over inner thoughts and feelings.56 Its goals are to increase adaptive behavior through positive and negative reinforcement, and to extinguish maladaptive behavior through punishment.56
Thieme et al performed a study to examine the efficacy of CBT in treating FM patients. 125 patients who fulfilled the ACR criteria for FM were randomized to either operant behavioral therapy (OBT) (n = 43), CBT (n = 42), or an attention-placebo group (n = 40) that consisted of discussions surrounding FM related problems. The results demonstrated that both OBT and CBT are effective modalities in treating FM.57
Bernardy et al recently performed the first meta-analysis of the efficacy of CBT in FM. The systematic review included 14 out of 27 studies with 910 subjects and a median treatment time of 27 hours over a median time range of 9 weeks. The primary endpoints were pain, sleep, fatigue, and HRQOL. Secondary endpoints included depressed mood, self-efficacy pain, and healthcare-seeking behavior. They demonstrated that CBT reduced depressed mood and self-efficacy pain post-treatment, but had no significant effects on pain, fatigue, sleep, or HRQOL after treatment or at follow-up.58 Furthermore, OBT was shown to significantly reduce the number of physician visits at follow-up. Thus CBT may be most beneficial in helping FM patients cope with pain and depression on their own and somewhat reduce dependence on health care providers.58
It is important to note that all CBT interventions are not identical, in that most incorporate only small elements of cognitive therapy with more focus on behavioral interventions.56 CBT may also be somewhat operator-dependent and offers specific therapies for predominant symptom-types [eg, insomnia (CBT-I); pain (CBT-P); stress (CBT-S)].
Relaxation techniques are commonly incorporated into CBT therapies.59 Techniques likely to be helpful for FM patients include progressive muscle relaxation (tightening and relaxing of muscle groups to improve anxiety);60 autogenic training (verbally repeating sets of visualizations to induce relaxation);61 guided imagery (engaging all senses to experience pleasant places/circumstances); and meditation.
Thieme and Gracely performed a literature search identifying 14 RCTs assessing CBT and OBT, 5 relaxation RCTs, 5 biofeedback RCTs, 5 hypnotherapy RCTs, and 2 writing intervention RCTs.62 The greatest effects on pain reduction (r = 0.53–2.14) were experienced after the CBT and OBT therapies.62
Finally, there is substantial evidence that multi-modal interventions may be most efficacious in treating FM. Häuser et al performed a systematic review of 9 RCTs with 1,119 subjects (median treatment time 24 hours).63 It revealed strong evidence that multi-component treatment improves pain, fatigue, depressive symptoms, poor QOL, and physical fitness post-treatment.63
Pharmacologic Overview of Duloxetine
Duloxetine is classified pharmacologically as a serotonin-norepinephrine reuptake inhibitor (SNRI) which possesses high ki values for monoamine transporters (eg, serotonin and norepinephrine transporters).32 The ki value reflects the potency of an inhibitor compound as the tightness affinity of binding to the monoamine transporter.32 Duloxetine inhibits serotonin reuptake significantly more than norepinephrine reuptake (in an approximate 10:1 ratio).64 Duloxetine is the (+)-(S) isomer of the racemic mixture with structural similarities to both fluoxetine and atomoxetine. 32 It possesses a secondary amine structure unlike venlafaxine, the first approved SNRI, which possesses a tertiary amine structure.32 Duloxetine is FDA approved for the following uses: FM, diabetic neuropathic pain, major depressive disorder, and generalized anxiety disorder.65 Thus FM patients with comorbid depression and/or anxiety could potentially be treated with duloxetine as single agent therapy.
Duloxetine is available in delayed-release enteric-coated capsules.32 Duloxetine exhibits a peak effect on platelet serotonin reuptake at 4–6 hours. Its inhibition persists for a duration of action of 7 days.32 The maximum plasma concentration (Cmax) is achieved 6 hours after a post-prandial dose.66,67 The pharmacokinetics of duloxetine exhibits linearity and the steady-state concentration (Css) is reached in approximately 3–5 days.65 Its absorption and bioavailability are demonstrated to be 30%–80%.66,67 Duloxetine exhibits a high degree of protein binding (90%) and binds primarily to albumin and alpha-1 acid glycoprotein.32
Duloxetine has a usual half-life of 8–17 hours.65 Its metabolic pathways include cytochrome P450 1A2 and 2D6. In addition to being a substrate, duloxetine may produce mild inhibition of CYP450 1A2 and moderate inhibition of 2D6.32 CYP450 2D6 does exhibit genetic polymorphism and could potentially lead to the existence of poor, extensive, and ultra-extensive metabolizers.32 Approximately 70% of duloxetine is renally excreted as metabolites, with <1% as the parent compound.32 Metabolites found in plasma and urine include 4-hydroxy duloxetine glucuronide and 5-hydroxy, 6-methoxy duloxetine sulfate, neither of which appear to be significantly pharmacologically active.68 Thus patients with renal impairment (CrCl of 30–80 mL/min) should receive an initial lower dosage (ie, 20 mg) with the dose increased gradually thereafter. Approximately 20% of duloxetine is excreted in the feces, possibly representing hepatobiliary secretion.32 Duloxetine’s bioavailability is decreased by approximately 1/3 in smokers.66,67
Abrupt discontinuation of any SNRI, including duloxetine, can cause a multitude of symptoms, including headache, dizziness, nightmares, irritability, paresthesias, and nausea/vomiting.32 Thus it is recommended that this agent be decreased in small, decremental amounts over an extended period of time. There is also risk of serotonin syndrome (diaphoresis, hyperthermia, tachycardia, hyperreflexia) with this drug, especially if used in conjunction with other serotonin agents.65 In patients with a history of alcohol use, there is an increased risk of liver damage, because preexisting hepatic damage may be present.32 In patients with controlled narrow-angle glaucoma, there may be an increased risk of mydriasis. Patients who are elderly or hypovolemic may develop hyponatremia with duloxetine. There has also been reports of the syndrome of inappropriate antidiuretic hormone secretion in patients taking duloxetine or other SNRIs. Finally, if use is initiated during pregnancy, it should be delayed until the third trimester (pregnancy category C).32
Adverse effects that may occur commonly (>10%) in patients include somnolence, dizziness, headaches, and insomnia.32 Possible cardiovascular effects include increase in blood pressure, orthostatic hypotension, syncope, and palpitations.65 Possible gastrointestinal effects include nausea, xerostomia, diarrhea, and constipation.32 Other adverse effects reported in FM patients include hyperhidrosis, sexual dysfunction, diminished appetite, and urinary hesitancy.32
Efficacy of Duloxetine
Several studies have been performed examining the efficacy of duloxetine specifically for the treatment of FM. The following 5 RCTs30,69–72 and analyses of pooled73,74 data provide detailed insight into that examination.
Arnold et al conducted a multicenter (18 centers), randomized, double-blinded, placebo-controlled trial assessing the efficacy of duloxetine in FM patients with or without concurrent major depressive disorder (MDD).30 After single-blinded placebo treatment for 1 week, patients were randomized to either duloxetine 60 mg twice daily (n = 104) or placebo (n = 103) for 12 weeks. Co-primary endpoints included the FIQ total score and FIQ pain score. Secondary endpoints included mean tender point pain threshold; number of tender points; FIQ fatigue, tiredness on awakening, and stiffness scores; Clinical Global Impression of Severity (CGI-S) score; Patient Global Impression of Improvement (PGI-I) score; Brief Pain Inventory (BPI short form); Medical Outcomes Study Short Form 36 (SF-36); Quality of Life in Depression Scale; and Sheehan Disability Scale [(SDS)30 assesses functional impairment through use of home, school/work, and social domains].75 Compared with placebo-treated subjects, duloxetine-treated subjects improved significantly more (P = 0.027) on the FIQ total score, but not significantly more on the FIQ pain score (P = 0.130).30 The FIQ pain score, however, might be limited in its capacity as an endpoint in that subjects must recall and rate their pain over the prior week, which may be more difficult to recall than pain over the past 24 hours. Compared with placebo-treated subjects, duloxetine-treated subjects had significantly greater reductions in BPI average pain severity score (P = 0.008), BPI average interference from pain score (P = 0.004), number of tender points (P = 0.002), and FIQ stiffness score (P = 0.048), and had significantly greater improvement in mean tender point pain threshold (P = 0.002), CGI-S score (P = 0.048), PGI-I score (P = 0.033), and QOL endpoints.30 To test the direct effect of duloxetine on pain reduction, a path analysis was performed utilizing three regression models to compare the response to mood symptoms using both the Beck Depression and Anxiety Inventories to the response to pain that allowed estimation of the percentage of direct and indirect effects on the total treatment effect.76 The path analysis demonstrated that duloxetine had a direct reduction in pain (61.1%–83.3%) that was independent of improvement in mood.30 Compared with placebo-treated female subjects (n = 92), duloxetine-treated female subjects (n = 92) demonstrated significantly greater improvement in most endpoints, while duloxetine-treated male subjects (n = 12) failed to improve significantly on any measure. The reasons for the sex differences in response are unclear, however sex differences in FM that affect treatment response cannot be ruled out.30 Another potential explanation for lack of significant response in men are the small male subgroup in the study (11%), reflecting the high prevalence of FM among women, and contributing to low statistical power.30
Another multicenter (21 centers), randomized, double-blinded, placebo-controlled trial conducted by Arnold et al assessed the efficacy of duloxetine exclusively in the treatment of females with or without MDD.69 The women were randomized to one of three treatment groups for a 12-week duration: duloxetine 60 mg/day (n = 118), duloxetine 60 mg twice daily (n = 116), or placebo (n = 120). The primary endpoint was pain severity as measured by the BPI average pain severity score.69 Response to treatment was defined as ≥30% reduction in this score. Secondary endpoints included the BPI items for severity of worst pain and least pain during the last 24 hours, pain right now, and pain interference with general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life; the FIQ score; the CGI-S score; the PGI-I score; the Hamilton Depression Rating Scale (HAMD17); the Quality of Life in Depression Scale; the SF-36; and the SDS. Compared with placebo, both duloxetine-treated groups improved significantly more (P < 0.001) on the BPI average pain severity score.69 A significantly higher percentage of duloxetine-treated patients had a decrease of ≥30% in this score (duloxetine 60 mg/day [55%; P < 0.001]; duloxetine 60 mg twice daily [54%; P = 0.002]; placebo [33%]). Path analysis utilizing two regression models demonstrated that 75.6% and 86.9% of the reduction in pain symptoms was due to the direct effect of duloxetine 60 mg/day and 60 mg twice daily, respectively, that was independent of improvement in mood.69 Furthermore it was shown that patients with and without MDD had similar reductions in pain symptoms. Compared with placebo, patients treated with duloxetine 60 mg/day or duloxetine 60 mg twice daily had significantly greater improvement in BPI pain interference scores, FIQ scores, CGI-S scores, PGI-I scores, and QOL endpoints.69 There were no significant differences between the duloxetine 60 mg/day and duloxetine 60 mg twice daily treatment groups in efficacy outcomes. However, only the duloxetine 60 mg twice daily dose, compared with placebo, significantly improved tender point assessments.69 Tender point assessment, however, is used predominantly for research purposes and its utilization as diagnostic criteria is waning.
Arnold et al pooled results from the prior two RCTs to analyze the effectiveness of duloxetine, specifically for improvement in pain, functional capacity, and QOL, in the treatment of female patients with FM. Pooling data is advantageous because the larger sample sizes may result in higher statistical power, thus leading to more precise treatment effects.73 Compared with placebo, the duloxetine group had significantly greater improvement in the BPI average pain severity score and the FIQ total score over the 12-week period (P < 0.001).73 The duloxetine group demonstrated significantly greater improvement in mean tender point threshold, CGI-S score, PGI-I score, average interference from pain scores, and other QOL endpoints. There were significant (P < 0.001) differences between duloxetine and placebo in the proportion of patients with at least 50% reduction in the BPI average pain score (duloxetine 95, 30%; placebo 27, 13%) and the FIQ average pain score (duloxetine 125, 39%; placebo 37, 18%).73 There were also significant (P < 0.001) differences between duloxetine and placebo in the proportion of patients with at least 30% reduction in the BPI average pain score (duloxetine 125, 39%; placebo 39, 19%) and the FIQ average pain score (duloxetine 163, 51%; placebo 67, 32%). The treatment effect was shown to be directly mediated through reduction in pain and not through improvement in mood.73 The results demonstrate that duloxetine is effective in women with or without MDD, but the higher numerical scores in duloxetine compared with placebo in MDD subgroups suggests that duloxetine could help improve the signs/symptoms of concurrent depression. Some limitations of these studies include the lack of generalizability to treatment periods longer than 12 weeks and to populations including patients with extensive psychopathology and secondary FM, as they were excluded from participation.73
A third study conducted by Russell et al also examined the efficacy of duloxetine for reducing pain severity in patients with or without current MDD over a 6 month period. It was a multicenter, randomized, double-blinded, placebo-controlled trial in which 520 patients were randomized to one of four groups: duloxetine 20 mg/day, 60 mg/day, 120 mg/day, or placebo.70 The duloxetine 20 mg/day group was titrated up to 60 mg/day after 3 months. The co-primary endpoints were the BPI average pain severity score and PGI-I score. The response rate was defined as ≥50% reduction in the average pain score.70 Secondary endpoints included the FIQ score, the CGI-S score, tender point pain assessments including mean tender point pain thresholds, the Multidimensional Fatigue Inventory (MFI), the HAMD17, the SDS, the SF-36, and the EuroQoL Questionnaire-5 Dimensions (EQ-5D). Compared with placebo, patients treated with duloxetine 120 mg/day improved significantly more on the co-primary endpoints at 3 months (change in BPI score [−2.31 vs. 1.39, P < 0.001] and PGI-I score [2.89 vs. 3.39, P = 0.004]) and at 6 months (change in BPI score [−2.26 vs. 1.43, P = 0.003] and PGI-I score [2.93 vs. 3.37, P = 0.012]). Compared with placebo, patients treated with duloxetine 60 mg/day also demonstrated significantly improved co-primary endpoints at 3 months and BPI score at 6 months.70 The number needed to treat (NNT) for duloxetine 20 mg/day, 60 mg/day, and 120 mg/day was 12, 10, and 7, respectively, at the end of 3 months. The NNT for duloxetine 20/60 mg/day, 60 mg/day, and 120 mg/day was 10, 7, and 7, respectively, at the end of 6 months. The majority of the duloxetine effect (62.2%–79.0%) was mediated by direct reduction in pain and not through improvement in mood.70 All doses of duloxetine significantly improved the MFI mental fatigue domain compared with placebo after 6 months of treatment. Since the mental fatigue domain consists of four questions relating to attention/concentration, it may be possible that duloxetine is efficacious in treating the cognitive dysfunction frequently reported by FM patients. Finally, this RCT succeeded in demonstrating similar improvement in the average pain severity score after 3 and 6 months of treatment in both male and female subjects.70
A study conducted by Chappell et al evaluated the efficacy of duloxetine in FM over a 1 year period.71 It was a phase 3 study which consisted of an 8-week open-label period followed by a 52-week double-blinded period. Patients received duloxetine 30 mg/day for 1 week, then 60 mg/day for 7 weeks, and were subsequently randomized to either 60 mg/day or 120 mg/day. The endpoints included the BPI average pain severity and interference item scores, the FIQ total score, the PGI-I score, the CGI-I score, the mean of the tender points pain thresholds, the number of tender points with a low threshold (≤4 kg/cm2), and the SDS.71 Patients with at least a 50% reduction in BPI average pain scores were considered treatment responders. Significant pain reduction was observed as assessed by numerous endpoints during the open-label phase of the study.71 This reduction in pain severity persisted throughout the double-blinded phase, as evidenced by additional mean decreases in the BPI average pain score within both duloxetine groups. This can be considered noteworthy given the unprecedented 60-week treatment period. Furthermore, evaluations of the long-term difference in efficacy between duloxetine 60 mg/day and 120 mg/day did not reveal dose-dependent differences in BPI pain responses.71
Another study conducted by Chappell et al analyzed the effectiveness of duloxetine (n = 162) compared with placebo (n = 168) in the treatment of FM patients for 6 months.72 It was a phase 3, parallel, double-blinded, placebo-controlled trial in which patients were initially randomized to duloxetine 60 mg/day or placebo. If a patient from the duloxetine-treated group did not have a ≥50% reduction in the BPI average pain score at week 13, then the patient was blindly escalated to duloxetine 120 mg/day. If the patient could not tolerate this dose, then the patient was allowed to return to the 60 mg/day dose.72 The co-primary endpoints were BPI average pain score and the PGI-I score. Secondary endpoints included the FIQ total score, CGI-S score, the tender point pain threshold, the mean of the thresholds, the number of tender points with a low threshold, area under the curve of pain relief, the BPI severity and interference pain score, the MFI, the HAMD17, Beck Depression Inventory-II (BDI-II), the SDS, the SF-36, and the EQ-5D.72 The BPI average score and PGI-I score both demonstrated greater numerical improvement in duloxetine-treated compared with placebo-treated groups, but the differences were not statistically significant (BPI average score P = 0.053, PGI-I P = 0.073). However, a significant treatment-by-investigator interaction was observed for these variables. The nature of this interaction could not be fully explained.72 Duloxetine-treated patients did improve significantly more than placebo-treated patients on the FIQ pain score, BPI least pain and interference pain score, CGI-S score, area under the curve of pain relief, MFI mental fatigue component, BDI-II score, and SF-36 mental component summary and mental health score. These secondary endpoints can be considered important factors in assessing treatment efficacy in patients with FM.72
Arnold et al pooled data from 4 of the prior RCTs so as to enable the assessment of precise treatment effects.74 Changes in the BPI average pain severity scores demonstrated significantly greater improvement in duloxetine-treated versus placebo-treated patients at week 1 and continuing through week 12 (P < 0.001). Duloxetine also showed significantly greater improvement compared with placebo on the BPI severity scores for least pain, worst pain, and pain right now and on the mean of the pain interference scores.74 Finally, duloxetine was statistically superior to placebo with respect to improvement in CGI-S scores (P < 0.001), FIQ total scores (P < 0.001), HAMD17 total scores (P = 0.003), PGI-I scores (P < 0.001), and QOL endpoints. The authors concluded that duloxetine 60–120 mg/day effectively improved FM symptoms and may offer benefits beyond pain relief, as evidenced by improvement in secondary endpoints.74 Some limitations of these studies include lack of generalizability to treatment with duloxetine beyond 12 weeks and to male, non-white patients (majority of subjects in the studies were middle-aged white women). Finally, additional research needs to be done combining duloxetine with non-pharmacologic interventions74 since there is substantial evidence supporting multi-modal therapy in the treatment of FM.63
Bradley et al also pooled data from four 12-week, randomized controlled trials to examine the time course in which duloxetine could produce minimal clinically significant improvement in FM.77 Patients were randomized to either duloxetine 60 mg/day, 120 mg/day, or placebo. A clinically significant treatment response was defined as ≥30% reduction in the BPI average pain severity score.77 The investigators demonstrated that nearly one-half (47%–49%) of the duloxetine-treated patients achieved a 30% reduction in pain severity from baseline. They also found that a significant proportion of these patients sustained this response throughout the treatment period (30% sustained response 37%–38% for duloxetine group vs. 23% for placebo group).77 Duloxetine-treated patients also experienced reductions in pain earlier during therapy. At weeks 1 and 2, duloxetine-treated subjects achieved 30% response rates twice those of placebo-treated subjects. Rates of 30% sustained response were also at least twice as high in the duloxetine group compared with the placebo group during the initial weeks of treatment. Furthermore, among patients that did not initially respond by weeks 1, 2, 4, and 8, the percentages of duloxetine 60-mg-treated patients who did respond by the endpoint of the study were 36.9%, 29.8%, 28.9%, and 26.9%, respectively.77
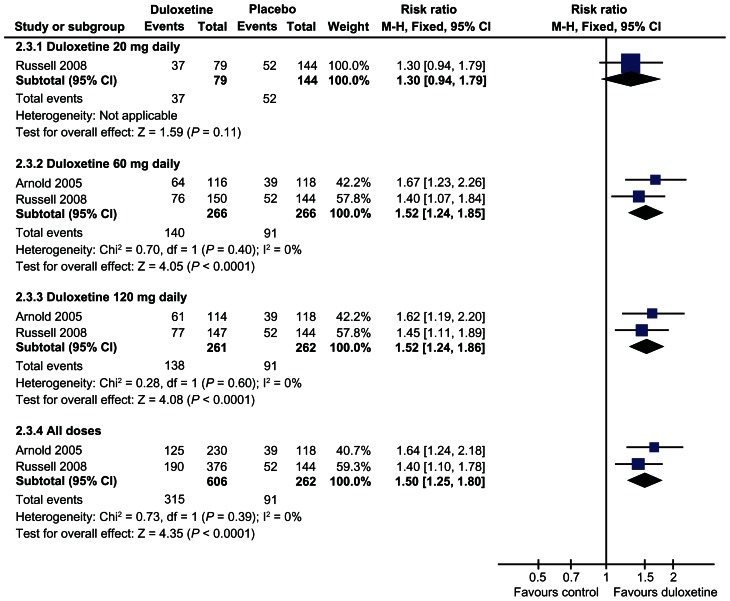
Finally in the 2010 Cochrane Review looking at outcome data based on FIQ and BPI scores, the risk ratio of improvement was significantly greater with duloxetine 60 mg (1.57, 95% CI 1.20 to 2.06) and with 120 mg daily (1.73, 95% CI 1.36 to 2.19) than with placebo for the primary outcome.78 The risk ratio of >30% improvement at 12 weeks or less was significantly greater than placebo with the duloxetine 60 mg dose (RR 1.52, 95% CI 1.24 to 1.85) and 120 mg dose (1.52, 95% CI 1.24 to 1.86) but not with the 20 mg dose78 (see Fig. 4).
Figure 4.
Duloxetine versus placebo in the treatment of fibromyalgia: >30% improvement <12 weeks.78
Safety and Tolerability of Duloxetine
In the first article by Arnold et al the investigators evaluated the safety of duloxetine in FM patients with or without MDD through examination of treatment-emergent adverse events (TEAEs), vital signs, physical findings, laboratory values, and EKG changes.30 There was no statistically significant difference between treatment groups in subject discontinuation due to TEAEs, with a total of 29 subjects discontinuing during the therapy phase [18 in the duloxetine group (17.3%) and 11 in the placebo group (10.7%); P = 0.229]. Duloxetine-treated subjects reported insomnia, dry mouth, and constipation more frequently than placebo-treated subjects, but these side effects were mostly mild or moderate in severity.30 Duloxetine-treated patients experienced small, but significant increases in heart rate (P = 0.005) and small, non-significant increases in systolic and diasto lic blood pressure. Three patients (2 from the duloxetine group and 1 from the placebo group) experienced sustained hypertension (supine diastolic blood pressure ≥90 mm Hg and an increase from baseline of ≥10 mm Hg for at least 3 consecutive visits, or supine systolic blood pressure ≥140 mm Hg and an increase from baseline of ≥10 mm Hg for at least 3 consecutive visits), but the treatment group difference was not statistically significant.30 Mean change in weight was not significantly different between the two groups. Compared with placebo-treated subjects, duloxetine-treated subjects exhibited small, but significant increases in aspartate transaminase, creatine phosphokinase, and cholesterol and significant decreases in calcium and chloride. These differences, however, were within normal reference ranges and were not considered to be clinically relevant. No patients exhibited increased corrected QT intervals during the study.30
In the second article by Arnold et al the authors assessed the safety/tolerability of duloxetine specifically in women with FM with or without MDD through analysis of TEAEs, vital signs, physical findings, and laboratory values.69 Significantly more subjects in the duloxetine 60 mg twice daily group discontinued due to TEAEs than placebo-treated subjects (P = 0.025) [duloxetine 60 mg/day, 25 (21.2%); duloxetine 60 mg twice daily, 27 (23.3%); placebo 14 (11.7%)]. This differs from the first study where there were no significant differences between groups due to discontinuation from TEAEs.69 Importantly, the majority of patients who did discontinue due to TEAEs did so during the first 4 weeks of therapy. This could potentially be explained by the slower titration in the former study (20 mg/day to 60 mg twice daily in two weeks) compared with the latter study (60 mg/day to 60 mg twice daily in 3 days).69 This suggests that patients would tolerate duloxetine better if started at a lower dose and slowly titrated. Patients in the duloxetine-treated groups reported nausea, dry mouth, constipation, decreased appetite, and anorexia more frequently than did placebo-treated patients.69 Compared with placebotreated patients, duloxetine-treated patients exhibited small, but significant increases in alkaline phosphatase and decreases in chloride. These differences were within normal reference ranges and not considered clinically significant. Duloxetine-treated subjects also experienced slight, but significant decreases in mean weight and increases in systolic (P = 0.03) and diastolic (P = 0.03) blood pressure, but these changes were not clinically relevant.69 Two patients (1 from the duloxetine 60 mg twice daily group and 1 from the placebo group) experienced sustained hypertension, but the group differences were not significant. There were no significantly different changes in heart rate among the groups. This study also included a 1-week tapering phase in which significantly more duloxetine-treated patients experienced discontinuation-emergent adverse events, most commonly dizziness, compared with placebo-treated patients. Thus, tapering the dose of duloxetine at the end of therapy may be recommended.69
Arnold et al pooled data from the prior 2 RCTs to allow for a more comprehensive evaluation of safety and tolerability.73 Subjects in the duloxetine-treated group reported nausea, insomnia, headache, dry mouth, fatigue, constipation, dizziness, somnolence, decreased appetite, increased sweating, anorexia, feeling jittery, nervousness, decreased libido, and tremor more often than placebo-treated subjects. A total of 90 patients discontinued due to TEAEs (68 from the duloxetine group and 22 from the placebo group; P = 0.001).73 Compared with placebo-treated patients, duloxetine-treated patients exhibited significant mean increases in alkaline phosphatase, alanine transaminase/serum glutamate pyruvate transaminase (ALT/SGPT), aspartate transaminase/serum glutamic oxaloacetic transaminase (AST/SGOT), cholesterol, and creatine phosphokinase, and mean decreases in total bilirubin, calcium, chloride, and inorganic phosphorus. Compared with duloxetine-treated patients, placebo-treated patients demonstrated a significant mean decrease in basophils.73 These differences were within normal reference ranges and not considered to be clinically significant. There were no significant differences between the groups in systolic blood pressure, pulse, and weight. There was a small but significant difference in mean change in diastolic blood pressure in the placebo group compared with the duloxetine group. 7 patients (5 in the duloxetine group and 2 in the placebo group) experienced sustained hypertension, but the differences between groups were not significant.73
In the third study Russell et al analyzed the safety/tolerability of duloxetine for treatment of FM patients with or without MDD through changes in vital signs, physical findings, laboratory values, and EKG findings.70 There was a significant difference among treatment groups regarding discontinuation due to TEAEs: duloxetine 20/60 mg/day (11.4%), duloxetine 60 mg/day (15.3%), duloxetine 120 mg/day (27.2%), and placebo (13.2%, P = 0.005). Fifteen TEAEs occurred in at least one of the duloxetine-treated groups at a frequency >5% and twice the rate of the placebo group, with nausea being the most commonly reported TEAE in all of the treatment groups.70 There were no significantly different changes in heart rate, supine diastolic blood pressure, weight, or corrected QT intervals among the groups, but there was a significant difference with regards to the supine systolic blood pressure (duloxetine 60 mg/day, 3.0 mm Hg; placebo, −1.1 mm Hg, P = 0.019). 10 patients (6 in the 60 mg/day group, 2 in the 120 mg/day group, and 2 in the placebo group) experienced sustained hypertension, but the group differences were not significant.70 Small, but significant differences (all P < 0.05) were observed between duloxetine-treated and placebotreated subjects for the following laboratory values: alkaline phosphatase, chloride, cholesterol, γ-glutamyl transferase (GGT), sodium, eosinophils, hematocrit, hemoglobin, mean cell hemoglobin, mean cell volume, monocytes, and platelet count. However, these changes were not considered to be clinically relevant. Significantly higher levels of creatine phosphokinase were observed in duloxetine-treated patients compared with placebo-treated patients (20/60 mg/day group [15.3%, P = 0.038], 120 mg/day group [13.6%, P = 0.036], placebo group [5.7%]), but the mean changes were not significantly different among the groups.70
In the study conducted by Chappell et al the safety of duloxetine in patients with FM was assessed through examination of TEAEs, discontinuation due to adverse events, laboratory values, vital signs, and physical findings (ie, weight).71 The TEAEs experienced by ≥15% of patients in the overall study phase were nausea, headache, insomnia, dizziness, constipation, and dry mouth. The majority of these events were mild or moderate in severity. Of the 350 patients who entered the double-blinded phase of the study, 74 discontinued due to a TEAE. TEAEs reported as the reason for discontinuation by ≥1% of patients were insomnia, vomiting, diarrhea, dizziness, and nausea.71 There were no significant differences in reasons for discontinuations between the treatment groups. Abnormally high laboratory values reported by ≥5% of subjects were alkaline phosphatase, ALT/SGPT, AST/SGOT, cholesterol, creatine phosphokinase, GGT, and urea nitrogen. Low abnormal values observed in ≥5% of subjects were total bilirubin, cholesterol, and potassium. High and potentially clinically significant abnormal values were noted for cholesterol (2.1%) and GGT (1.5%). The mean change (SD) in sitting systolic blood pressure (mm Hg) was −0.1 (14.4), in sitting diastolic blood pressure was −0.2 (9.6), in sitting pulse rate was 1.9 (10.4) bpm, and in weight was 0.7 (4.3) kg. The increases in the sitting pulse rate (P < 0.001) and weight (P < 0.005) were found to be statistically significant, but were not considered clinically relevant.71
In another study conducted by Chappell et al the safety of duloxetine for the treatment of FM was assessed through evaluation of TEAEs, reasons for discontinuation, laboratory tests, vital signs, weight, and EKG findings.72 TEAEs that occurred in ≥5% of duloxetine-treated subjects and twice the rate of placebo-treated subjects include: nausea, headache, dry mouth, diarrhea, constipation, hyperhidrosis, arthralgia, somnolence, dyspepsia, and sleep disorder. 49 (14.8%) patients discontinued from the therapy phase due to a TEAE, with no significant differences between the two groups (duloxetine, 30 [18.5%]; placebo, 19 [11.3%]; P = 0.088). The most common adverse events reported as reasons for discontinuation in the duloxetine group were nausea, dizziness, diarrhea, lethargy, somnolence, and vomiting, as compared with the placebo group in which dizziness and irritability were the most commonly reported reasons.72 There were significant differences between the duloxetine-treated and the placebo-treated groups for the mean change in alkaline phosphatase, ALT/SGPT, total bilirubin, cholesterol, uric acid, sitting pulse rate (P = 0.016), diastolic blood pressure (P = 0.004), PR interval (P < 0.001), and heart rate (P < 0.001). Three patients in each group experienced sustained hypertension, but the differences between the groups were not significant. There were no significant differences between groups for the mean change in corrected QT interval or QRS interval.72
Choy et al pooled data from the prior 5 RCTs30,69–72 to reliably assess the safety and tolerability of duloxetine in the treatment of patients with FM. The most commonly reported pooled TEAEs with duloxetine were nausea (33.4%), headache (25.2%), dry mouth (19.2%), insomnia (16.9%), fatigue (12.3%), constipation (16.7%), diarrhea (12.9%), and dizziness (15.1%)79 (see Table 1). Most TEAEs were mild to moderate in severity and emerged early in treatment. About 20% of patients discontinued due to TEAEs in both the short-term and 1-year studies. This rate is somewhat higher than the discontinuation rate due to adverse events seen with duloxetine and other SNRIs for MDD (9%–11%).79 Clinicians should be aware of this discontinuation risk and weigh it against the benefits of treatment response in individual patients. Serious adverse events (SAEs) were uncommon, and there were no significant differences in SAEs between groups. Mean changes in vital signs and weight were small.79 Although duloxetine’s noradrenergic effect suggests that it may slightly increase heart rate, only 0.5% of patients in the 3- and 6-month studies, 0.1% of patients enrolled for 6 months or more, and 0.6% of patients in the 1-year study had a clinically relevant increase in pulse rate. Rates of clinically significant laboratory and EKG changes were low, with the exception of ALT values being >5 times the upper limit of normal in duloxetine-treated patients (0.6%) compared with placebo-treated patients (0%).79 However, the lack of cases that met criteria for Hy’s rule during either short- or long-term use suggests that the risk of hepatotoxicity for duloxetine in FM is very low. In the 1-year study, four patients (1.1%) had suicide- related behavior. However, without the presence of a placebo, this rate is difficult to interpret, especially because high rates of suicide have been demonstrated for patients with widespread pain syndromes like FM.79
Table 1.
Treatment emergent adverse events for duloxetine in fibromyalgia.
| Duloxetine-induced adverse effect | Placebo (n = 535) % | Duloxetine (n = 1947) % |
|---|---|---|
| Nausea | 11.4 | 33.4 |
| Headache | 12 | 25.2 |
| Dry mouth | 5.2 | 19.2 |
| Insomnia | 9.2 | 16.9 |
| Fatigue | 7.1 | 12.3 |
| Constipation | 3.6 | 16.7 |
| Diarrhea | 7.9 | 12.9 |
| Dizziness | 6.7 | 15.1 |
| Somnolence | 2.8 | 11.8 |
| Hyperhidrosis | 1.1 | 10.6 |
| Decreased appetite | 0.6 | 5.6 |
Finally, it is conceivable that duloxetine may lead to moderate changes in glycemia in diabetic patients with diabetic peripheral neuropathic pain.80 There does not seem to be, however, any robust evidence about duloxetine affecting glucose control in nondiabetic patients (with or without FM) or in diabetic patients with FM.
Comparative Efficacy and Harms
As of yet, there have not been any direct head-to-head comparisons of the three FDA approved drugs for FM.81 Häuser et al however, recently compiled data from 11 RCTs enrolling 6, 388 patients, which indirectly compared the benefits and harms of duloxetine, milnacipran, and pregabalin specifically in FM.81 The endpoints analyzed were reductions in pain, fatigue, sleep disturbance, depressed mood, HRQOL, and adverse events. They found that all 3 drugs were superior to placebo except for the following symptom-types: duloxetine for fatigue, milnacipran for sleep disturbances, and pregabalin for depressed mood.81 Häuser found the pooled NNTs for a 30% pain reduction to be as follows: duloxetine 7.2, milnacipran 19, and pregabalin 8.6. The authors showed that there was no significant difference among the three drugs in achieving a minimum 30% reduction in pain and discontinuation rates due to adverse events were similar.81 There were substantial differences in symptom-type alleviated and adverse effects produced for each particular drug. Duloxetine and pregabalin were superior to milnacipran for pain and sleep disturbance. Duloxetine was superior to milnacipran and pregabalin for depressed mood.81 Milnacipran and pregabalin were superior to duloxetine for fatigue. The risk of headache and nausea was higher with duloxetine and milnacipran compared with pregabalin. The risk of diarrhea was higher with duloxetine compared with milnacipran and pregabalin. The most frequent adverse effects noted in pregabalin-treated patients were weight gain and peripheral edema. Rare but serious adverse events reported were liver failure and suicidality for duloxetine and milnacipran, and heart failure for pregabalin. Häuser found the numbers needed to harm (NNHs) for discontinuation due to adverse effects to be as follows: duloxetine 14.9, milnacipran 7.6, and pregabalin 7.6.81
Based on these observations, choice of treatment medication should be tailored to fit individual patient needs and preferences, and appropriate monitoring and precautions should be taken to prevent adverse effects. For example, because of their inherent noradrenergic activity, duloxetine and especially milnacipran should be utilized with caution in patients with tachycardia and/or significant hypertension.81 Additionally, SNRIs may be suboptimal first-line agents in patients with significant chronic liver disease. Patients with concurrent depression should be monitored for suicidal ideation while being treated with duloxetine or milnacipran.81 Pregabalin might be preferred in patients with dyspepsia and irritable bowel syndrome, as gastrointestinal side effects are higher with the other two agents. Patients with tension headache or migraine should be monitored for exacerbations when treated with duloxetine and milnacipran. Pregabalin should be used with caution in patients with congestive heart failure and obesity, as well as patients with significant pre-existing peripheral edema.81
Conclusion
In conclusion, duloxetine 60–120 mg/day is demonstrated in clinical trials to be effective in reducing key symptoms of FM, such as pain, reduced functional capacity, and poor QOL. It is recommended that the dose be started low and gradually titrated so as to reduce or prevent adverse effects. In addition to its pain reducing effects, duloxetine was shown to produce greater effects on outcome measures in MDD subgroups compared with placebo, and to reduce depressed mood in FM patients with this predominant symptom-type. Since approximately 30% of FM patients suffer from MDD,82 this is an important observation as it suggests the use of duloxetine as single use therapy for both disorders. The utilization of duloxetine must be considered under the impression that there are other therapeutic options available that may better fit individual patient needs and preferences. For example, duloxetine was demonstrated to be inferior to milnacipran and pregabalin in reducing fatigue. It was shown, however, that duloxetine significantly improved “mental fatigue” compared with placebo after 6 months of therapy, a frequently reported cognitive complaint in FM patients. Thus, duloxetine appears to be a safe and efficacious treatment for FM and may be partially well-suited for patients with a predominant component of depressed mood, sleep disturbances, and/or mental fatigue.
It has been suggested that FM is a heterogeneous disorder, analogous to autoimmune disease or hypertension, and involves many neurotransmitter abnormalities that converge onto a final common pathway creating hyperalgesia.45 If this proves to be the case, the use of multiple medications simultaneously could possibly produce better outcomes in FM patients.45 Furthermore, the use of non-pharmacologic therapy, such as exercise, education, and CBT, which has been shown to be efficacious in certain aspects of FM, should be more frequently utilized in clinical practice.83,84
Acknowledgements
The authors would like to acknowledge Pya Seidner for her important efforts in the preparation of this manuscript.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Burckhardt CS, Clark SR, Bennett RM. Fibromyalgia and quality of life: a comparative analysis. J Rhematol. 1993;20:475–9. [PubMed] [Google Scholar]
- 3.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmona L, Ballina J, Gabriel R, Laffon A EPISER Study Group. The burden of musculoskeletal diseases in the general population of Spain: results from a national survey. Ann Rheum Dis. 2001;60:1040–5. doi: 10.1136/ard.60.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard AL, Prince A, Edsall P. Quality of life issues for fibromyalgia patients. Arthritis Care Res. 2000;13:42–50. [PubMed] [Google Scholar]
- 6.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–6. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 7.Xu XJ, Dalsgaard CJ, Wiesenfeld-Hallin Z. Spinal substance P and N-methyl-D-aspartate receptors are coactivated in the induction of central sensitization of the nociceptive flexor reflex. Neurosci. 1992;51:641–8. doi: 10.1016/0306-4522(92)90303-j. [DOI] [PubMed] [Google Scholar]
- 8.Bradley LA, Alberts KR, Alarcón GS, et al. Abnormal brain regional cerebral blood flow (rCBF) and cerebrospinal fluid (CSF) levels of substance P (SP) in patients and non-patients with fibromyalgia (FM) Arthritis Rheum. 1996;39(9 Suppl):S212. [Google Scholar]
- 9.Vaerøy H, Helle R, Førre O, Kåss E, Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain. 1988;32:21–6. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- 10.Russell IJ, Orr MD, Littman B, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593–601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Welin M, Bragee B, Nyberg F. A high-recovery extraction procedure for quantitative analysis of substance P and opioid peptides in human cerebropinal fluid. Peptides. 2000;21:853–60. doi: 10.1016/s0196-9781(00)00219-9. [DOI] [PubMed] [Google Scholar]
- 12.Bradley LA, Alarcóm GS. Is Chiari malformation associated with increased levels of substance P and clinical symptoms in persons with fibromyalgia? Arthritis Rheum. 1999;42:2731–2. doi: 10.1002/1529-0131(199912)42:12<2731::AID-ANR36>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Sarchielli P, Di Filippo M, Nardi K, Calabresi P. Sensitization, glutamate, and the link between migraine and fibromyalgia. Curr Pain Headache Rep. 2007;11:343–51. doi: 10.1007/s11916-007-0216-2. [DOI] [PubMed] [Google Scholar]
- 14.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for classification of fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 16.Macfarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol. 1996;23:1628–32. [PubMed] [Google Scholar]
- 17.Carville SF, Arendt-Nielsen S, Bliddal H, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536–41. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatolol. 2003;30:369–78. [PubMed] [Google Scholar]
- 19.Wilke WS. New developments in the diagnosis of fibromyalgia syndrome: Say goodbye to tender points? Cleve Clin J Med. 2009;76:345–52. doi: 10.3949/ccjm.76a.08062. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe F, Clauw D, Fitzcharles MA, et al. Clinical Diagnostic and Severity Criteria for Fibromyalgia Presentation Number: 567. ACR/ARHP Scientific MeetingPhiladelphia, PA2009 Oct 18(abstract) [Google Scholar]
- 21.Wolfe F, Clauw D, Fitzcharles MA, et al. The Instability of Fibromyalgia Diagnosis: Association with Measures of Severity. Presentation Number: 86. ACR/ARHP Scientific Meeting; Philadelphia, PA. 2009 Oct 18; (abstract) [Google Scholar]
- 22.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–33. [PubMed] [Google Scholar]
- 23.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating-characteristics, and uses. Clin Exp Rheum. 2005;23:S154–62. [PubMed] [Google Scholar]
- 24.Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11:R120. doi: 10.1186/ar2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB. Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol. 2009;36:1304–11. doi: 10.3899/jrheum.081090. [DOI] [PubMed] [Google Scholar]
- 26.Uçeyler N, Häuser W, Sommer C. A systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndrome. Arthritis Rheum. 2008;59:1279–98. doi: 10.1002/art.24000. [DOI] [PubMed] [Google Scholar]
- 27.Arnold LM, Keck PEJ, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41:104–13. doi: 10.1176/appi.psy.41.2.104. [DOI] [PubMed] [Google Scholar]
- 28.Goldenberg DL. Treatment of fibromyalgia syndrome. Rheum Dis Clin North Am. 1989;15:61–71. [PubMed] [Google Scholar]
- 29.Sayar K, Aksu G, Ak I, Tosum M. Venlafaxine treatment of fibromyalgia. Ann Pharmacother. 2003;37:1561–5. doi: 10.1345/aph.1D112. [DOI] [PubMed] [Google Scholar]
- 30.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–84. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 31.Gendreau RM, Thorn MD, Gendreau JF, et al. Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol. 2005;20:2975–85. [PubMed] [Google Scholar]
- 32.Smith HS, Barkin RL. Fibromyalgia syndrome: a discussion of the syndrome and pharmacotherapy. Am Ther. 2010;17:418–39. doi: 10.1097/MJT.0b013e3181df8e1b. [DOI] [PubMed] [Google Scholar]
- 33.Vitton O, Gendreau M, Gendreau J, Kranzler J, Rao SG. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum Psychopharmacol. 2004;19:S27–35. doi: 10.1002/hup.622. [DOI] [PubMed] [Google Scholar]
- 34.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30:1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Mease PJ, Clauw DJ, Gemdreau RM, et al. The efficacy and safety of milnacipran for treatment of fibromyalgia, a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36:398–409. doi: 10.3899/jrheum.080734. [DOI] [PubMed] [Google Scholar]
- 36.Häuser W, Bernardy K, Uçeyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—a meta-analysis of randomized controlled trials. Pain. 2009;145:69–81. doi: 10.1016/j.pain.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Clauw DJ. Alpha-2-delta ligands in fibromyalgia: Is the glass half empty or full? Pain. 2009;145:8–9. doi: 10.1016/j.pain.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Crofford LJ, Mease PJ, Simpson SL, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalin. Pain. 2008;136:419–31. doi: 10.1016/j.pain.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336–44. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 40.Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatol. 2003;30:1070–4. [PubMed] [Google Scholar]
- 41.Holman AJ, Myers RR. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005;52:2495–505. doi: 10.1002/art.21191. [DOI] [PubMed] [Google Scholar]
- 42.Bennett RM, Kamin M, Mrim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114:537–45. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 43.Russell IJ, Kamin M, Bennett RM, Schnitzer TJ, Gren JA, Katz WA. Efficacy of Tramodol in Treatment of Pain in Fibromylagia. J Clin Rheumatol. 2000;6:250–7. doi: 10.1097/00124743-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Russell IJ, Michalek JE, Xiao Y, Haynes W, Vertiz R, Lawrence RA. Therapy with a central alpha 2-adrenergic agonist (tizanidine) decreases cerebrospinal fluid substance P, and may reduce serum hyaluronic acid as it improves the clinical symptoms of the fibromyalgia syndrome. Arthritis Rheum. 2002;46:S614. [Google Scholar]
- 45.Clauw DJ. Pain management: Fibromyalgia drugs are ‘as good as it gets’ in chronic pain. Nat Rev Rheumatol. 2010;6:439–40. doi: 10.1038/nrrheum.2010.120. [DOI] [PubMed] [Google Scholar]
- 46.Jones KD, Liptan GL. Exercise interventions in fibromyalgia: clinical applications from the evidence. Rheum Dis Clin N Am. 2009;35:373–91. doi: 10.1016/j.rdc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Thomas EN, Blotman F. Aerobic exercise in fibromyalgia: a practical review. Rheumatol Int. 2010;30:1143–50. doi: 10.1007/s00296-010-1369-6. [DOI] [PubMed] [Google Scholar]
- 48.Figueroa A, Kingsley JD, McMillan V, Panton LB. Resistance exercise training improves heart rate variability in women with fibromyalgia. Clin Physiol Funct Imaging. 2008;28:49–54. doi: 10.1111/j.1475-097X.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 49.Valkeinen H, Alen M, Hakkinen A, Hannonen P, Kukkonen-Harjula K, Häkkinen K. Effects of concurrent strength and endurance training on physical fitness and symptoms in postmenopausal women with fibromyalgia: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:1660–6. doi: 10.1016/j.apmr.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 50.Jones KD, Burckhardt CS, Clark SR, Bennett RM, Potempa KM. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J Rheumatol. 2002;29:1041–8. [PubMed] [Google Scholar]
- 51.Matsutani LA, Marques AP, Ferreira EA, et al. Effectiveness of muscle stretching exercises with and without laser therapy at tender points for patients with fibromyalgia. Clin Exp Rheumatol. 2007;25:410–5. [PubMed] [Google Scholar]
- 52.Haak T, Scott B. The effect of Qigong on fibromyalgia (FMS): a controlled randomized study. Disabil Rehabil. 2008;30:625–33. doi: 10.1080/09638280701400540. [DOI] [PubMed] [Google Scholar]
- 53.da Silva GD, Lorenzi-Filho G, Lage LV. Effects of yoga and the addition of Tui Na in patients with fibromyalgia. J Altern Complement Med. 2007;13:1107–13. doi: 10.1089/acm.2007.0615. [DOI] [PubMed] [Google Scholar]
- 54.Rooks DS, Gautam S, Romeling M, et al. Group exercise, education, and combination self-management in women with fibromyalgia: a randomized trial. Arch Intern Med. 2007;167:2192–200. doi: 10.1001/archinte.167.20.2192. [DOI] [PubMed] [Google Scholar]
- 55.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–84. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 56.Hassett AL, Gevirtz RN. Nonpharmacologic treatment for fibromyalgia: patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum Dis Clin North Am. 2009;35:393–407. doi: 10.1016/j.rdc.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thieme K, Flor H, Turk D. Psychological pain treatment in fibromyalgia syndrome: efficacy of operant behavioural and cognitive behavioural treatments. Arthritis Res Ther. 2006;8:121–32. doi: 10.1186/ar2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome—a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- 59.Allen LA, Woolfolk RL, Escobar JI, Gara MA, Hamer RM. Cognitive-behavioral therapy for somatization disorder: a randomized controlled trial. Arch Intern Med. 2006;166:1512–8. doi: 10.1001/archinte.166.14.1512. [DOI] [PubMed] [Google Scholar]
- 60.Jacobson E. Progressive relaxation. Chicago: University of Chicago Press; 1938. [Google Scholar]
- 61.Luthe W, Schultz JH. Autogenic therapy. New York: The British Autogenic Society; 1969. First published by Grune and Stratton, Inc. Republished in 2001. [Google Scholar]
- 62.Thieme K, Gracely RH. Are psychological treatments effective for fibromyalgia pain? Curr Rheumatol Rep. 2009;11:443–50. doi: 10.1007/s11926-009-0065-6. [DOI] [PubMed] [Google Scholar]
- 63.Häuser W, Bernardy K, Arnold B, Offenbächer M, Schiltenwolf M. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61:216–24. doi: 10.1002/art.24276. [DOI] [PubMed] [Google Scholar]
- 64.Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–47. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 65.Hansten PD, Horn JR. The top 100 Drug Interactions-a Guide to Patient Management. Freeland, WA: H&H Publicatons, LLP; 2010. [Google Scholar]
- 66.Wickersham RM. Senior Managing editor, Drug Facts and comparisons. E-answers 2010 Wolter Kluwer, Health Inc Referred 3/1/2010
- 67.Barkin RL. Duloxetine: A Pharmacological Overview. Pain Medicine Network. 2007 Fall;22:8–11. [Google Scholar]
- 68.Curran MP. Duloxetine: in patients with fibromyalgia. Drugs. 2009;69:1217–27. doi: 10.2165/00003495-200969090-00006. [DOI] [PubMed] [Google Scholar]
- 69.Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119:5–15. doi: 10.1016/j.pain.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 70.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebocontrolled, fixed-dose trial. Pain. 2008;136:432–44. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Chappell AS, Littlejohn G, Kajdasz DK, Scheinberg M, D’Souza DN, Moldofsky H. A 1-year safety and efficacy study of duloxetine in patients with fibromyalgia. Clin J Pain. 2009;25:365–75. doi: 10.1097/AJP.0b013e31819be587. [DOI] [PubMed] [Google Scholar]
- 72.Chappell AS, Bradley LA, Wiltse C, Detke MJ, D’Souza DN, Spaeth M. A six-month double-blind, placebo-controlled, randomized clinical trial of duloxetine for the treatment of fibromyalgia. Int J Gen Med. 2008;1:91–102. doi: 10.2147/ijgm.s3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnold LM, Pritchett YL, D’Souza DN, Kajdasz DK, Iyengar S, Wernicke JF. Duloxetine for the treatment of fibromyalgia in women: pooled results from two randomized, placebo-controlled clinical trials. J Womens Health (Larchmt) 2007;16:1145–56. doi: 10.1089/jwh.2006.0213. [DOI] [PubMed] [Google Scholar]
- 74.Arnold LM, Clauw DJ, Wohlreich MM. Efficacy of duloxetine in patients with fibromyalgia: pooled analysis of 4 placebo-controlled clinical trials. Prim Care Companion J Clin Psychiatry. 2009;11:237–44. doi: 10.4088/PCC.08m00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;3:89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 76.Retherford RD, Choe MK. Statistical methods for caused analysis. New York: John Wiley & Sons; 1993. [Google Scholar]
- 77.Bradley LA, Wohlreich MM, Bradley LA, et al. Pain response profile of patients with fibromyalgia treated with duloxetine. Clin J Pain. 2010;26:498–504. doi: 10.1097/AJP.0b013e3181dee80e. [DOI] [PubMed] [Google Scholar]
- 78.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev. 2009;4:CD007115. doi: 10.1002/14651858.CD007115.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Choy EH, Mease PJ, Kajdasz DK, et al. Safety and tolerability of duloxetine in the treatment of patients with fibromyalgia: pooled analysis of data from five clinical trials. Clin Rheumatol. 2009;28:1035–44. doi: 10.1007/s10067-009-1203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hardy T, Sachson R, Shen S, Armbruster M, Boulton AJ. Does treatment with duloxetine for neuropathic pain impact glycemic control? Diabetes Care. 2007;30:21–6. doi: 10.2337/dc06-0947. [DOI] [PubMed] [Google Scholar]
- 81.Häuser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain. 2010;11:505–21. doi: 10.1016/j.jpain.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 82.White KP, Nielson WR, Harth M, Ostbye T, Speechley M. Chronic wide-spread musculoskeletal pain with or without fibromyalgia: psychological distress in a representative community adult sample. J Rheumatol. 2002;29:588–94. [PubMed] [Google Scholar]
- 83.Chou R, Huffman LH American Pain Society; American College of Physicians. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 84.Williams DA. In: Fibromyalgia and other central pain syndromes. Wallace DJ, Clauw DJ, editors. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 343–52. [Google Scholar]