Abstract
Objective
To evaluate whether antioxidant supplements presumed to target specific cellular compartments affected cerebrospinal fluid (CSF) biomarkers.
Design
Double-blind, placebo-controlled clinical trial.
Setting
Academic medical centers.
Participants
Subjects with mild to moderate Alzheimer disease.
Intervention
Random assignment to treatment for 16 weeks with 800 IU/d of vitamin E (α-tocopherol) plus 500 mg/d of vitamin C plus 900 mg/d of α-lipoic acid (E/C/ALA); 400 mg of coenzyme Q 3 times/d; or placebo.
Main Outcome Measures
Changes from baseline to 16 weeks in CSF biomarkers related to Alzheimer disease and oxidative stress, cognition (Mini-Mental State Examination), and function (Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale).
Results
Seventy-eight subjects were randomized; 66 provided serial CSF specimens adequate for biochemical analyses. Study drugs were well tolerated, but accelerated decline in Mini-Mental State Examination scores occurred in the E/C/ALA group, a potential safety concern. Changes in CSF Aβ42, tau, and P-tau181 levels did not differ between the 3 groups. Cerebrospinal fluid F2-isoprostane levels, an oxidative stress biomarker, decreased on average by 19% from baseline to week 16 in the E/C/ALA group but were unchanged in the other groups.
Conclusions
Antioxidants did not influence CSF biomarkers related to amyloid or tau pathology. Lowering of CSF F2-isoprostane levels in the E/C/ALA group suggests reduction of oxidative stress in the brain. However, this treatment raised the caution of faster cognitive decline, which would need careful assessment if longer-term clinical trials are conducted.
Trial Registration
clinicaltrials.gov Identifier: NCT00117403
Oxidative damage is associated with aging and is widespread in the brain in Alzheimer disease (AD).1 Free radical species mediate damage to proteins, lipids, mitochondria, and DNA and may activate the cell cycle; overwhelm endogenous antioxidant defenses in the brain; and contribute to neuronal damage.1,2 Observational studies suggest that an antioxidant-rich diet may reduce the risk of AD.3 However, prevention trials of combinations of antioxidant dietary supplements in elderly subjects did not influence age-associated cognitive decline.4,5 Antioxidant randomized clinical trials in AD have had mixed results. High doses of vitamin E (α-tocopherol) in moderate to severe AD delayed clinical milestones without benefiting cognition.6 High-dose α-tocopherol did not influence progression in amnestic mild cognitive impairment.7
We hypothesized that if antioxidant treatment affects key pathogenic mechanisms in patients with AD, this would be reflected by cerebrospinal fluid (CSF) biomarker changes. Aided by an expert panel of advisors, we reviewed antioxidant studies for evidence of benefit in transgenic mouse and other models relevant to AD, determined dose ranges likely to be safe and potentially efficacious in humans, and identified combinations that could provide synergism8 (eg, vitamin C helps to quench reactive oxygen intermediates when combined with vitamin E).9 We decided to study a combination of vitamin E, vitamin C, and α-lipoic acid (ALA); co-enzyme Q (CoQ) alone (to our knowledge, this study would provide the first safety data in AD), and placebo.
In transgenic mouse models of amyloid deposition, effects of antioxidant supplements depend on time and dose. In an amyloid protein precursor transgenic mouse, early treatment with vitamin E (α-tocopherol) attenuated plaque formation but was not effective if started after amyloid deposits were present.10 Treatment with vitamin C plus a medium dose of vitamin E decreased oxidative damage and improved performance on tests of spatial memory; these benefits were not seen with vitamin C plus a high dose of vitamin E.11 Treatment with vitamin E improved the morphology of neurites surrounding plaque cores.12
α-Lipoic acid is a mitochondrial coenzyme with anti-oxidant actions; it also induces the transcription factor Nrf2, which induces many antioxidant enzymes.9 Aged dogs given a diet rich in antioxidant supplements, including ALA, showed better cognitive performance, decreased amyloid burden, and reduced oxidative damage.13 Studies of long-term dietary supplementation with ALA in amyloid protein precursor transgenic mice have reported amelioration of deficits on memory testing14 and decreased oxidative markers without changes in amyloid pathology.15 A cohort of patients with mild AD who received 600 mg/d of ALA, compared with patients who did not receive ALA, showed cognitive stabilization over 48 months.16
Coenzyme Q is a naturally occurring antioxidant that helps to protect mitochondria from reactive oxidative species generated during oxidative metabolism. It is currently under investigation in amyotrophic lateral sclerosis and Parkinson disease.17 Administration of CoQ to aged amyloid protein precursor/presenilin 1 mice led to a decrease in cortical levels of Aβ42 and reduction of markers of oxidative stress.18 Aged mice treated with CoQ showed decreased levels of brain carbonyls and other oxidative stress markers.19
METHODS
PARTICIPANTS
The Alzheimer’s Disease Cooperative Study (ADCS) Anti-oxidant Biomarker study was a double-blind, randomized multicenter clinical trial, conducted between 2006 and 2008 at 12 centers across the United States, funded by the National Institute on Aging. Subjects diagnosed with probable AD20 with Mini-Mental State Examination (MMSE)21 scores of 16 or more of 30 were enrolled. Further inclusion criteria were age between 50 and 85 years, presence of a caregiver to supervise study medications, and neuroimaging (computed tomography or magnetic resonance imaging) results within the past 24 months consistent with AD but lacking evidence of significant vascular disease (infarction that could cause dementia) or other intracranial disease processes. Stable anti-AD treatment with a cholinesterase inhibitor, memantine, or both for at least 3 months was required. Medications for behavioral symptoms were allowed only if doses were stable for at least 4 weeks before screening.
Subjects were excluded if they had a diagnosis of dementia other than AD (eg, vascular dementia), a neurological disorder or major psychiatric disorder, or drug or alcohol abuse or dependence. Subjects with contraindications to lumbar puncture were excluded, eg, treatment with oral anticoagulants, prior surgery or deformity of the lumbosacral spine, or a platelet count less than 100×103/μL. Medical factors or conditions that could increase systemic oxidative stress were also exclusions: diabetes mellitus; obesity (body mass index >30 [calculated as weight in kilograms divided by height in meters squared]); cardiac, renal, or pulmonary failure; smoking; inflammatory diseases; and regular use of nonsteroidal anti-inflammatory drugs, immunosuppressive drugs, or drugs for Parkinson disease. Because supplements and vitamins are widely used in elderly individuals, subjects were allowed to continue taking their current antioxidant supplements provided that daily doses were less than the following thresholds: 100 IU/d or less for α-tocopherol, 200 mg/d or less for vitamin C, 60 mg/d or less for CoQ, and 100 IU/d or less for ALA.
STUDY DESIGN
Subjects who met study criteria and provided informed consent were assigned to a 16-week double-blind period of treatment in 1 of 3 arms: 800 IU/d of vitamin E (α-tocopherol) plus 500 mg/d of vitamin C plus 900 mg/d of α-lipoic acid (E/C/ALA); 400 mg of CoQ 3 times/d; or placebo. To maintain blinding, all subjects received 3-times/d dosing, including placebos matching CoQ and the E/C/ALA combination as needed. Study drugs were dispensed in coded containers.
Study visits occurred at screening, baseline (within 4 weeks of screening), 8 weeks, and 16 weeks. Lumbar punctures were performed at the baseline and 16-week visits. Investigators received training regarding research lumbar punctures, including the use of atraumatic small-gauge needles. Vitaline Inc. provided CoQ and a matching placebo, in the form of chewable wafers, taken at mealtime. They also supplied encapsulated α-tocopherol, vitamin C, and racemic ALA and matching placebo capsules. Subjects, care-givers, and investigators were blind to treatment allocation.
The study was conducted in accordance with the principles of the Declaration of Helsinki and with the laws and regulations of the locality in which the research was conducted. The study protocol was approved by the institutional review boards of the participating centers. The study was conducted under an Investigational New Drug Application from the Food and Drug Administration. A data safety monitoring board reviewed the study data every 3 months.
OUTCOME MEASURES
Safety was assessed by interviewing subjects and informants about adverse events, measuring vital signs, brief physical examination, and a panel of blood tests at baseline, week 8, and week 16. Electrocardiograms were obtained at baseline and 16 weeks. Adverse experiences were recorded at each study visit and defined according to Medical Dictionary for Regulatory Activities terminology.
Cognition was assessed with the MMSE at baseline and 16 weeks; scores range from 0 to 30. Functional abilities were rated by informant interview using the ADCS Activities of Daily Living Scale (ADCS-ADL); scores range from 0 to 78.22
The primary outcome measures were levels of 4 biochemical markers measured in CSF.23 Aβ42 is a 42–amino acid peptide that aggregates and is deposited in the brain to form plaques in AD. Cerebrospinal fluid levels of Aβ42 are decreased in AD, presumably because binding of this peptide to aggregated amyloid in the brain results in less being cleared into the CSF.24 The microtubule-associated protein tau is an integral component of neurofibrillary tangles and neuritic dystrophy in AD. Cerebrospinal fluid levels of tau and tau phosphorylated at specific sites (P-tau) are increased in AD, related to damage to neurons and their axonal processes.24 To evaluate oxidative damage to the central nervous system, F2-isoprostane levels, which are stably oxidized forms of arachidonic acid, were measured.25 Brain levels of F2-isoprostanes increase in patients with AD and in transgenic mouse models.26 Cerebrospinal fluid levels of F2-isoprostanes are increased in AD25,26 and in cognitively intact subjects who carry mutations in the amyloid protein precursor or presenilin 1 genes associated with familial AD.27
Cerebrospinal fluid cell counts and total protein and glucose levels were measured by local laboratories. Only samples with a red blood cell count less than 500/mL were used for biomarker measurements. Within 30 minutes of the lumbar puncture, CSF samples were aliquoted into polypropylene cryo-tubes, frozen, shipped to a central laboratory, then sent to the University of California, San Diego, and stored at −80°C until biomarkers were measured. Levels of Aβ42, total tau, and P-tau181 were measured using multiplex sandwich assays (INNO-BIA AlzBio3; Innogenetics), following established methods.28 F2-isoprostane levels were measured in the laboratory of one of us (T.J.M., University of Washington, Seattle) by stable isotope dilution assay using gas chromatography–mass spectrometry with selective ion monitoring.26 Assays were measured by technicians who were blind to subjects and treatment groups.
DATA ANALYSIS
Statistical analyses were conducted by the ADCS Data Core. The primary efficacy analysis was a between-group comparison of the changes from baseline in CSF levels of Aβ42, total tau, P-tau181, and F2-isoprostanes. Secondary analyses compared safety data and changes in MMSE and ADCS-ADL scores among the 3 groups. The primary analyses were performed in subjects who completed a baseline and follow-up lumbar puncture that yielded analyzable CSF. For clinical and safety outcomes, modified intention-to-treat analyses were performed, including subjects who received at least 1 dose of double-blind medication, had a baseline evaluation, and had at least 1 evaluation after the start of treatment.
Baseline data were compared between groups, using the Kruskal-Wallis test for continuous variables and the Fisher exact test for categorical variables. For analysis of change in CSF biomarkers, analysis of covariance was used. Age, sex, and baseline MMSE score did not differ across groups, and models with and without these as covariates were analyzed. Models were fitted comparing each treatment group with placebo, and the P values were adjusted using the Hochberg method for multiple comparison.29 The adjusted P values were compared with α=.05.
Power calculations for CSF biomarkers were based on published data on serial CSF levels of tau.30 With 25 subjects in each treatment arm, there would be 80% power to detect a 40% or more change in CSF tau level, with α=.05 and σ2 (variance)=382.
RESULTS
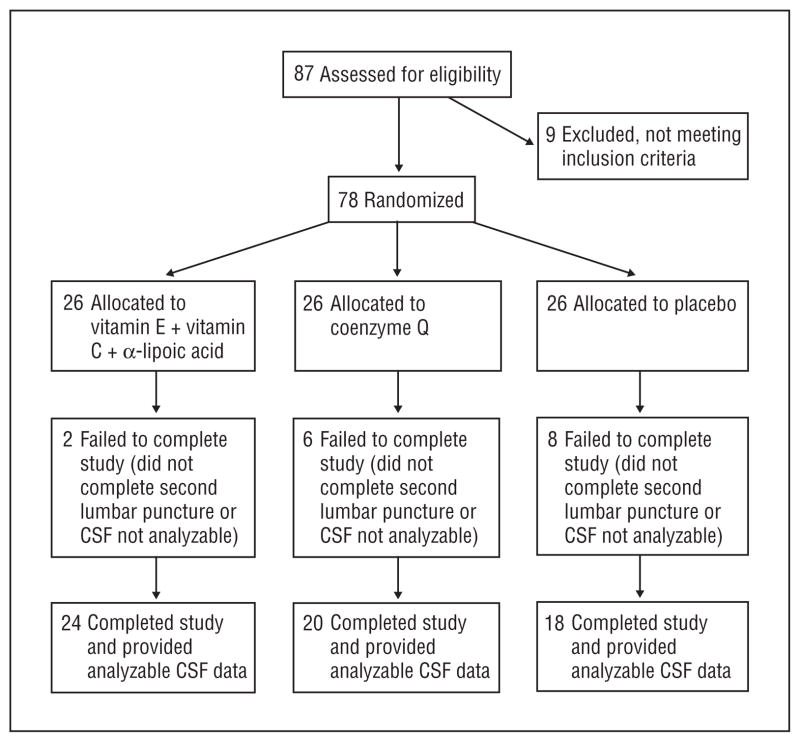
Eighty-seven subjects were screened at 12 sites, and 78 were randomized to E/C/ALA, CoQ, or placebo, using permuted block randomization (blocks of 3 subjects at each site), generated by the Coordinating Center. The flow of subjects is shown in the Figure, and demographic and clinical data, in Table 1. The groups were well matched at baseline.
Figure.
Flowchart of subject participation in the study. CSF indicates cerebrospinal fluid.
Table 1.
Demographic and Clinical Features of 78 Study Participantsa
| E/C/ALA (n = 28) | CoQ (n = 25) | Placebo (n = 25) | |
|---|---|---|---|
| Age, y, mean (SD) | 73.6 (9.1) | 71.4 (8.4) | 73.2 (9.5) |
| Women, % | 46 | 44 | 48 |
| Education, y, mean (SD) | 14.6 (3.7) | 15.0 (2.5) | 15.8 (2.4) |
| BMI, mean (SD) | 26.5 (4.1) | 27.0 (4.2) | 25.8 (4.1) |
| Acetyl cholinesterase inhibitor use, % | 88 | 84 | 100 |
| Memantine use, % | 44 | 40 | 50 |
| Concomitant vitamin or supplement use, %b | 52 | 64 | 43 |
| MMSE score, mean (SD) | 23.1 (3.9) | 23.3 (4.4) | 23.1 (3.4) |
| MMSE score change at 16 wk, mean (SD) | −2.8 (2.9)c | −1.0 (2.5) | −0.9 (2.5) |
| ADCS-ADL score, mean (SD) | 61.1 (16.3) | 65.2 (12.8) | 65.4 (8.7) |
| ADCS-ADL score change at 16 wk, mean (SD) | −4.6 (7.9) | −2.4 (6.7) | −2.3 (5.9) |
Abbreviations: ADCS-ADL, Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CoQ, coenzyme Q; E/C/ALA, vitamin E plus vitamin C plus α-lipoic acid; MMSE, Mini-Mental State Examination.
Intention to treat. Demographic or clinical variables listed in Table 1 did not differ at baseline among the treatment arms.
Concomitant vitamins or supplements were allowed only if they contained vitamin E, vitamin C, α-lipoic acid, or CoQ in amounts less than defined thresholds, much lower than the doses used in the treatment arms of this trial.
P = .04, analysis of covariance, adjusted for multiple comparisons. In a logistic model that compared MMSE score changes in the E/C/ALA and placebo arms, and included age, sex, and baseline MMSE score as covariates, treatment arm was significant (P = .02).
The study drugs were well tolerated. There were no significant changes in vital signs or body mass index from baseline to the end of the study in any treatment arm and no group differences in the frequencies of reported adverse events. There were no significant changes in results of a panel of hematological and biochemical blood tests in any of the treatment groups. Three serious adverse events were reported, in different subjects, and were judged to be unrelated to the study drug. One subject was hospitalized briefly after a pacemaker lead fell off. One subject fell and sustained a wrist fracture, which was treated. One subject developed dizziness and headache about 7 days before the week 16 visit. That subject successfully completed the week 16 lumbar puncture but was noted to have drowsiness, worsening mental abilities, and mild left hemiparesis about 5 days later. Brain magnetic resonance imaging revealed a tumor subsequently diagnosed as glioblastoma multiforme. There was no evidence of a tumor on a head computed tomography reviewed when this subject was screened, which was obtained about 18 months before the subject entered the study.
Clinical measures of cognitive abilities (MMSE) and function (ADCS-ADL) did not differ between groups at baseline. The MMSE scores showed a greater decline in the E/C/ALA group relative to placebo. There were no differences between groups in changes in ADCS-ADL total scores, or in scores for subscales of basic or instrumental ADL, although there were trends toward greater decline in the E/C/ALA group.
Cerebrospinal fluid levels of Aβ42, tau, P-tau181, and F2-isoprostanes were measureable in 66 subjects at baseline and week 16 (Table 2). Levels of each biomarker did not differ between the 3 groups at baseline. At 16 weeks, mean changes in each of the biomarkers were small. Changes in levels of Aβ42, tau, and P-tau181 did not differ between the 3 groups. Levels of F2-isoprostanes decreased in the E/C/ALA group from baseline but did not change in the CoQ or placebo groups. This was significant before and after adjustment for age and sex and represented a mean decrease of about 19%.
Table 2.
CSF Biomarker Data
| Mean (SD)
|
|||
|---|---|---|---|
| E/C/ALA (n = 24) | CoQ (n = 20) | Placebo (n = 22) | |
| A342 level at baseline, pg/mL | 190 (43) | 185 (54) | 201 (57) |
| A342 level change | −8 (23) | −15 (31) | −6 (37) |
| Tau level at baseline, pg/mL | 123 (60) | 109 (64) | 114 (84) |
| Tau level change | −23 (39) | −9 (21) | −12 (19) |
| P-tau181 level at baseline, pg/mL | 68 (33) | 70 (37) | 58 (35) |
| P-tau181 level change | −6 (19) | −4 (17) | 0.5 (19) |
| F2-isoprostane level at baseline, pg/mL | 38 (15) | 34 (15) | 35 (12) |
| F2-isoprostane level change | −7 (10)a | 0 (8) | 0 (6) |
Abbreviations: CoQ, coenzyme Q; CSF, cerebrospinal fluid; E/C/ALA, vitamin E plus vitamin C plus α-lipoic acid.
P = .04, analysis of covariance, adjusted for multiple comparisons.
Correlations were examined between clinical scores (MMSE and ADCS-ADL) and CSF biomarker indices (levels of tau, P-tau, Aβ42, and F2-isoprostanes), analyzing baseline and 16-week change data. Significant correlations were found among all subjects for baseline MMSE scores vs ADCS-ADL scores (R = 0.42) and vs CSF total tau level (R = −0.32). No other correlations were significant for baseline or change data, among all subjects or within specific treatment arms.
COMMENT
Basic and observational studies provide strong support for antioxidant treatment in AD. Oxidative stress is a widespread cellular process and lacks a typical treatment target such as a receptor or a single major metabolic pathway. This is a challenge to designing and measuring treatment interventions. Approaches to combat oxidative stress typically involve augmenting antioxidant defenses, eg, with nutritional supplements or vitamins. Key questions include what doses to use, whether defenses are more critical in relation to mitochondrial or cytosolic oxidative stress, and whether a combination of antioxidants is more effective than a single agent at quenching oxidative reactions. A related question, whether a diet high in naturally occurring antioxidants may reduce the risk of developing AD, is difficult to address in an intervention study.
The present study examined the effects of a high dose of CoQ, a naturally occurring antioxidant that may protect mitochondria from reactive oxidative species generated during oxidative metabolism. Oral administration can achieve brain antioxidant effects in animal models.19,31 A phase 2 trial in Parkinson disease suggested that 400 mg of CoQ 3 times/d resulted in slowing of motor progression,32 and a phase 3 trial is currently in progress. A phase 2 trial in amyotrophic lateral sclerosis used a high-dose range of CoQ (1800 and 2700 mg/d) but found no difference in clinical progression over 9 months of treatment compared with placebo.33 Although our study found that CoQ was safe and well tolerated in patients with AD, the absence of a biomarker signal in CSF suggests that CoQ, at the dose tested, does not improve indices of oxidative stress or neurodegeneration. These results do not support further clinical trial development of CoQ in AD. Idebenone, a benzoquinone derivative structurally related to CoQ, has been studied widely in AD and other disorders. A placebo-controlled 12-month clinical trial that assessed 3 different doses of idebenone in mild to moderate AD found no differences between treatment and placebo.34
Doses for the E/C/ALA combination were selected based on expert opinion and clinical trials for other indications. A similar combination of E/C/ALA improved indices of skeletal muscle metabolism in elderly subjects.35 Although prior studies of vitamin E in AD and other neurodegenerative disorders have used high doses, typically 2000 IU/d, we limited vitamin E to 800 mg/d because of a meta-analysis of clinical trials and prevention studies that raised the possibility that more than 800 IU/d of vitamin E may be associated with increased all-cause mortality.36
Increased decline on the MMSE and a trend in this direction on the ADCS-ADL in the E/C/ALA group raises a concern that this combination could adversely affect cognition in AD. The lack of correlation of changes in these measures with changes in CSF biomarkers suggests that the cognitive changes may not be due to worsening of AD-related pathology. Although a mechanism is uncertain, this cognitive finding raises a caution and will need to be carefully monitored if longer-term studies are planned.
The combination of E/C/ALA did not affect CSF biomarkers related to Aβ, tau, or P-tau. The half-life of Aβ42 in CSF is extremely short, measured in hours.37 Tau and P-tau persist for a few weeks in CSF after acute stroke.38 Our clinical trial was therefore long enough to be able to detect a potential anti-AD effect on these bio-markers. Our findings suggest that this antioxidant combination did not influence pathways related to amyloid and tau pathology. However, E/C/ALA did result in a significant decrease in CSF levels of F2-isoprostanes, consistent with antioxidant effects in the brain. These findings are analogous to the transgenic mouse model study cited earlier, in which vitamin E plus vitamin C decreased neuroprostane levels in the brain, without affecting measures of Aβ, plaques, or neuritic changes; in this model, there was improvement of performance on spatial memory testing.11
It is unclear whether the relatively small reduction in CSF F2-isoprostane level seen in this study may lead to clinical benefits in AD. The more rapid MMSE score decline raises a caution and indicates that cognitive performance would need to be assessed if a longer-term clinical trial of this antioxidant combination is considered.
Acknowledgments
Funding/Support: The study was supported by NIA grant UO1 AG10483.
Role of the Sponsors: The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
ADCS Investigators: The ADCS investigators and sites in this clinical trial were David Weisman, MD (site principal investigator [PI]), Mary Margaret Pay (study coordinator), University of California, San Diego; Alan Lerner, MD (site PI), Elaine Ziol (study coordinator), Case Western Reserve University, Cleveland, Ohio; Joseph Quinn, MD (site PI), Samantha Raphael (study coordinator), Oregon Health and Sciences University, Portland; Ruth Mulnard, RN, DNSc, FAAN (site PI), Catherine McAdams-Ortiz (study coordinator), University of California, Irvine; John Ringman, MD (site PI), Jenny Bardens (study coordinator), University of California, Los Angeles; Gregory Jicha, MD, PhD (site PI), Sarah Carr(study coordinator), University of Kentucky, Lexington; Christopher M. Clark, MD (site PI), Cassie Pham (study coordinator), University of Pennsylvania, Philadelphia; Elaine Peskind, MD (site PI), Linda Mandelco (study coordinator), University of Washington, Seattle; Aljoeson Walker, MD (site PI), Stephanie Kirbach (study coordinator), Medical University of South Carolina, Charleston; Steven DeKosky, MD (site PI), Carolyn Mishler-Rickard (study coordinator), University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania; Smita Kittur, MD (site PI), Seema Mirje (study coordinator), Neurological Care of Central NewYork, Buffalo.
Additional Contributions: The ADCS Data Coordinating Center provided oversight for the conduct and monitoring of the clinical trial.
Author Contributions: The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Galasko, Cotman, Thomas, and Aisen. Acquisition of data: Galasko, Peskind, Clark, Quinn, Ringman, Jicha, Cotman, Cottrell, Montine, and Aisen. Analysis and interpretation of data: Galasko, Quinn, Ringman, Jicha, Cottrell, Thomas, and Aisen. Drafting of the manuscript: Galasko, Cottrell, and Thomas. Critical revision of the manuscript for important intellectual content: Galasko, Peskind, Clark, Quinn, Ringman, Jicha, Cotman, Montine, Thomas, and Aisen. Statistical analysis: Thomas. Obtained funding: Galasko, Montine, and Aisen. Administrative, technical, and material support: Galasko, Peskind, Ringman, Jicha, Cottrell, Thomas, and Aisen. Study supervision: Galasko, Montine, Thomas, and Aisen.
Financial Disclosure: None of the authors have any disclosures regarding the study; the specific antioxidants that were examined; Vitaline Inc (the company that supplied the study drugs and placebo); or the CSF assays that were measured. Dr Galasko serves as editor of Alzheimer’s Disease Research and Treatment; serves on data safety monitoring boards for Elan and Janssen; is an investigator in clinical trials sponsored by Eli Lilly and Avid; and receives research support from National Institutes of Health (NIH)grants P50AGO5131 (PI) and U01 AG10483 (coinvestigator). Dr Peskind is an investigator in clinical trials sponsored by Eli Lilly, Bristol-Myers Squibb, Novartis, and Medivation and receives research support from NIH grant R01 AGO33133, the Department of Veterans Affairs (VA Cooperative Study 563 [co-PI] and Rehabilitation Research and Development Merit Review), and an anonymous foundation. Dr Clark is the medical director of Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly and Company. Prior to January 2010, he was the director of the Center of Excellence for Research on Neurodegenerative Diseases at the University of Pennsylvania and served on data safety monitoring committees for Elan Pharmaceuticals and Myriad and the diagnostic adjudication committee for a Bristol-Myers Squibb treatment trial. Dr Quinn is on the speakers bureau for Pfizer, Forest, and Novartis. He is an investigator in clinical trials sponsored by Elan, Bristol-Meyers Squibb, Pfizer, and Danone. He is a coinventor on a patent for the use of docosahexaenoic acid for the treatment of AD (Martek Biosciences). He also receives research support from the Department of Veterans Affairs (merit review funding) and NIH grants P50AGO0817 (PI) and U01 AG10483 (project director). Dr Ringman currently receives research support from National Institute on Aging (NIA) grants P50 AG16570 (PI) and U01 AG032438 (site PI). He has served on an advisory board for Taked a Pharmaceuticals. Dr Jicha is an investigator on clinical trials sponsored by Janssen, Baxter, Pfizer, Medivation, and Danone. He has consulted for Medivation, Martek, and Avanir in clinical trial development. He receives research support from NIH grants P30AG028383 (coinvestigator), R01 AG019241 (coinvestigator), U01 AG010483 (coinvestigator), R01 HD064993-0110 (coinvestigator), and L30 AG032934 (PI) and from Alzheimer’s Association grant NIRG-07-59967 (PI). Dr Cotman receives research support from NIH grants AR47752, AG000096, AG00538, and AG016573. Dr Montine serves as senior editor for Brain Pathology and on the editorial board of the American Journal of Pathology and receives research support from NIH grants P50 NS62684 (PI), R01 ES16754 (PI), T32 ES07032 (PI), R01 AG31892 (PI), U01 AG32984 (coinvestigator), and P50 AGO5136 (coinvestigator). Dr Thomas has served as a consultant for Medivation and Bristol-Myers Squibb. He has also served on the data safety monitoring board for a trial sponsored by Myriad. Dr Aisen serves on a scientific advisory board for NeuroPhage; serves as a consultant to Elan Corporation, Wyeth, Eisai Inc, Schering-Plough Corp, Bristol-Myers Squibb, Eli Lilly and Company, NeuroPhage, Merck & Co, Roche, Amgen, Genentech Inc, Abbott, Pfizer Inc, Novartis, Bayer, Astellas, Dainippon, Biomarin, Solvay, Otsuka, Daiichi, AstraZeneca, Janssen, Medivation, Theravance, Cardeus, and Anavex; receives research support from Pfizer Inc, Baxter International Inc, and NIH NIA grants U01 AG10483 (PI), U01 AG024904 (Coordinating Center director), and R01 AG030048 (PI) and NIH grant R01 AG16381 (coinvestigator); and has received stock options from Medivation and NeuroPhage.
References
- 1.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res. 2007;85(14):3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 2.Montine TJ, Neely MD, Quinn JF, et al. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med. 2002;33(5):620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 3.Morris MC. The role of nutrition in Alzheimer’s disease: epidemiological evidence. Eur J Neurol. 2009;16(suppl 1):1–7. doi: 10.1111/j.1468-1331.2009.02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaffe K, Clemons TE, McBee WL, Lindblad AS Age-Related Eye Disease Study Research Group. Impact of antioxidants, zinc, and copper on cognition in the elderly: a randomized, controlled trial. Neurology. 2004;63(9):1705–1707. doi: 10.1212/01.wnl.0000142969.19465.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 6.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336(17):1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Thomas RG, Grundman M, et al. Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 8.Floyd RA, Hensley K. Oxidative stress in brain aging: implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23(5):795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 9.Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11(9):1135–1146. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- 10.Sung S, Yao Y, Uryu K, et al. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18(2):323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 11.Harrison FE, Allard J, Bixler R, et al. Antioxidants and cognitive training interact to affect oxidative stress and memory in APP/PSEN1 mice. Nutr Neurosci. 2009;12(5):203–218. doi: 10.1179/147683009X423364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Alloza M, Dodwell SA, Meyer-Luehmann M, Hyman BT, Bacskai BJ. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. J Neuropathol Exp Neurol. 2006;65(11):1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- 13.Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15(4):685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- 14.Quinn JF, Bussiere JR, Hammond RS, et al. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28(2):213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Siedlak SL, Casadesus G, Webber KM, et al. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res. 2009;43(2):156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hager K, Kenklies M, McAfoose J, Engel J, Münch G. Alpha-lipoic acid as a new treatment option for Alzheimer’s disease: a 48 months follow-up analysis. J Neural Transm Suppl. 2007;72(72):189–193. doi: 10.1007/978-3-211-73574-9_24. [DOI] [PubMed] [Google Scholar]
- 17.Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36(4):381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34(2):165–171. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- 19.Wadsworth TL, Bishop JA, Pappu AS, Woltjer RL, Quinn JF. Evaluation of co-enzyme Q as an antioxidant strategy for Alzheimer’s disease. J Alzheimers Dis. 2008;14(2):225–234. doi: 10.3233/jad-2008-14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer’s disease. J Am Geriatr Soc. 2004;52(7):1070–1076. doi: 10.1111/j.1532-5415.2004.52303.x. [DOI] [PubMed] [Google Scholar]
- 23.Thal LJ, Kantarci K, Reiman EM, et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):6–15. doi: 10.1097/01.wad.0000191420.61260.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 25.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med. 2010;4(1):27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montine TJ, Beal MF, Cudkowicz ME, et al. Increased CSF F2-isoprostane concentration in probable AD. Neurology. 1999;52(3):562–565. doi: 10.1212/wnl.52.3.562. [DOI] [PubMed] [Google Scholar]
- 27.Ringman JM, Younkin SG, Pratico D, et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008;71(2):85–92. doi: 10.1212/01.wnl.0000303973.71803.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51(2):336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. doi: 10.2307/2336325. [DOI] [Google Scholar]
- 30.Blennow K, Zetterberg H, Minthon L, et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett. 2007;419(1):18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 31.Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci U S A. 1998;95(15):8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shults CW, Oakes D, Kieburtz K, et al. Parkinson Study Group. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59(10):1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann P, Thompson JL, Levy G, et al. QALS Study Group. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66(2):235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thal LJ, Grundman M, Berg J, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology. 2003;61(11):1498–1502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- 35.Wray DW, Nishiyama SK, Monnet A, et al. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009;297(5):H1870–H1875. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 37.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12(7):856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phosphotau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297(3):187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]