Abstract
During axon pathfinding, growth cones commonly show changes in sensitivity to guidance cues that follow a cell-intrinsic timetable. The cellular timer mechanisms that regulate such changes are, however, poorly understood. Here we have investigated microRNAs (miRNAs) in the timing control of sensitivity to the semaphorin Sema3A in Xenopus laevis retinal ganglion cell (RGC) growth cones. A developmental profiling screen identified miR-124 as a candidate timer. Loss of miR-124 delayed the onset of Sema3A sensitivity and concomitant neuropilin-1 (NRP1) receptor expression and caused cell-autonomous pathfinding errors. CoREST, a cofactor of a NRP1 repressor, was newly identified as a target and mediator of miR-124 for this highly specific temporal aspect of RGC growth cone responsiveness. Our findings indicate that miR-124 is important in regulating the intrinsic temporal changes in RGC growth cone sensitivity and suggest that miRNAs may act broadly as linear timers in vertebrate neuronal development.
Axons navigate in a complex and changing environment to establish connections with their targets. Chemotropic cues in this environment attract and repel growing axons1,2, and growth cones must modulate their responsiveness en route to avoid stalling at attractive intermediate targets or invading non-targets. Growth cones of commissural neurons in the vertebrate spinal cord, for example, are initially attracted to Netrin-1 and unresponsive to Slits, but, after crossing the midline (an intermediate target), they become unresponsive to Netrin-1 and repelled by Slits3,4. Similarly, RGC axons change their responsiveness to several cues as they advance along the pathway5–7 with growth cones initially showing attraction to Netrin-1 and neutral responses to repellents (Sema3A and Slit2) and later gaining sensitivity to repellents and switching the polarity of their response to Netrin-1. This developmental regulation helps to match growth cone sensitivity appropriately with pathway stimuli and thereby ensures correct navigation.
The timetable of changes in growth cone sensitivity can be remarkably precise, much like other aspects of neuronal differentiation, raising the question of how it is controlled. Whereas evidence in spinal cord commissural neurons favors extrinsic midline-mediated control of growth cone sensitivity3,4,8, in RGC axons, the developmental changes are primarily under intrinsic control. Pathway-naive RGC growth cones in vitro, for example, show the same program of changes in cue sensitivity, polarity switching and receptor expression and with a similar timetable as pathway-experienced growth cones in vivo5,6. These findings point to the involvement of an intrinsic molecular clock, or ‘linear timer’. Such timers regulate the linear progression of serial events in these postmitotic neurons and could serve to synchronize the sensitivity of advancing growth cones with their changing environment. Whereas much is known about the molecular basis of cyclical clocks that control repetitive processes such as the cell cycle and somite segmentation9,10, little is known about linear timers that control the sequential program of cell differentiation.
miRNAs are emerging as key molecules regulating the linear timing of developmental events in Caenorhabditis elegans11–13 and vertebrates14. miRNAs are small, noncoding RNAs of ~22 nucleotides that pair to the 3′ untranslated region (UTR) of target mRNAs to repress their expression by inducing either their degradation or translational repression15. Of interest, several miRNAs have been reported to be specifically distributed within the nervous system, including in differentiating neurons16,17. Thus, miRNAs are prime candidate regulators of growth cone age-related changes. We have therefore investigated whether miRNAs contribute to the intrinsic temporal regulation of growth cone responsiveness to Sema3A. We identify miR-124 as a strong candidate in RGCs, on the basis of developmental miRNA profiling of different age retinas and in situ hybridization (ISH). Using a loss-of-function approach, we demonstrate that miR-124 regulates the onset of growth cone responsiveness to Sema3A. Moreover, we show that miR-124 acts by targeting the mRNA encoding CoREST (alternative name, rcor1), a known cofactor of REST (repressor element 1 silencing transcription factor), which in turn represses transcription of NRP1, encoding a Sema3A receptor. We propose that in normal development miR-124 causes the upregulation of NRP1 and the onset of sensitivity of growth cones to Sema3A, which is essential for normal axon guidance.
RESULTS
miRNA profiling identifies miR-124 as candidate timer
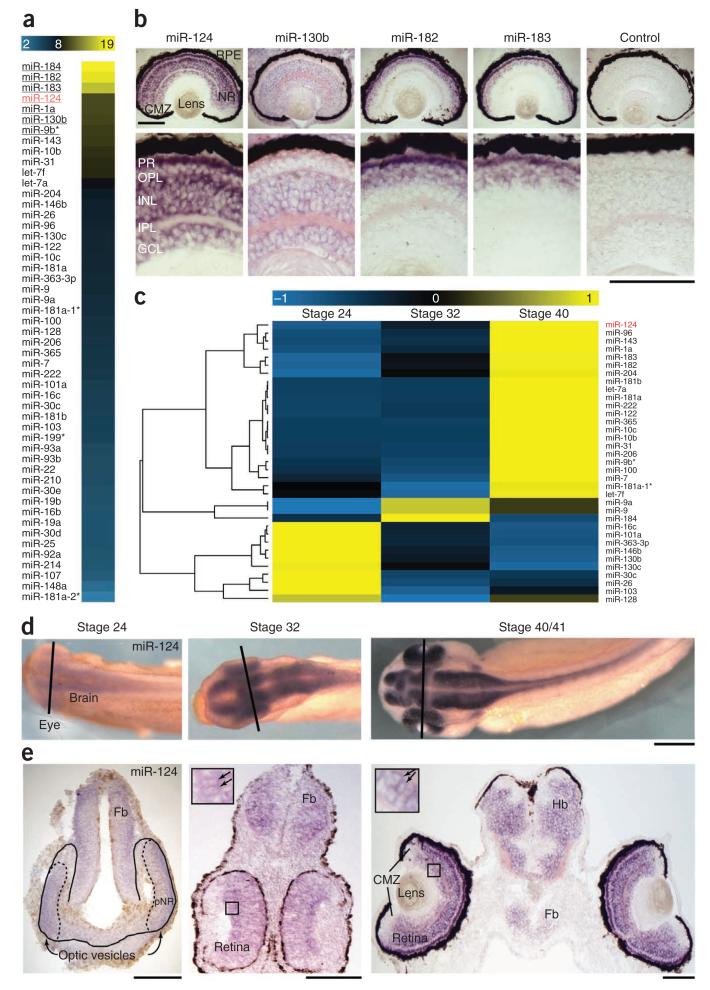
To determine which miRNA(s) might be involved in RGC growth cone aging, we identified the repertoire of miRNAs in Xenopus RGCs. We first profiled miRNAs in stage 40 retinas, when most RGCs have already been born, using Illumina sequencing, which detected 51 miRNAs at stage 40 (Fig. 1a). Previously identified timer miRNAs, including orthologs of miRNAs shown to regulate developmental timing in C. elegans18 (let-7a-i, miR-98, miR-125a and miR-125b), were either absent or were not among the most abundant miRNAs in the retina (Fig. 1a). ISH analysis of the distribution of the most abundant (>1,000 reads) miRNAs showed miR-124, miR-130b, miR-182 and miR-183 in the neural retina (Fig. 1b), whereas miR-184 and miR-1a were detected solely in extra-retinal tissues (Supplementary Fig. 1). miR-124 and miR-130b were the only two to be detected in RGCs, although they were also present in other retinal cells (Fig. 1b). miR-130b, however, was found to be ubiquitously distributed in all cells of the Xenopus head, whereas miR-124 was present exclusively in postmitotic neurons (Fig. 1b), in agreement with mammalian studies19,20. These initial results, therefore, identified the neuron-specific miR-124 as a candidate of interest.
Figure 1.
Expression profiling of miRNAs in the developing retina. (a) Heat map representing the log2 of the normalized number of reads for the 51 miRNAs profiled in stage 40 retina (neural retina and retinal pigmented epithelium) using Illumina sequencing. The names of miRNAs with >1,000 reads are underlined. (b) Retinal distribution of the most abundant miRNAs at stage 40 by ISH. Only miR-124 and miR-130b are distributed in RGCs, along with other retinal cells, whereas miR-182 and miR-183 are located in the developing photoreceptor layers. (c) Hierarchical clustering of the 35 miRNAs detected with >50 reads at any of the stages studied. The heat map shows the relative standardized miRNA expression (Z-score) indicated in the color key. There were 10, 22 and 3 miRNAs that appeared, respectively, to decrease over time, increase over time or peak at stage 32. (d,e) ISH on whole mounts (d) or tissue sections (e) showing miR-124 detection in the spinal cord, brain and retina of Xenopus embryos, including in differentiating RGCs (arrows in insets). Lines in d indicate approximate planes of section shown below in e; boxed regions in e are magnified in insets. miR-124 was absent or low in proliferating cells and in the ciliary marginal zone (CMZ). Fb, forebrain; GCL, ganglion cell layer; Hb, hindbrain; INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; PR, photoreceptor layer; NR, neural retina; pNR, presumptive NR (dashed outline) within optic vesicles (solid outline); RPE, retinal pigmented epithelium. Scale bars, 100 μm (b,e); 250 μm (d).
We reasoned that a linear timer would be expected to show progressive changes in expression with time, as shown for miRNAs involved in developmental timing11–13,18. Therefore, we next quantified miR-124 expression at three developmental stages (24, 32 and 40) that span the period of cell proliferation and RGC differentiation, including axon initiation, elongation and guidance. Pioneer RGC axons reach the optic chiasm at stage 32, arrive at the tectum at stage 37/38 and have arborized by stage 40 to form functional visual connections21. As determined by Illumina sequencing analysis, we found that miR-124 increased over the three stages analyzed (Fig. 1c) by a factor of 1.33 between stages 24 and 32, and by a factor of 1.64 between stages 32 and 40. ISH confirmed this temporal regulation (Fig. 1d,e). A miR-124-associated signal was faintly present at stage 24 in the presumptive neural retina, brain and spinal cord, and the intensity of the signal in these tissues increased at stage 32, then further at stage 40 (Fig. 1d,e). This progressive increase was particularly evident in the neural retina, including RGCs (Fig. 1e). miR-124 expression did not seem to change after stage 40, when axons have started to arborize (data not shown).
miR-124 knockdown does not alter RGC differentiation
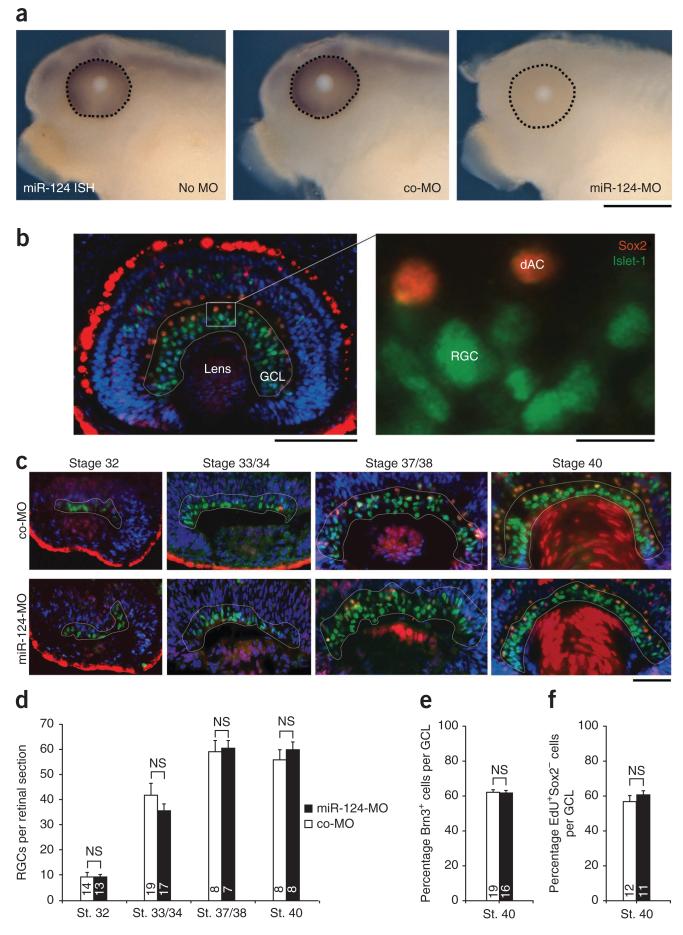
We next investigated whether miR-124 is implicated in RGC growth cone age-related changes, using a loss-of-function approach. miR-124 is a highly conserved miRNA expressed by only one gene in Xenopus tropicalis and is the sole member of its family (miRBase release 16)22. We therefore designed a miR-124 morpholino antisense oligonucleotide, miR-124-MO, to block the function of endogenous miR-124. miR-124-MO was injected at the eight-cell stage into both dorsal blastomeres fated to give rise to the central nervous system, including the neural retina. In miR-124-MO injected embryos, endogenous miR-124 was absent at stage 40 (approximately 72 h post-fertilization (hpf)), whereas a strong ISH signal was present in the retina, brain and spinal cord in uninjected or control morpholino (co-MO)-injected embryos (Figs. 1d,e and 2a). This indicates that morpholino-mediated miR-124 knockdown was both effective and long-lasting. Morpholinos act by preventing the maturation of miRNA23, and because miR-124-MO was introduced before miR-124 was expressed, the absence of an endogenous miR-124 signal likely reflected the absence of mature miR-124. Although miR-124 is abundant and highly specific to postmitotic neurons16,17,20, we detected no gross morphological phenotype in the miR-124 morphant (Supplementary Fig. 2).
Figure 2.
miR-124 knockdown does not affect the timing of RGC genesis and differentiation. (a) Morpholino-mediated knockdown of endogenous miR-124 was confirmed at stage 40 by whole-mount ISH. No signal was detected in embryos injected with miR-124-MO, unlike in controls (uninjected or co-MO injected). (b) Representative stage 40 retina stained with Islet-1 (green), Sox2 (red) and DAPI (blue). The white outline delineates the developing RGC layer. A cell was considered a RGC when it was located in the innermost part of the retina, positive for Islet-1 and negative for Sox2. dAC, displaced amacrine cell. (c) Illustrative retinas stained for Islet-1 and Sox2 from stage 32–40 embryos microinjected with co-MO or miR-124-MO. (d) Numbers of RGCs per retinal section in stage (st.) 32–40 retina of embryos injected with co-MO or miR-124-MO. (e,f) Proportion of Brn3-positive cells (e) and EdU-positive, Sox2-negative cells (f) per GCL of embryos injected with co-MO or miR-124-MO. All samples passed Kolmogorov-Smirnov normality test. (d) Analysis of variance (ANOVA) followed by Bonferroni post-test. (e,f) Unpaired Student’s t-test. Values are mean ± s.e.m. NS, not significant. Numbers of retinas analyzed are indicated in bars. Up to ten 12-μm sections were analyzed per retina; about 25,000 (d), 20,000 (e) and 8,000 (f) cells were counted in total. Scale bars, 250 μm (a); 100 μm (b, left), 10 μm (b, right); 50 μm (c).
We first examined whether miR-124 influences the general progression of RGC differentiation, which in turn might affect the intrinsic maturation of growth cones. Indeed, the exact role of miR-124 in differentiation is not clear24. We therefore counted differentiated RGCs at four developmental stages, from early RGC differentiation (stage 32) to maturation (stage 40), to determine whether miR-124 knockdown altered cell numbers. RGCs were identified as Islet-1 positive, Sox2 negative. The RGC layer is composed of RGCs and displaced amacrine cells, both of which are positive for Islet-1 (ref. 25). Displaced amacrine cells are also positive for Sox2 (ref. 25) (Fig. 2b). At all stages studied, the number of Islet-1+Sox2− RGCs was similar in miR-124 morphant and control retinas, indicating that miR-124 knockdown does not influence the general timetable of RGC differentiation (Fig. 2c,d). We further assessed the timing of RGC cell differentiation using a different RGC maker, Brn3 (ref. 26), and found no difference between the number of Brn3-positive cells in the RGC layer at stage 40 in miR-124 morphants and controls (Fig. 2e and Supplementary Fig. 3a). Finally, we investigated whether the timing of RGC neurogenesis was impaired. We incubated stage 27–28 embryos with a birthdating agent, 5-ethynyl-2′-deoxyuridine (EdU), when about half of the RGCs had been born and measured the number of EdU-positive, Sox2-negative cells in the ganglion cell layer (GCL) at stage 40 (Fig. 2f and Supplementary Fig. 3b). Again, no significant difference was found in the percentage of labeled RGCs in miR-124 morphant and control retinas. Although these results do not agree with reported evidence that miR-124 promotes neuronal differentiation in vivo27,28, they are consistent with several other reports showing that the loss of miR-124 does not affect neuronal differentiation29,30.
miR-124 knockdown delays onset of Sema3A sensitivity
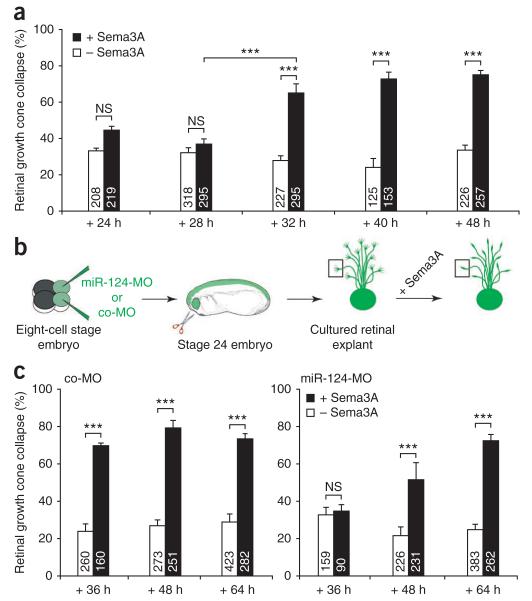
RGC axonal growth cones show intrinsic temporal regulation of cue sensitivity exemplified by the switching on of Sema3A-induced collapse responses and NRP1 expression several hours after axon initiation in neurons without pathway experience5,6. We investigated whether the temporal regulation of this subcellular aspect of differentiation (growth cone cue sensitivity) was influenced by miR-124. We reasoned that if miR-124 acts as a timer, disrupting its function would induce either precocious or retarded cue responses. First we determined the normal time of onset of Sema3A sensitivity. We explanted embryonic retinal tissue at stage 24, before axonogenesis21,31, to ensure de novo axon growth and tested the responsiveness of growth cones to Sema3A at various times using collapse assays. A significant increase in Sema3A-induced collapse response occurred within a 4 h time frame between 28 h (36% ± 3, s.e.m. collapse, equivalent to background collapse) and 32 h (65% ± 5) after plating. At 32 h a full collapse response was reached, as it did not significantly differ from that observed at later time points (40 h and 48 h) (Fig. 3a). The gain in Sema3A sensitivity thus occurred abruptly between 28 h and 32 h.
Figure 3.
Responsiveness of growth cones to Sema3A is delayed in miR-124 morphants. (a) Measure of the number of collapsed growth cones in stage 24 retinal explants grown in culture for 24–48 h in the presence (+) or absence (−) of Sema3A. The onset of retinal growth cone responsiveness to Sema3A occurred between 28 and 32 h in culture by collapse assay. (b) Schematic representation of the experiment; 125 μM co-MO or miR-124-MO were injected. Collapsed growth cones (box) have retracted lamellipodia and have less than two filopodia. (c) Number of collapsed growth cones in stage 24 retinal explants dissected from embryos injected with either co-MO or miR-124-MO and grown in culture for 36–64 h. Growth cone responsiveness to Sema3A was delayed in miR-124 morphants compared to control. All samples passed Kolmogorov-Smirnov normality test. ANOVA followed by Bonferroni post-test. Values are mean ± s.e.m.; NS, not significant; ***P < 0.001. Number of growth cones analyzed are indicated in bars. Stage 24 embryo image in b modified from ref. 46, copyright 1994 P.D. Nieuwkoop and J. Faber. Reproduced by permission of Taylor and Francis Group, LLC, a division of Informa plc.
We next assessed whether the onset of Sema3A responsiveness was altered in retinal explants from miR-124 morphants (Fig. 3b). At 36 h, a full collapse response was observed in control cultures (Fig. 3c), but by contrast, miR-124 morphant retinal growth cones did not respond to Sema3A. At 48 h, miR-124 morphant RGC growth cones responded significantly (P < 0.001) to Sema3A (52% ± 9) compared to BSA control (21% ± 5), but the collapse response was significantly (P < 0.001) lower than that of control (co-MO) growth cones (79% ± 4; Fig. 3c). At 64 h, a full Sema3A-mediated collapse response was observed in miR-124 morphants (72% ± 3) that did not differ significantly (P > 0.05) from that observed in co-MO controls (73% ± 3) (Fig. 3c). The full collapse response was thus observed 32 h later than normal. Because miR-124 is absent until at least 72 hpf, when the delayed response begins, this indicates that miR-124 affects the timing rather than simple repression of responsiveness.
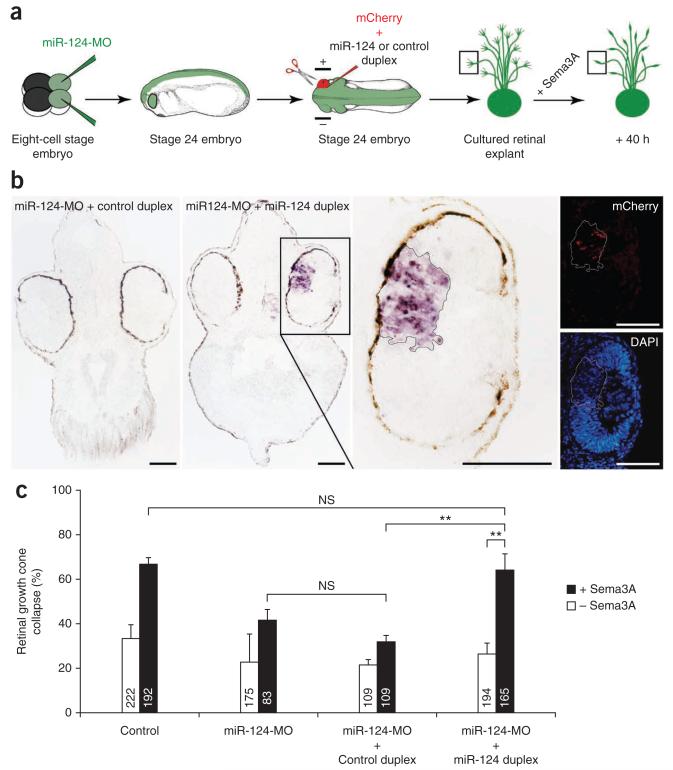
To test the specificity of the miR-124-MO phenotype, we performed rescue experiments using a miR-124 duplex, a mimic of endogenous double-stranded mature miR-124. We electroporated the miR-124 duplex into stage 24 eyes of embryos that had been injected with miR-124-MO at the eight-cell stage (Fig. 4a). Sectioned material verified that the duplex-electroporated regions of retina positive for CAAX-mCherry, a construct encoding the red fluorescent protein mCherry targeted to the plasma membrane by the CAAX box, showed strong ISH staining for miR-124 in an otherwise miR-124-negative morphant background (Fig. 4b). This indicates that the electroporated duplex restored mature miR-124 to a substantial degree after morpholino-mediated knockdown. Retinal explants cultured from eyes that were electroporated with high efficiency (assessed by mCherry fluorescence) with both the miR-124 duplex and miR-124-MO showed high Sema3A-induced collapse (64% ± 7) at 40 h in culture, similar to uninjected control growth cones at this time (Fig. 4c). This response was significantly higher than that observed in miR-124 morphant growth cones, whether electroporated with control duplex (32% ± 2) or not (41% ± 5). The miR-124 duplex thus rescued the loss of Sema3A responsiveness observed in miR-124 morphants.
Figure 4.
Electroporated miR-124 duplex rescues normal onset of Sema3A response. (a) Schematic representation of the experiment and illustrative collapsed growth cone (box). Positive and negative electrodes are shown. We electroporated 50 μM miR-124 or control duplexes, along with 400 ng μl−1 CAAX-mCherry to assess the electroporation success. (b) ISH of stage 37/38 Xenopus head sections. Exogenous miR-124 duplex, but not control duplex, was detected in electroporated retinal areas (delineated by a solid black (brightfield image) or white (fluorescence images) line) in a miR-124 morphant background. The decrease in fluorescent DAPI signal is likely due to a masking effect of the ISH BM Purple precipitate. (c) Number of collapsed growth cones in stage 24 retinal explants grown in culture for 40 h. Growth cone responsiveness to Sema3A was restored to control levels in miR-124 morphants electroporated with miR-124 duplex but not with control duplex. All samples passed Kolmogorov-Smirnov normality test. ANOVA followed by Bonferroni post-test. Values are mean ± s.e.m.; NS, not significant; **P < 0.01. Number of growth cones analyzed are indicated in bars. Scale bars, 100 μm. Stage 24 embryo images in a modified from ref. 46, copyright 1994 P.D. Nieuwkoop and J. Faber. Reproduced by permission of Taylor and Francis Group, LLC, a division of Informa plc.
To determine whether miR-124 controls the onset of sensitivity to other cues, we examined two other repulsive cues, Slit2 and lysophosphatidic acid (LPA). Like Sema3A, collapse assays show that RGC growth cones gain responsiveness to Slit2 (refs. 6,7) and LPA between 24 h and 48 h in culture (Supplementary Fig. 4a). We observed full collapse with LPA and Slit2 in miR-124 morphant growth cones at 48 h (Supplementary Fig. 4b,c). This contrasts with Sema3A, which induced significantly less collapse at 48 h in miR-124-MO growth cones (see above), and indicates that miR-124 does not regulate the onset of all repellent cue sensitivity.
miR-124 knockdown induces a delay in expression of NRP1
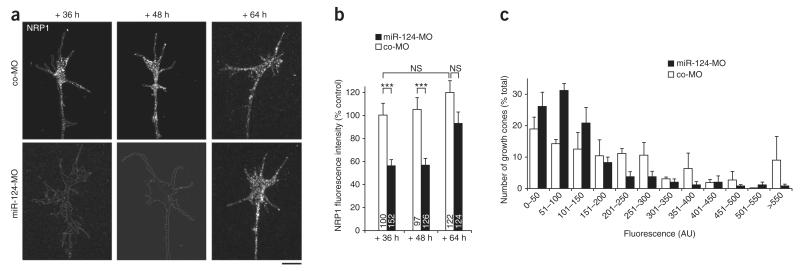
NRP1 is a Sema3A receptor, and its precocious overexpression in young Xenopus RGC growth cones, normally unresponsive to Sema3A, confers Sema3A sensitivity6. NRP1 is thus sufficient to induce Sema3A responsiveness. Therefore, we reasoned that miR-124 might alter Sema3A responsiveness by regulating the temporal expression of NRP1. To assess this, we compared NRP1 immunoreactivity in RGC growth cones using quantitative immunofluorescence.
The NRP1 signal intensity (mean pixel intensity per unit area) was significantly lower on average in stage 24 miR-124 morphant explants grown for 36 h and 48 h in culture than in controls (Fig. 5a,b). Although most individual morphant growth cones showed weak NRP1 staining, we noted some variability, with occasional growth cones displaying high levels of NRP1 (Fig. 5c). By 64 h, however, NRP1 had increased significantly (P < 0.001) compared to 36 h and 48 h, being on average lower than but not significantly different from controls (Fig. 5b). Increasing the morpholino concentration by a factor of two to four did not alter the change in NRP1 expression observed in miR-124 morphants (Supplementary Fig. 5), indicating that the effect was not simply due to morpholino titration over time. This is consistent with the possibility that miR-124 regulates the temporal expression of NRP1 in growth cones. In addition, the data showed that NRP1 expression in miR-124 morphants reached control levels at a similar time to the onset of Sema3A responsiveness. The delay in NRP1 expression in miR-124 morphant growth cones is thus likely to underlie the concomitant delay in Sema3A sensitivity.
Figure 5.
Expression of NRP1 in growth cones is delayed in miR-124 morphants. (a) Illustrative growth cones stained for NRP1; the white lines delineate the boundaries of the growth cone and the distal axon shaft. Contrast and brightness were both increased for illustration purposes. Scale bar, 10 μm. (b) Fluorescence intensity associated with NRP1 immunostaining measured in growth cones from stage 24 explants grown in culture for 36–64 h. The fluorescence intensity was lower in retinal growth cones from miR-124 morphants than in controls after 36 and 48 h in culture but not after 64 h. Kruskal-Wallis test followed by Dunn post-test. Values are mean ± s.e.m.; NS, not significant; ***P < 0.001. Numbers of growth cones analyzed are indicated in bars. (c) Distribution of growth cone NRP1 fluorescence intensity from stage 24 explants grown in culture for 48 h. AU, arbitrary units.
miR-124 morphant RGC axons make targeting errors in vivo
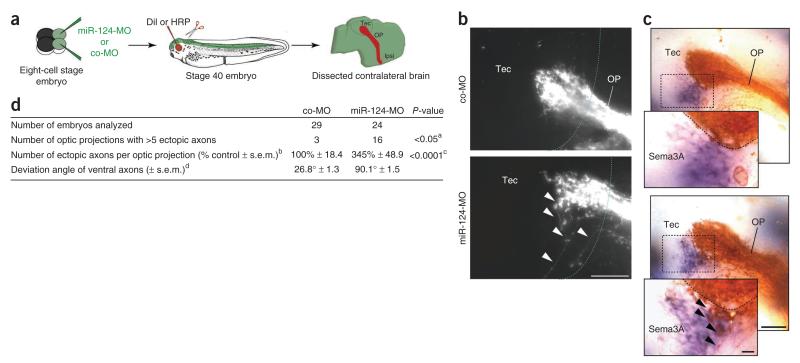
We next examined whether the delay of Sema3A responsiveness shown in vitro in growth cones lacking miR-124 could also lead to errors in pathfinding in vivo. Sema3A is encountered in discrete domains in the distal parts of the optic pathway, including the mid-optic tract, the ventral border of the optic tectum (Supplementary Fig. 6) and the caudal midbrain6. We raised miR-124 morphant embryos until stage 40, when pioneer retinal axons have reached and terminated in the tectum31, and traced RGC axons by anterograde filling using the lipophilic dye DiI (Fig. 6a). We found that a subset of RGC axons consistently failed to terminate in the tectum and, instead, continued to project ventro-caudally, often exiting the tectum at its ventral border (Fig. 6b) and aberrantly trespassing into the Sema3A domain (Fig. 6c). This phenotype occurred in 67% of miR-124 morphant brains analyzed, but rarely (10%) in controls, (P < 0.05) and was characterized by a significant (P < 0.0001) 3.5-fold increase in the number of ectopic axons (Fig. 6d). Measurement of the mean angle of deviation from the optic projection median showed that the mistargeting axons deviated by 90.1°, compared with 26.8° in control axons in the ventral tectal area (Fig. 6d and Supplementary Fig. 6). Long-range guidance through the optic tract did not seem to be affected. miR-124 morphant RGC axons thus showed errors at the ventral tectal border close to where Sema3A is expressed, suggesting a failure to respond to Sema3A in this area. Consistent with this, we found that NRP1 expression was significantly (P < 0.01) reduced by 50.2% ± 6.1 in miR-124 morphant RGC axons in vivo (Supplementary Fig. 7b,c).
Figure 6.
RGC axons are misrouted in the ventral tectum in miR-124 morphants. (a) Schematic representation of the experiment. We microinjected 125 μM co-MO or miR-124-MO. (b) DiI-labeled RGC axons visualized in the contralateral brain (lateral view). The dotted blue line represents the anterior boundary of the tectum. (c) HRP-labeled RGC axons (brown) visualized along with Sema3A mRNA (purple). The black dotted line delineates the ventral tectal boundary of the optic projection. In brains from miR-124 morphant embryos (b,c, bottom), a subset of ventral axons (arrowheads) adjacent to a tectal site of Sema3A production failed to stop and appeared misrouted toward the ventral part of tectum. (d) Quantitative analysis of the phenotype. aFisher exact test. A conservative cut-off value (>5 aberrantly targeting axons) was chosen to determine whether a given OP was considered abnormal. bValues were normalized to the width of the OP at the anterior boundary of the tectum to account for variation in DiI filling. cUnpaired Mann-Whitney U test. dDeviation angles formed by the 5–8 most ventral axons (co-MO) and ventral ectopic axons (miR-124-MO) to the median axon of the OP (see also Supplementary Fig. 6). We counted 213 ectopic axons in total, and the angles formed by 337 axons were measured in total. Images were adjusted for brightness and contrast. HRP, horseradish peroxidase; Ipsi, ipsilateral; OP, optic projection; Tec, tectum. Scale bars, 50 μm (b,c); inset (c), 10 μm. Stage 40 embryo image in a modified from ref. 46, copyright 1994 P.D. Nieuwkoop and J. Faber. Reproduced by permission of Taylor and Francis Group, LLC, a division of Informa plc.
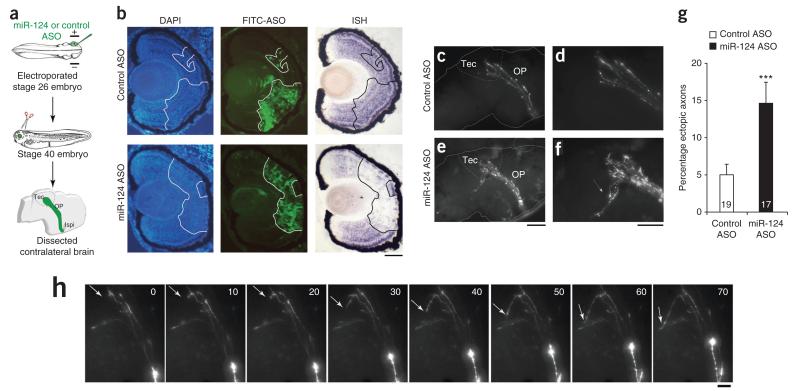
To address whether this is a cell-autonomous phenotype, we knocked down miR-124 exclusively in retinal cells by electroporating antisense oligonucleotides (ASOs) (either morpholino or LNA knockdown probes) in one eye of stage 26 embryos, when the first RGCs start to differentiate21,31 (Fig. 7a). We judged the knockdown of miR-124 to be effective, as ASO electroporated retinal cells were devoid of miR-124 signal by ISH at stage 40 (Fig. 7b and Supplementary Fig. 8). Pathfinding of miR-124 ASO-electroporated RGC axons was analyzed at stage 40. Again, retinotectal axons misprojected at the ventral tectal border (Fig. 7c–f) like those in miR-124 morphants. The percentage of ectopic axons was approximately three times that in controls: 15% ± 2.9 miR-124 ASO-electroporated RGC axons aberrantly exited the ventral tectum, compared to 5% ± 1.4 in controls (Fig. 7g). Time-lapse recording of elongating pioneer axons at stage 39/40 captured a few axons entering the tectum and then misprojecting beyond its ventral boundary (Fig. 7h). Although a non-autonomous function of tectal miRNA-124 cannot be completely excluded, these data collectively suggest that miR-124 acts intrinsically in the retina to promote accurate targeting of RGC axons to the tectum.
Figure 7.
miR-124 acts autonomously to cause retinal axon misrouting (a) Schematic representation of the experiment. ASOs (100 μM morpholino or 18.75 μM LNA knockdown probe) were electroporated. (b) ISH performed on embryos electroporated with fluorescein isothiocyanate (FITC)-labeled morpholino and fixed at stage 40. Cells electroporated with miR-124 ASO were devoid of endogenous miR-124–associated signal, whereas controls showed a strong miR-124–associated signal throughout the retina, even in electroporated cells. (c–f) Fluorescing RGC axons projecting to the contralateral brain (c,e), with corresponding zoomed images (d,f). A subpopulation of axons from RGCs, electroporated with miR-124 ASO, did not stop in the tectum but misprojected ventrally (e,f, arrow), whereas RGC axons all stopped in the tectum in controls (c,d). (g) Number of ectopic axons normalized to the number electroporated RGC axons in the tectum. Unpaired Mann-Whitney U test. Values are mean ± s.e.m., ***P < 0.001. Numbers of optic projections examined are indicated in bars; 556 axons were analyzed in total. (h) Time-lapse imaging of axons misprojecting in the ventral tectum. After reaching the tectum, an axon took a sharp, 90° turn and was misrouted in a distal-ventral direction, instead of stopping and branching within the tectum (arrow). Time is shown in minutes. Images were adjusted for brightness and contrast. Ipsi, ipsilateral; OP, optic projection; Tec, tectum. Scale bars, 50 μm (b); 100 μm (c,e); 50 μm (d,f); 20 μm (h). Embryo images in a modified from ref. 46, copyright 1994 P.D. Nieuwkoop and J. Faber. Reproduced by permission of Taylor and Francis Group, LLC, a division of Informa plc.
miR-124 regulates responsiveness to Sema3A via CoREST
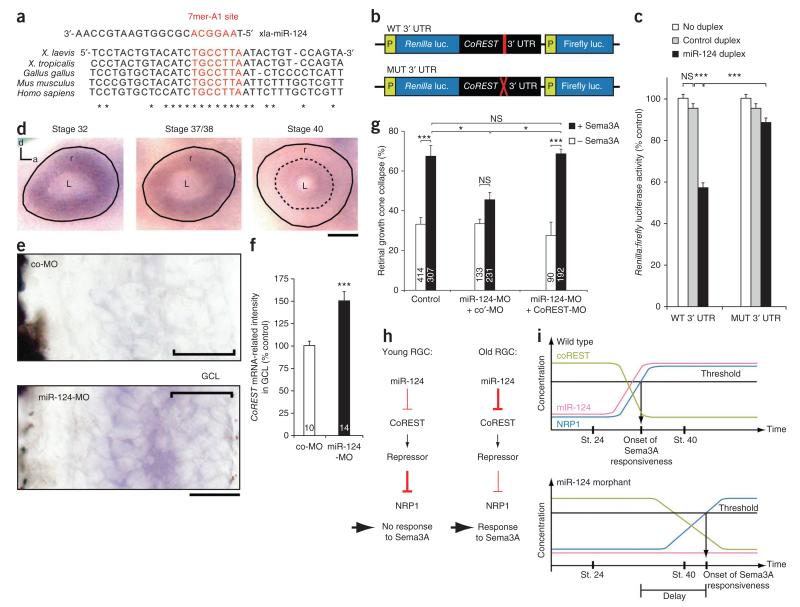
As miR-124 knockdown leads to the downregulation of NRP1 in the growth cone and as miRNAs generally repress the expression of their target mRNAs, miR-124 is likely to regulate NRP1 indirectly. We entertained the hypothesis that such regulation might occur through silencing of a repressive effector. Although little is known about the molecules regulating NRP1 expression, a recent study reported that the transcriptional repressor REST represses the expression of NRP1 in a human keratinocyte cell line, leading to a loss of sensitivity to Sema3A32. Of note, the 3′ UTR of REST’s primary cofactor, CoREST, but not that of REST itself, has a predicted binding site for miR-124 (7mer-A1) that is highly conserved from Xenopus to human (Fig. 8a).
Figure 8.
CoREST mediates miR-124 action on Sema3A responsiveness. (a) Sequence alignment of the 3′ UTR of CoREST from various species. (b) Schematic representation of Xenopus CoREST 3′ UTR, with wild-type (WT) or mutated (MUT) miR-124 binding site, subcloned downstream of a dual Renilla:firefly luciferase (luc.) reporter. (c) Quantification of reporter activity after transfection of 1–5 pmol Xenopus miR-124 or control duplex in HEK (human embryonic kidney)-293T cells. (d) Ocular distribution of CoREST mRNA detected by ISH. Solid black line delineates the outer boundary of the retina and the dashed line demarcates the peripheral area devoid of staining. (e) Retinal CoREST mRNA distribution in stage 40 embryos injected with 125 μM co-MO or miR-124-MO. (f) Quantification of CoREST mRNA signal intensity in the GCL. (g) Number of collapsed growth cones in stage 24 retinal explants grown in culture for 40 h after microinjection of 25 μM (1.1 ng) CoREST-MO, standard commercially available control morpholino (co′-MO) and/or 125 μM miR-124-MO. (h,i) Model of miR-124-mediated regulation of growth cone aging (see text for explanation). Statistics: (c,g) All samples passed Kolmogorov-Smirnov normality test. ANOVA followed by Bonferroni post-test. (f) Unpaired Mann-Whitney U test. Values are mean ± s.e.m. throughout figure; NS, not significant; *P < 0.05, ***P < 0.001. Numbers in bars indicate number of retinas examined (f; up to seven 18-μm sections were analyzed per retina) or number of growth cones analyzed (g). a, anterior; d, dorsal; L, lens; P, promoter; r, retina; xla, Xenopus laevis. Scale bars, 100 μm (d), 25 μm (e).
We tested, therefore, whether the predicted miR-124-binding site in the CoREST 3′ UTR was sufficient for miR-124–mediated inhibition of expression, using a dual Renilla:firefly luciferase reporter assay (Fig. 8b). miR-124, but not the control duplex, induced a 43% ± 2.6 decrease in the ratio of Renilla to firefly luciferase activity. By contrast, miR-124 had no significant effect (P > 0.05) on luciferase activity compared to control duplex when the seed of the miR-124 binding site in the CoREST 3′ UTR was disrupted by mutation to give a 3-nt mismatch (Fig. 8c). Taken together, these results demonstrate that CoREST 3′ UTR–driven translation or stability is negatively regulated by miR-124.
We next assessed the developmental expression pattern of CoREST in the retina. If CoREST is a bone fide miR-124 target in vivo, it would be predicted to decline as miR-124 increases. Indeed, ISH analysis revealed a strong CoREST signal at stage 32 that decreased progressively to barely detectable levels in the RGC layer at stage 40, although a weak signal persisted in the inner nuclear layer (Fig. 8d,e). Similar results were obtained with two riboprobes complementary to different regions of the CoREST coding sequence (data not shown). Moreover, a significant (P < 0.001) CoREST signal persisted in the RGC layer in miR-124 morphant retinas at stage 40 compared to that in co-MO controls (Fig. 8e,f), indicating that miR-124 regulates CoREST mRNA stability in RGCs. High CoREST activity in RGCs when their axons enter the tectum would be predicted to repress NRP1. CoREST might thus act downstream of miR-124 to determine the time of onset of growth cone sensitivity to Sema3A.
We therefore next examined whether CoREST, as a miR-124 target, is responsible for the altered Sema3A responsiveness seen in miR-124 morphants. If this is the case, knocking down CoREST in a miR-124 morphant should restore growth cone responsiveness to Sema3A. We thus knocked down both CoREST and miR-124 by morpholino microinjection and observed that this double knockdown led to the rescue of Sema3A responsiveness in stage 24 explants at 40 h (Fig. 8g). Finally, we tested whether the CoREST-MO–induced rescue of Sema3A sensitivity in miR-124 morphants was due to a change in NRP1 abundance. Upon microinjection of CoREST-MO, the NRP1 signal intensity was significantly (P < 0.01) higher in miR-124-MO growth cones than in co-MO growth cones in stage 24 explants at 40 h (Supplementary Fig. 9). Together the findings support a model in which miR-124–mediated silencing of CoREST progressively leads to increased NRP1 expression and the concomitant induction of growth cone sensitivity to Sema3A (Fig. 8h,i).
DISCUSSION
Growth cones change their responsiveness to cues en route to their target3,5,33, and, in Xenopus RGCs, this change seems to be regulated by an intrinsic clock of unknown mechanism5. Using a loss-of-function approach, we have demonstrated that miR-124 controls the timing of expression of NRP1 and thereby confers growth cone sensitivity to Sema3A at the right time and place during navigation. Our data also revealed that miR-124 promotes proper targeting to the tectum, consistent with a miR-124–mediated regulation of retinal growth cone sensitivity to this repulsive guidance cue. We identified CoREST as a miR-124 target and showed that it mediates miR-124’s influence on growth cone aging. Taken together, these results indicate that miR-124 intrinsically regulates specific age-related changes in the behavior of growth cones and thus promotes accurate pathfinding.
Growth cone aging in Xenopus RGCs is characterized by intrinsic changes in the response to various cues and in the abundance of their cognate receptors over time5,6. We have shown here that miR-124 affects the temporal change in growth cone responsiveness to Sema3A, but not to LPA or Slit2. This suggests that miR-124 does not act as a master timer regulating growth cone aging overall but rather as an auxiliary timer regulating only some aspects of the program of growth cone changes. Other molecules, perhaps other miRNAs, might act in concert with miR-124 to regulate other aspects of growth cone aging. It is of interest that other miRNAs profiled in the Xenopus retina, such as miR-9, are also predicted to target guidance receptors that change over time. In addition, the temporally coordinated action of a set of miRNAs has been reported to control progenitor cell fate by silencing multiple targets in vertebrates14. Individual miRNAs may thus have a more restricted role in regulating developmental timing in vertebrates, as previously proposed18, than in Caenorhabditis elegans.
Our results revealed that knockdown of CoREST rescues the reduced Sema3A responsiveness of miR-124 morphant growth cones through a partial restoration of NRP1 to the growth cone. Therefore, it seems reasonable to infer that CoREST mediates miR-124’s effect on the regulation of NRP1 and associated growth cone responsiveness to Sema3A. Of note, other validated miR-124 targets are present in RGCs34 (Supplementary Table 1), suggesting that miR-124 might affect additional pathways. Although none of these targets has been shown to be biologically linked to neuropilin-1 or CoREST function in neurons, we cannot rule out cross-talk between these potential miR-124 regulated pathways and CoREST-mediated regulation of NRP1 and growth cone sensitivity to Sema3A.
The loss of miR-124 did not lead to the sustained loss of NRP1 but to its delayed expression. miR-124 was absent from embryos until at least 72 hpf—similar to observations in zebrafish, where miR-124-MO induces complete knockdown of endogenous miR-124 until at least 72–96 hpf (ref. 23). This period spans the time frame for which we observed the resumption of growth cone sensitivity to Sema3A. In addition, the observed phenotype was not secondary to the morpholino being cleared from the RGCs. Thus NRP1 expression and Sema3A sensitivity are eventually switched on in the absence of miR-124, arguing for an effect on timing rather than simple recovery from repression through return of endogenous miR-124.
Our results showed that CoREST and miR-124 had opposite expression profiles in the retina over the same time period, such that CoREST decreased while miR-124 increased. In addition, miR-124 knockdown delayed the developmental decline in CoREST mRNA expression in the GCL. The miR-124–mediated post-transcriptional silencing of CoREST that we report here might thus be due to accelerated clearance of the remaining CoREST mRNAs in RGCs. We therefore suggest that miR-124, rather than acting as a simple off switch like C. elegans heterochronic miRNAs11–13, sharpens the window over which CoREST mRNA clearance occurs. Such interaction has been described for other developmental processes15, such as the miR-430–mediated accelerated degradation of maternal mRNAs in zebrafish35. Along these lines, we propose a model whereby the temporal increase of miR-124 over the period of RGC differentiation leads to the accelerated decay of CoREST expression, promoting, in turn, rapid NRP1 expression in growth cones, synchronizing the onset of Sema3A sensitivity with Sema3A encounters. Overall, the increase in miR-124 determines the time at which NRP1 is expressed above the level that is necessary for growth cones to show sensitivity to Sema3A in areas where Sema3A is expressed, such as the ventral tectal border. In absence of miR-124, this level would be reached later in development, desynchronizing growth cone sensitivity from exposure to Sema3A in the pathway (Fig. 8h,i).
Previous studies have demonstrated a function for cue-induced local translation in axons and growth cones in guidance, with the main class of translated proteins identified as cytoskeletal regulators such as RhoA and β-actin36–38. Of interest, CoREST mRNA is present in RGC somata but not in growth cones throughout the period of pathfinding34. Similarly here, miR-124 appeared enriched in the cell soma and was only weakly detected in growth cones by ISH (data not shown). The increase in NRP1 at the growth cone may thus be a direct consequence of transcriptional de-repression and subsequent transport of the protein to this compartment. This possibility is supported by the recent findings that NRP1 mRNA is absent from RGC growth cones34. This is also consistent with other studies that show transcriptional control of guidance receptors in axons; for instance, Robo39. mRNAs encoding receptors for guidance cues such as neuropilin-2 (a receptor for Sema3F and Sema3C40), activin and Eph receptors are detected in RGC growth cones34, and EphA2 has been shown to be translated in commissural axons41. This suggests a receptor and context-specific translational regulation in growth cones. Overall, this implies that miR-124–induced growth cone aging may be centrally and not locally regulated, and this central regulation might lead to progressive changes in the molecular makeup of the growth cone over time.
miR-124 knockdown resulted in the failure of a subset of RGC axons to stall at the ventral border of the tectum. The subtlety of this in vivo phenotype contrasted with the robust in vitro defect and likely reflects molecular redundancy. Several different guidance mechanisms combine to accurately guide axons to their targets in vivo, and retinal axons are guided by multiple cues to the tectum42. For instance, double but not single knockdown of Sema3A and Slit leads to a strong guidance phenotype of the retinotectal projection43. Similarly, in studies of retinotectal topography, a multiple-knockdown approach is needed to fully impair even a single mapping axis (for example, ref. 44). As miR-124 affects the responsiveness to Sema3A but not to the repulsive tectal cue Slit or LPA, repulsive action of other cues likely contributes to the subtle phenotype reported in our study. The axons that project erroneously out of the tectum originated from axons positioned very close to the Sema3A domain that borders the ventral tectum, and these axons may be particularly vulnerable because they may rely more heavily on Sema3A than other cues at this point in the pathway.
Despite its subtlety, the in vivo targeting defect is consistent with the possibility that miR-124 morphant RGC growth cones are not appropriately repelled by Sema3A owing to delayed NRP1 expression. This is supported by our findings that upregulation of NRP1, and concomitant growth cone responsiveness to Sema3A, was delayed and NRP1 abundance was significantly lower in most miR-124 morphant RGC growth cones than in controls, at an in vitro stage corresponding to stage 40 in vivo, and in stage 40 RGC axons in vivo. Overall, miR-124 might ensure that RGC growth cones express sufficient NRP1 to enable an adequate repulsive response to tectal Sema3A at the right time, and targeting of axons in the tectum. miR-124 would thus regulate axon guidance. A previous report45 revealed that miRNAs, as a class of molecule, are involved in axon guidance in the developing visual system. However, the miRNA(s) responsible for this phenotype were not identified. In addition, the study did not prove that the phenotype was directly caused by lack of miRNAs in RGCs. The present work is thus, to our knowledge, the first report demonstrating that a specific miRNA directly regulates axon pathfinding.
In summary, we provide evidence that miR-124, through the direct repression of CoREST, specifically and intrinsically regulates the timing of NRP1 expression, ensuring that the onset of growth cone responsiveness to its cognate ligand, Sema3A, occurs at the right time in the development of the optic pathway. miR-124 thus contributes to the intrinsic regulation of Xenopus RGC growth cone aging and thereby ensures proper axonal targeting to the tectum. Our findings further suggest that, by acting as a cellular timer in RGCs, miR-124 is important in synchronizing the responsiveness of RGC growth cones to relevant cues in their local microenvironment.
ONLINE METHODS
Embryos
X. laevis embryos were obtained by in vitro fertilization as previously described47, raised in 0.1× modified Barth’s saline (MBS; 0.88 mM NaCl, 0.01 mM KCl, 0.024 mM NaHCO3, 0.1 mM HEPES, 8.2 μM MgSO4, 3.3 μM Ca(NO3)2, 4.1 μM CaCl2) at 14–22 °C and staged according to ref. 46. All animal experiments were approved by the University of Cambridge Ethical Review Committee.
High-throughput sequencing
One hundred eyes each were extracted from stage 24, 32 and 40 embryos and placed in RNAlater (Ambion). Developing lenses were dissected out to obtain eyes composed of the neural retina and retinal pigmented epithelium. Total RNA was subsequently extracted using a standard Trizol protocol for small sample size (Invitrogen). RNA quantity and quality was assessed by NanoDrop analysis (Thermo Scientific).
Ten micrograms of total RNA were size-selected to 16–30 nucleotides on a denaturing polyacrylamide gel and small RNAs were cloned through ligation of a 5′ and 3′ adaptor according to the manufacturer’s protocol (Illumina). Libraries were sequenced on an Illumina Genome Analyzer.
Fastq data files were processed using custom Perl scripts. Reads with missing bases were excluded. 3′ adaptor sequences were identified on the basis of at least six matching nucleotides and removed, allowing up to two mismatches. Trimmed reads shorter than 16 nucleotides were discarded, along with reads without recognizable adaptor sequences. The resulting 16–30 nt inserts were aligned to the X. tropicalis genome (xenTro2) downloaded from the UCSC Genome Browser website (http://genome.ucsc.edu/) using the ELAND module in the Illumina Genome Analyzer Pipeline software, v0.3.0. Only perfect alignments were considered. In the case of reads aligning to more than one location, the read depth was scaled by the number of possible alignments. Read counts were obtained for mature miRNAs by assigning each miRNA the total number of reads (normalized for multiple alignments) overlapping the genomic loci of the mature miRNA (miRBase release 16; ref. 22). To determine which miRNAs change over time, sequencing at each stage were reproduced in triplicate. Read numbers were normalized at every locus to the total number of reads per sample and expression values were log2 transformed. Before hierarchical clustering, miRNA profiles were standardized to have mean 0 and s.d. 1. Heat maps were generated using R (http://www.r-project.org/).
In situ hybridization for miRNAs
LNA oligonucleotides complementary to X. tropicalis (xtr)-miR-124 (5′-TGGCATTCACCGCGTGCCTTAA-3′), xtr-miR-1a (5′-TACATACTTCTTTACATTCCA-3′), xtr-miR-9b* (5′-ACGTTCGGTTGTCTAGCTTTA-3′), xtr-miR-130b (5′-ATGCCCTTTCATCATTGCACTG-3′), xtr-miR-182 (5′-TGTGAGTTCTACCATTGCCAAA-3′), xtr-miR-183 (5′-CAGTGAATTCTACCAGTGCCATA-3′) and xtr-miR-184 (5′-AGCCTTATCAGTTCTCCGTCCA-3′) were purchased from Exiqon. Standard scrambled probes (5′-TTCACAATGCGTTATCGGATGT-3′) were used as a negative control.
For whole-mount ISH, stage 24, 32 and 40 embryos were processed as previously described17. Probes were labeled by transferring a digoxigenin (DIG) tail to the oligonucleotide using a tailing kit (Roche) and used at 1:100. Embryos were photographed on an agarose bed using Leica MZ FLIII inverted microscope equipped with a Micropublisher 5.0 RTV camera (Qimaging). To analyze histological distribution, embryos were embedded in OCT compound (Tissue-Tek) and cryosectioned.
ISH on sections was carried out on transverse cryosections of fixed stage 37–40 embryos essentially as previously described48. LNA oligonucleotides were purchased already DIG-labeled and used at 1 nM. Sample examination and photographs were taken with a Zeiss Axoskop microscope equipped with Micropublisher 5.0 RTV camera.
Antisense oligonucleotides and duplexes
xtr-miR-124 (5′-TGGCATTCACCGCGTGCCTTAA-3′), X. laevis CoREST (5′-ATATTTCTGCTCCCTTCTCGATCAT-3′), control (co) (5′-GTGTAACACGTCTATACGCCCA-3′) and standard control morpholinos (co′-MO) (5′-CCTCTTACCTCAGTTACAATTTATA-3′) were obtained from Gene Tools. xtr-miR-124 (5′-TGGCATTCACCGCGTGCCTTAA) and control (5′-GTGTAACACGTCTATACGCCCA-3′) LNA knockdown antisense oligonucleotides were purchased from Exiqon. miR-124 duplex (miRIDIAN mimic, 5′-UAAGGCACGCGGUGAAUGCCAA-3′) and control duplexes (miRIDIAN mimic negative control #1, cel-miR-67, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were purchased already annealed from Thermo Fisher.
Blastomere microinjection
Morpholinos or duplexes were injected in both dorsal blastomeres of eight-cell-stage embryos. When these were unlabeled, EGFP mRNA (1 ng) was included to screen for appropriately injected embryos.
Electroporation
DNA, duplexes, carboxyfluorescein-morpholino or LNA knockdown antisense oligonucleotides were electroporated in one eye of stage 24–26 embryos with conditions similar to those previously described49.
Retinal explant culture
Whole retinas or hemi-retinas from stage 24 embryos were dissected and cultured at 20 °C for 6–64 h in 60% L15 minimal medium (Invitrogen) on glass coverslips (Bellco) coated with poly-l-lysine (10 μg ml−1, Sigma) and laminin (10 μg ml−1, Sigma).
Collapse assay
Retinal explants were bathed in 0.45–2 μg ml−1 human recombinant Sema3A-FC (stored diluted in 0.1% protease-free BSA, R&D), conditioned medium from Slit2-expressing cells (kindly provided by E. Hohenester, Imperial College London) or 1 μM l-α-lysophosphatidic acid (LPA) for 10 min and then fixed in 2% paraformaldehyde (PFA), 7.5% (wt/vol) sucrose. BSA (0.1%), control conditioned medium or sterile double-distilled water were used as controls for Sema3A, Slit2 and LPA, respectively. To avoid subjective bias, all collapse analysis was done blind to experimental condition. Growth cones were considered collapsed when they possessed no filopodia, or two or fewer filopodia each shorter than 10 μm.
Quantitative immunofluorescence
Quantitative immunofluorescence for NRP1 was carried out as previously described6. Immunocytochemistry was first performed on growth cones using monoclonal antibodies (A5)6 to Xenopus NRP1 (1:15). For digital quantitation, isolated growth cones were selected free of adjacent cells at random with phase optics at 60× on a Nikon Eclipse 80i microscope. To avoid subjective bias, analyses were performed blind to experimental condition.
Immunostaining of retinal sections
Transverse Xenopus cryosections were fixed at stage 32–40 and stained with mouse anti–Islet-1 (40.2D6, 1:500, Developmental Studies Hybridoma Bank), rabbit anti-Sox2 (1:500, Millipore) or goat anti-Brn3 (1:500, Santa-Cruz) and then with secondary antibodies goat anti-mouse Alexa Fluor 488–, goat anti-rabbit Alexa Fluor 555– and donkey anti-goat Alexa Fluor 547–conjugated F(ab′)2 fragments (Invitrogen), respectively. Sections were visualized with a Nikon Eclipse TE2000-U inverted fluorescent microscope and photographed with a Hamamatsu ORCA-ER camera.
EdU staining
Stage 27–28 embryos were allowed to develop in EdU solution (Invitrogen, 5 mM diluted in 0.1× MBS) until stage 40. Embryos were then processed for Sox2 immunostaining as described above, except that an extra 30-min incubation was carried out, before coverslipping, with EdU reaction buffer containing Alexa Fluor 488 azide as per manufacturer’s instructions.
DiI axon tracing
Stage 40 embryos were fixed in 4% PFA and stored at 4 °C until use. DiI crystals (Invitrogen) were dissolved in chloroform and the solution loaded into a glass capillary. The left lens was removed and the DiI solution was injected into the ocular cavity and incubated for 48 h at 20–23 °C. Brains were then dissected out and flat-mounted in PBS. DiI-labeled RGC axons were visualized using a Nikon Eclipse 80i upright fluorescence microscope and photographed with a Hamamatsu ORCA-ER camera. z-stacks of serial images comprising the entire contralateral optic pathway were captured using Openlab software (Improvision).
Horseradish peroxidase axon tracing
Stage 40 embryos were anesthetized with 1.5 mM MS222 (3-aminobenzoic acid ethyl ester methanesulfonate salt, Sigma) and their left lens was removed. Horseradish peroxidase (grade I, Roche) was reconstituted in a filtered 1% (wt/vol) L-α-lysolecithin (Calbiochem) solution. The paste thus obtained was applied in the lensless ocular cavity. Embryos were subsequently fixed in 4% PFA and brains were dissected out and incubated with a 3,3′-diaminobenzidine tetrahydrochloride (Sigma) solution containing 0.1% H2O2.
In situ hybridization for mRNAs
The synthesis of pCS2-XSema3A (the open reading frame of Xenopus Sema3A subcloned in the pCS2+ vector) has been previously described6.
Total RNA from stage 24 retina was reverse transcribed into cDNA using random hexamers (Invitrogen). Two fragments spanning two separate regions of the coding sequence (CDS) of Xenopus CoREST cDNA were then amplified using Taq DNA polymerase (Roche). Forward 5′-GCATGACTCGAGATGATCGAGAAGGGAGCAGA-3′ and reverse 5′-GCATGACTCGAGGCGAGCATGTCGATCCAT-3′ primers generated a 591-bp amplicon of the 5′ end of Xenopus CoREST CDS mRNA (CoREST-5′CDS), and forward 5′-GCATGATTCGAAGAGAAAGAGAAGGAAGTGGTGA-3′ and reverse 5′-GCATGACTCGAGTCATGATGCAGAGGGGTATG-3′ primers generated a 696-bp amplicon of the 3′ end of CoREST CDS mRNA (CoREST-3′CDS). These PCR fragments were then subcloned into pCRII-TOPO vector following manufacturer’s instructions (Invitrogen). The sequences of pCRII-CoREST-5′CDS and pCRII-CoREST-3′CDS thus obtained, and of pCS2-XSema3A, were validated by the University of Cambridge Biochemistry Department DNA core facility.
For probe synthesis, pCRII-CoREST-3′CDS and pCRII-CoREST-5′CDS vectors were linearized and used as a template for the production of DIG-labeled antisense and sense riboprobes according to the manufacturer’s instructions.
Whole embryos, brains or whole eyes were processed for ISH as described previously6. Probes were used at 2 ng μl−1. No signal was detected with sense probes (data not shown). Samples were examined and photographs taken with a Zeiss Axoskop microscope equipped with Micropublisher 5.0 RTV camera. Irregular illumination due to the microscope-derived vignetting effect was digitally adjusted on low-magnification brightfield images.
Immunostaining and quantitative immunofluorescence on retinal sections
Immunostaining was carried out using anti-mouse NRP1 (1:250) and rabbit anti–α-tubulin (1:5,000, clone B-5-1-2, Sigma), or mouse anti–neurofilament-associated antigen (3A10, 1:50, Developmental Studies Hybridoma Bank). Anti-rabbit Alexa Fluor 488– and anti-mouse Alexa Fluor 647–conjugated secondary antibodies were used to detect α-tubulin and NRP1, respectively. These fluorophores were chosen to avoid any possible bleed-through between fluorescent channels.
For digital quantitation, pixel saturation was strictly avoided and the same gain and exposure settings were used for digital capture of fluorescence images for each experiment. To define the area for digital quantitation, the outline of the optic nerve head (ONH) was traced with a drawing pad on the green fluorescence image (stained for α-tubulin) and superimposed on the red fluorescence image (stained for NRP1). Openlab software (Improvision) was used to calculate NRP1 fluorescent intensity in the ONH, giving a measurement of mean pixel intensity per unit area. The background fluorescence was similarly measured in an area devoid of biological material and located immediately adjacent to the retinal section to record the background pixel intensity per unit area. This reading was then subtracted from the ONH NRP1 reading, resulting in the background-corrected intensity.
Quantification of ISH signal in the GCL
miR-124 morphant or control embryos and eyes were incubated in BM Purple (Roche) for an identical period of time, fixed, sectioned and photographed. Retinal sections of each experiment were photographed the same day with identical microscope settings. For digital quantitation, identical gain and exposure settings for digital capture of brightfield images were used. To define the area for digital quantitation, images were converted to 32-bit gray pictures. The outline of the GCL and of an adjacent background region were traced on each micrograph and background-corrected mean pixel intensity per unit area was measured using ImageJ (http://rsbweb.nih.gov/ij/).
In vivo time-lapse imaging
Stage 39 embryos were anesthetized with 1.5 mM MS222 and the dorsal diencephalon and optic tectum contralateral to the electroporated eye were exposed by removing epidermis. Embryos were mounted in a closed chamber containing 1× MBS, 0.3 mM MS222. Fluorescing elongating RGC axons were visualized using a Nikon Eclipse TE2000-U inverted fluorescence microscope. For all movies, images were collected with Openlab software at 10-min intervals in 1-μm steps along the z axis. Images from z-stacks series taken at the indicated intervals were compiled, aligned and converted into QuickTime movies with Openlab software.
Dual luciferase reporter assay
The construct CoREST-WT-3′UTR was obtained by annealing 58 bp of the wild-type X. laevis CoREST-3′ UTR 5′-TCGAGAATAATTCCAGTCCTACTGTACATCTGCCTTAATACTGTCCAGTATCCACACACTCTTGC-3′, containing a putative miR-124 binding site (underlined), to its complementary sequence. CoREST-MUT-3′UTR was obtained by annealing the mutant CoREST-3′ UTR 5′-TCGAGAATAATTCCAGTCCTACTGTACATCTGCGCCAATACTGTCCAGTATCCACACACTCTTGC-3′, whose putative miR-124 binding site (underlined) was mutated (italics), to its complementary sequence. We then ligated them into the multiple cloning site of the Renilla: firefly luciferase–expressing vector psiCHECK-2 (Promega). The sequences of psiCHECK-2-CoREST-WT-3′UTR and psiCHECK-2-CoREST-MUT-3′UTR thus obtained were validated.
For the assay, 100 ng of psiCHECK-2-CoREST-WT-3′UTR or psiCHECK2-CoREST-MUT-3′UTR were transfected either alone or with 1–5 pmol control duplex or miR-124 duplex using 0.5 μl Lipofectamine 2000 (Invitrogen) into HEK-293T cells plated 8–10 h earlier on 96-well plates. The activity of both firefly and Renilla luciferase was assessed 36 h after transfection using the Dual Luciferase Reporter Kit (Promega) and a DLReady TD-20/20 single-tube luminometer (Turner Biosystems).
miR-124 target prediction
Most target prediction algorithms do not include Xenopus miRNAs; therefore, we generated our own list of predicted targets by directly running TargetScan50. All available X. laevis and X. tropicalis mRNAs were obtained from RefSeq (downloaded June 2010) and 3′ UTR sequences were extracted if they were at least 20 nt long. Seed-matching sites were located using a custom Perl script and targetscan_50_context_scores.pl was used to calculate context scores for each potential target.
Statistical analysis
Each experiment was conducted at least three times unless otherwise stated. For all tests, the significance level was α = 0.05. Data were analyzed with Instat3 or Prism (GraphPad).
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank J. Woolford for her contribution to the analysis of high-throughput sequencing data, H. Lynn for help on cloning and M. Wood for participation in collapse analysis. We also thank A. Dwivedy, L. Strochlic, J. Falk, K. Leung, M. Agathocleous, A. Lin, S. McFarlane and A. Almeida for technical assistance. We thank W. Harris, A.C. Lee, J. Yoon and M. Agathocleous for critically reading the manuscript, as well as all the members of the Harris/Holt laboratory for their input. This work was supported by postdoctoral fellowships from the National Research Council of Canada (M.-L.B.) and Alberta Heritage Foundation for Medical Research (M.-L.B.), by Wellcome Trust Programme and UK Medical Research Council grants (C.E.H.) and by a European Framework 6 grant (SIROCCO) (E.A.M.).
Footnotes
Accession codes. Gene Expression Omnibus: GSE33444.
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS C.E.H. and M.-L.B. conceived the project. M.-L.B. performed most of the experiments and data analysis. M.-L.B., K.H.Z., C.A.-G., J.A., C.B. and L.D.G. participated in profiling experiments and analyses. K.H.Z. contributed to Slit2 and LPA collapse assays. C.A.-G. determined the putative targets of miR-124. A.M. performed ISH on cryosections of wild-type Xenopus. E.A.M. designed and discussed profiling experiments. M.-L.B. and C.E.H. wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 2.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 3.Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 4.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 5.Shewan D, Dwivedy A, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat. Neurosci. 2002;5:955–962. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- 6.Campbell DS, et al. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J. Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piper M, et al. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirasaki R, Katsumata R, Murakami F. Change in chemoattractant responsiveness of developing axons at an intermediate target. Science. 1998;279:105–107. doi: 10.1126/science.279.5347.105. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MH, Day ML. Egg timers: how is developmental time measured in the early vertebrate embryo? Bioessays. 2000;22:57–63. doi: 10.1002/(SICI)1521-1878(200001)22:1<57::AID-BIES10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Aulehla A, Pourquié O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb. Perspect. Biol. 2010;2:a000869. doi: 10.1101/cshperspect.a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 12.Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 13.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 14.Decembrini S, et al. MicroRNAs couple cell fate and developmental timing in retina. Proc. Natl. Acad. Sci. USA. 2009;106:21179–21184. doi: 10.1073/pnas.0909167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wienholds E. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 18.Moss EG. Heterochronic genes and the nature of developmental time. Curr. Biol. 2007;17:R425–R434. doi: 10.1016/j.cub.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Smirnova L, et al. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 20.Sempere LF, et al. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt CE. A single-cell analysis of early retinal ganglion cell differentiation in Xenopus: from soma to axon tip. J. Neurosci. 1989;9:3123–3145. doi: 10.1523/JNEUROSCI.09-09-03123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths-Jones S, Grocock RJ, Van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RHA. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007;5:e203. doi: 10.1371/journal.pbio.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao F-B. Context-dependent functions of specific microRNAs in neuronal development. Neural Dev. 2010;5:25. doi: 10.1186/1749-8104-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taranova OV, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocking JC, Hehr CL, Bertolesi GE, Wu JY, McFarlane S. Distinct roles for Robo2 in the regulation of axon and dendrite growth by retinal ganglion cells. Mech. Dev. 2010;127:36–48. doi: 10.1016/j.mod.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Visvanathan J, Lee S, Lee B, Lee JW, Lee S-K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng L-C, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark AM, et al. The microRNA miR-124 controls gene expression in the sensory nervous system of Caenorhabditis elegans. Nucleic Acids Res. 2010;38:3780–3793. doi: 10.1093/nar/gkq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X, Pfaff SL, Gage FH. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holt CE. Does timing of axon outgrowth influence initial retinotectal topography in Xenopus? J. Neurosci. 1984;4:1130–1152. doi: 10.1523/JNEUROSCI.04-04-01130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurschat P, Bielenberg D, Rossignol-Tallandier M, Stahl A, Klagsbrun M. Neuron restrictive silencer factor NRSF/REST is a transcriptional repressor of neuropilin-1 and diminishes the ability of semaphorin 3A to inhibit keratinocyte migration. J. Biol. Chem. 2006;281:2721–2729. doi: 10.1074/jbc.M507860200. [DOI] [PubMed] [Google Scholar]
- 33.Diefenbach TJ, Guthrie PB, Kater SB. Stimulus history alters behavioral responses of neuronal growth cones. J. Neurosci. 2000;20:1484–1494. doi: 10.1523/JNEUROSCI.20-04-01484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zivraj KH, et al. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J. Neurosci. 2010;30:15464–15478. doi: 10.1523/JNEUROSCI.1800-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giraldez AJ. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 36.Jung H, O’Hare CM, Holt CE. Translational regulation in growth cones. Curr. Opin. Genet. Dev. 2011;21:458–464. doi: 10.1016/j.gde.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung K-M, et al. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu KY, et al. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keleman K, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. doi: 10.1016/s0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 41.Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 42.Mann F, Harris WA, Holt CE. New views on retinal axon development: a navigation guide. Int. J. Dev. Biol. 2004;48:957–964. doi: 10.1387/ijdb.041899fm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson-Leadbeater K, et al. Dynamic expression of axon guidance cues required for optic tract development is controlled by fibroblast growth factor signaling. J. Neurosci. 2010;30:685–693. doi: 10.1523/JNEUROSCI.4165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldheim DA, et al. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25:563–574. doi: 10.1016/s0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- 45.Pinter R, Hindges R. Perturbations of microRNA function in mouse dicer mutants produce retinal defects and lead to aberrant axon pathfinding at the optic chiasm. PLoS ONE. 2010;5:e10021. doi: 10.1371/journal.pone.0010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Garland, New York and London: 1994. [Google Scholar]
- 47.Cornel E, Holt C. Precocious pathfinding: retinal axons can navigate in an axonless brain. Neuron. 1992;9:1001–1011. doi: 10.1016/0896-6273(92)90061-h. [DOI] [PubMed] [Google Scholar]
- 48.Obernosterer G, Martinez J, Alenius M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat. Protoc. 2007;2:1508–1514. doi: 10.1038/nprot.2007.153. [DOI] [PubMed] [Google Scholar]
- 49.Falk J, et al. Electroporation of cDNA/Morpholinos to targeted areas of embryonic CNS in Xenopus. BMC Dev. Biol. 2007;7:107. doi: 10.1186/1471-213X-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimson A, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.