Abstract
Aim
To assess serum Cu/Zn SOD (Superoxide Dismutase) concentration in children with ADHD and evaluate its possible relationship to Cu and Zn levels.
Subjects and methods
Serum from 22 children with ADHD and 20 healthy control children without ADHD and 19 autistic children without ADHD were tested for Cu/Zn SOD using ELISAs and levels of serum Cu and Zn using inductively-coupled plasma-mass spectrometry.
Results
Serum Cu/Zn SOD levels of ADHD children were significantly lower than age and gender matched healthy non-ADHD controls (P < 0.001). Serum Cu/Zn SOD of ADHD children was significantly lower in individuals with high serum copper (P = 0.024). There was no significant correlation between Cu/Zn SOD levels and Zinc or Cu/Zn in ADHD individuals.
Discussion
These results suggest an association between Cu/Zn SOD serum levels and ADHD, particularly ADHD children with high serum copper.
Keywords: ADHD, Cu/Zn SOD, super oxide dismutase, copper
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood disorders and can continue through adolescence and adulthood. Symptoms include difficulty staying focused and paying attention, difficulty controlling behavior, and hyperactivity.1
Molecular, genetic and pharmacological studies suggest the involvement of the dopaminergic, serotonergic and noradrenergic neurotransmitter systems in the pathogenesis of ADHD, and polymorphic variants in several genes involved in regulation of the dopamine, and related neurotransmitter pathways are reported to be associated with the disease.2–4
There are few studies evaluating the biochemical basis of ADHD. However, in 2008, Selek et al5 found that adults with ADHD had high levels of the oxidant nitrous oxide, and low super oxide dismutase activities, suggesting an oxidative imbalance.
In vivo, oxygen radicals are produced as byproducts of normal oxidative metabolism.6 Hence, activated cells with increased metabolism produce more oxygen radicals. It has long been known that control of the intracellular redox environment is vital for proper cellular function. To protect themselves from the constant oxidative challenge, cells have developed defense mechanisms that ensure a proper balance between pro- and antioxidant molecules.7 Cu/Zn superoxide dismutase (SOD-1) is a key enzyme in the dismutation of superoxide radicals resulting from cellular oxidative metabolism, converting them into hydrogen peroxide,8 and, as a result, serves a key antioxidant role. In fact, mice lacking SOD die several days after birth, amidst massive oxidative stress.11
Copper, and zinc participate in SOD enzymatic mechanisms that protect against free radicals ad therefore serve an important adjunct role in oxidative balance.12
Cu appears to have many important functional roles in the body that apparently relate to, among others, the maintenance of immune function, bone health and haemostasis.9 The element is needed in trace amounts, but excess is toxic. It increases lipid per-oxidation and depletes glutathione reserves, which makes the organism more vulnerable to other oxidative challenges.10
Zinc to copper ratio is abnormally low in individuals with other disorders associated with hyperactivity, such as autism13 and in boys with oppositional defiant disorder.14 Low zinc may be associated with ADHD individuals16 and has been directly related to low Cu/Zn SOD concentration.15
Our study was designed to determine Cu/Zn SOD serum levels, and the relationship between these levels and copper and zinc concentration, in ADHD individuals.
Materials and Methods
ELISA to measure serum Cu/Zn SOD (Bender Systems)
All reagents and specimens were equilibrated to room temperature before the assay was performed. A 1:51 dilution of the patient samples was prepared by mixing 10 μl of the patient’s sera with 0.5 ml of Serum Diluent. One hundred microliters of calibrators (.08–2.5 ng/ml Cu/Zn SOD), serum diluent alone, and diluted patient samples were added to the appropriate microwells of a microculture plate (each well contained affinity purified polyclonal IgG to Cu/Zn SOD). Wells were incubated for 60 minutes (±5 min) at room temperature, then washed 4× with wash buffer. One hundred microliters of pre-diluter anti-human Cu/Zn SOD IgG conjugated with HRP was added to all microwells, incubated for 30 minutes (±5 min) at room temperature, then washed 4× with wash buffer. One hundred microliters of enzyme substrate was added to each microwell. After approximately 30 minutes at room temperature, the reaction was stopped by adding 50 μl of 1M sulfuric acid, then the wells were read at 450 nm with an ELISA reader (BioRad Laboratories, Inc., Hercules, CA, USA).
Copper and zinc serum concentration
Copper and zinc plasma concentration was performed by LabCorp, Inc. (NAPERVILLE, IL 60563) using inductively-coupled plasma-mass spectrometry.
Subjects
Experimental
Serum from ADHD individuals (n = 22) was obtained from patients presenting at the Health Research Institute/Pfeiffer Treatment Center. Most of these children (median age 10.2 years; 17 male) were diagnosed using Connor’s Parent or Teacher scale before presenting at Pfeiffer, and had no known co-morbidities.
Controls
Serum and medical history of controls (n = 20) were obtained from the Autism Genetic Resource Exchange—AGRE.a Through analysis of an extensive medical history and parental questionnaire provided by AGRE, it was established that none of these individuals had family history of ADHD or any other similar behavioral disorders. The controls were age and gender matched to experimental group (media age 12.8 years; 18 males).
Serums
Experimental (Pfeiffer Treatment Centerb) and control (AGRE) serums were all morning draws and then treated in an identical fashion—frozen at −70C immediately after collection and cell/serum separation, then stored at −70C until thawed for use in ELI-SAs or sent to LabCorp for Cu and Zn analysis.
Statistics
Inferential statistics were derived from t-test and odds ratios with 95% confidence intervals. ANOVA analysis was used to do an analysis of variance and multiple comparisons.
Results
Serum from 22 ADHD children, and 20 non-ADHD age/gender matched controls and 19 non-ADHD autistic age/gender matched controls was tested for Cu/Zn SOD plasma concentration using an ELISA (described above). Each assay was repeated two or more times, with multiple wells for each serum in each assay.
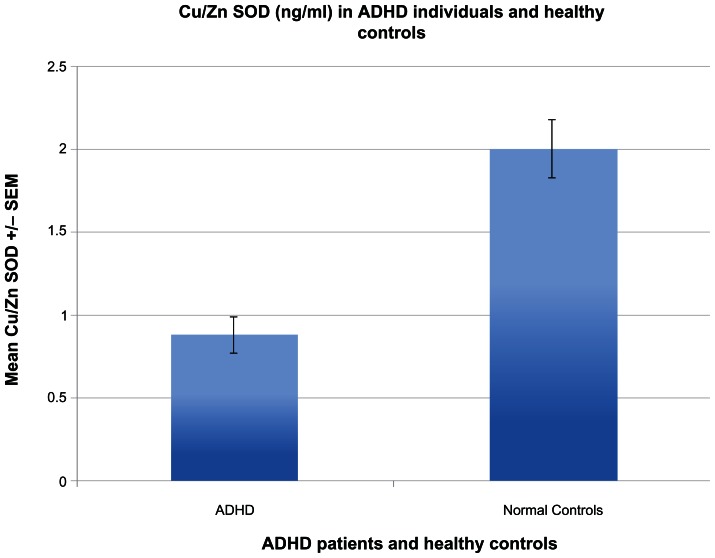
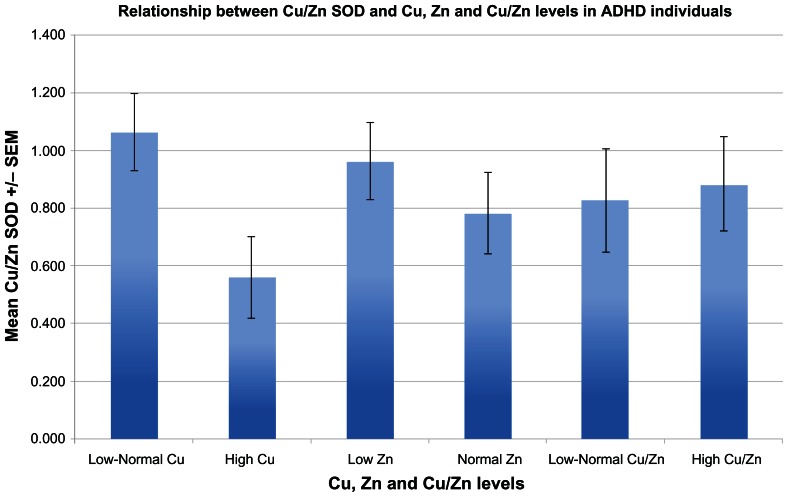
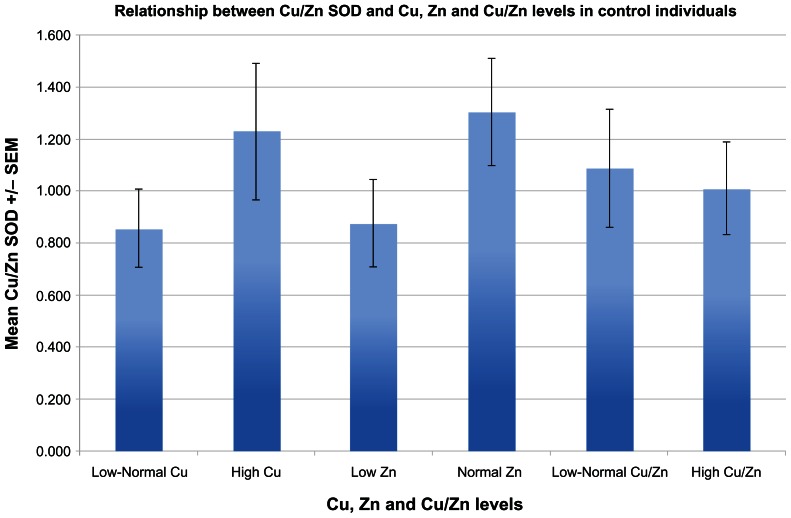
Serum Cu/Zn SOD levels of ADHD children were significantly lower than all non-ADHD controls (P < 0.001) (Fig. 1). Serum Cu/Zn SOD in ADHD children was significantly lower in individuals with high serum copper (P = 0.024) (Fig. 2), whereas serum Cu/Zn SOD of controls (autistic) were not found to be related to copper levels (Fig. 3).
Figure 1.
Shows the significant difference between Cu/Zn SOD serum levels in ADHD individuals, compared to normal healthy controls.
Figure 2.
Shows the relationship between Cu/Zn SOD and Cu, Zn and Cu/Zn levels in ADHD individuals (n = 20). Cu/Zn SOD levels were significantly lower when accompanied by high Cu (P = 0.024), but not zinc (P = 0.431) or Cu/Zn (P = 0.823).
Figure 3.
Shows the relationship between Cu/Zn SOD and Cu, Zn and Cu/Zn levels in non-ADHD controls (autistic) (n = 19). Cu/Zn SOD levels were not significantly different when accompanied by high Cu (P = 0.220), but not zinc (P = 0.127) or Cu/Zn (P = 0.778).
We did not find any significant difference between Cu/Zn SOD concentration and zinc levels or ratio of zinc to copper in experimental or control groups (Figs. 2 and 3).
Discussion
Oxidative stress has been implicated in the pathogenesis of a diverse group of disease states, and, because the brain has comparatively greater vulnerability to oxidative damage, may be a common pathogenic mechanism underlying many major psychiatric disorders.
A small number of studies have focused upon oxidative stress in humans with ADHD, demonstrating increased levels of 8-oxoguanine,17 n-3 fatty acid oxidation,18 urinary catecholamines,19 glutathione,20 and lower levels of long-chain polyunsaturated fatty acids.21
Studies have also shown changes in antioxidant enzyme activities, particularly Cu/Zn SOD, following methylphenidate therapy.22
Our preliminary data shows that ADHD individuals have significantly lower levels of serum Cu/Zn SOD and supports these previous findings.
Zinc and copper are essential trace elements and, as such, members of major subgroups of micronutrients that have attained prominence in human nutrition and health. Several studies have investigated the role of zinc in the etiology of ADHD and have suggested that zinc deficiency is associated with the pathophysiology of the disease.23–25 Research has also demonstrated that zinc treatment may be efficacious for ADHD individuals.26
Abnormal plasma levels of copper have also been found in hyperactive children.27
This study suggests that high copper is associated with low Cu/Zn SOD levels in ADHD individuals. Recent studies have shown that ADHD individuals have lower dopamine activity in the caudate and limbic system,31 as well as lower dopamine receptor levels in the midbrain and accumbens.30 Since it has been suggested that high copper induces damage of dopaminergic neurons through destroying antioxidant defenses, such as lowering Cu/Zn SOD levels in rats,28,29 we suggest that a similar mechanism may be involved in the etiology of ADHD.
Footnotes
The Autism Genetic Resource Exchange (AGRE) is the first collaborative gene bank for the study of autism spectrum disorders and one of the world’s largest shared resources for the study of autism and related disorders, with a collection of over 900 well-characterized multiplex and simplex families made available to the greater scientific community. Founded by Cure Autism Now (CAN) in 1997, AGRE is currently funded by the National Institute of Mental Health (NIMH) and Autism Speaks (AS), which merged with CAN in 2006.
The Pfeiffer Treatment Center is a comprehensive treatment and research center, specializing in the care of children with neurological disorders, including ADHD.
Disclosure
This manuscript has been read and approved by the author. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The author and peer reviewers of this paper report no conflicts of interest. The author confirms that they have permission to reproduce any copyrighted material.
References
- 1.DSM-IV-TR workgroup. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; [Google Scholar]
- 2.Asherson P. The IMAGE Consortium: Attention-deficit hyperactivity disorder in the post-genomic era. Eur Child Adolesc Psychiatry. 2004;13:50–70. doi: 10.1007/s00787-004-1006-6. [DOI] [PubMed] [Google Scholar]
- 3.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Thapar A, Langley K, Owen MJ, O’donovan MC. Advances in genetic findings on attention deficit hyperactivity disorder. Psychol Med. 2007;37:1681–92. doi: 10.1017/S0033291707000773. [DOI] [PubMed] [Google Scholar]
- 5.Selek S, et al. Oxidative imbalance in adult attention deficit/hyperactivity disorder. Journal of Biopsychology. 2008;06:005. [Google Scholar]
- 6.Malmstrom BG. Enzymology of oxygen. Annu Rev Biochem. 1982;51:21. doi: 10.1146/annurev.bi.51.070182.000321. [DOI] [PubMed] [Google Scholar]
- 7.Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 9.Bonham M, O’Connor JM, Hannigan BM, Strain JJ. The immune system as a physiological indicator of marginal copper status? Br J Nutr. 2002 May;87(5):393–403. doi: 10.1079/BJNBJN2002558. [DOI] [PubMed] [Google Scholar]
- 10.Ozcelik D, et al. Copper-mediated oxidative stress in rat liver. Biological Trace Element Research. 2007;96:209–15. doi: 10.1385/BTER:96:1-3:209. [DOI] [PubMed] [Google Scholar]
- 11.Horvath K, Perman JA. Autism and gastrointestinal symptoms. Curr Gastroenterol Rep. 2002;4:251–8. doi: 10.1007/s11894-002-0071-6. [DOI] [PubMed] [Google Scholar]
- 12.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006 Sep-Oct;10(5):377–85. [PubMed] [Google Scholar]
- 13.Faber S, Zinn GM, Kern JC, 2nd, Kingston HM. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers. 2009;14:171–80. doi: 10.1080/13547500902783747. [DOI] [PubMed] [Google Scholar]
- 14.Yorbik O, Olgun A, Kirmizigül P, Akman S. Plasma zinc and copper levels in boys with oppositional defiant disorderTurk Psikiyatri Derg. Winter. 2004;15(4):276–81. [PubMed] [Google Scholar]
- 15.Pandey Nalini, a, Pathak Girish Chandra, a, Singh Amit Kumar, a, Sharma Chandra Prakash. Enzymic changes in response to zinc nutrition. Journal of Plant Physiology. 2002;159:1151–3. [Google Scholar]
- 16.Yorbik O, et al. Potential effects of zinc on information processing in boys with attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Apr 1;32(3):662–7. doi: 10.1016/j.pnpbp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Chovanová Z, et al. Effect of polyphenolic extract, Pycnogenol, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorderFree. Radic Res. 2006 Sep;40(9):1003–10. doi: 10.1080/10715760600824902. [DOI] [PubMed] [Google Scholar]
- 18.Ross BM, McKenzie I, Glen I, Bennett CP. Increased levels of ethane, a non-invasive marker of n-3 fatty acid oxidation, in breath of children with attention deficit hyperactivity disorder. Nutr Neurosci. 2003 Oct;6(5):277–81. doi: 10.1080/10284150310001612203. [DOI] [PubMed] [Google Scholar]
- 19.Dvoráková M, et al. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): modulation by a polyphenolic extract from pine bark (pycnogenol) Nutr Neurosci. 2007 Jun-Aug;10(3–4):151–7. doi: 10.1080/09513590701565443. [DOI] [PubMed] [Google Scholar]
- 20.Dvoráková M. The effect of polyphenolic extract from pine bark, Pycnogenol on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD) Redox Rep. 2006;11(4):163–72. doi: 10.1179/135100006X116664. [DOI] [PubMed] [Google Scholar]
- 21.Antalis CJ. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2006 Oct-Nov;75(4–5):299–308. doi: 10.1016/j.plefa.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Gomes KM, et al. Antioxidant enzyme activities following acute or chronic methylphenidate treatment in young rats. Neurochem Res. 2008 Jun;33(6):1024–7. doi: 10.1007/s11064-007-9544-1. [DOI] [PubMed] [Google Scholar]
- 23.Toren P, Elder S, Sela BA, et al. Zinc deficiency in attention deficit hyperactivity disorder. Biol Psychiat. 1996;40:1308–10. doi: 10.1016/S0006-3223(96)00310-1. [DOI] [PubMed] [Google Scholar]
- 24.Bekarglu M, Aslan Y, Gedik Y, et al. Relation between serum free fatty acids and zinc, and attention deficit hyperactivity disorder: A research note. J Child Psyhol Psychiat. 1996;37:225–7. doi: 10.1111/j.1469-7610.1996.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 25.Kirby K, Floriani V, Bernstein H. Diagnosis and management of attention-deficit hyperactivity disorder in children. Cur Opinion in Pediatr. 2001;13:190–9. doi: 10.1097/00008480-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Bilici M, Yildirim F, Kandil S, et al. Double blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder. Prog in Neuropsychopharm and Biol Psychiat. 2004;28:181–90. doi: 10.1016/j.pnpbp.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Kozielec T, Starobrat-Hermelin B, Kotkowiak L. Deficiency of certain trace elements in children with hyperactivity. Psychiatr Pol. 1994 May-Jun;28(3):345–53. [PubMed] [Google Scholar]
- 28.Yu WR, Jiang H, Wang J, Xie JX. Copper (Cu2+) induces degeneration of dopaminergic neurons in the nigrostriatal system of rats. Neurosci Bull. 2008 Apr;24(2):73–8. doi: 10.1007/s12264-008-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Li-Min, et al. Mitochondria dysfunction was involved in copper-induced toxicity in MES23.5 cells. Neurosci Bull. 2008;24(2):79–83. doi: 10.1007/s12264-008-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, et al. Evaluating Dopamine Reward Pathway in ADHD: Clinical Implications. JAMA. 2009;302:1084–91. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkow ND, et al. Depressed Dopamine Activity in Caudate and Preliminary Evidence of Limbic Involvement in Adults With Attention-Deficit/Hyperactivity. Disorder Arch Gen Psychiatry. 2007;64(8):932–40. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]