Abstract
Background
The anatomical spatial distribution of microencapsulated islets transplanted into the peritoneal cavity of large animals remains a relatively unexplored area of study. In this study, we developed a new implantation approach using laparoscopy in order to avoid microcapsule amalgamation. This approach constitutes a clinically relevant method, which can be used to evaluate the distribution and in vivo biocompatibility of various types of transplanted microcapsules in the future.
Materials and Methods
Two healthy baboons were implanted intraperitoneally with microencapsulated islets through mini-laparotomy and observed at 76 days after implantation. Nine baboons underwent laparoscopic implantation of approximately 80,000 empty microcapsules. Microcapsule distribution was observed by laparoscopic camera during and after implantation at 1, 2, and 4 weeks. At each time point, microcapsules were retrieved and evaluated with brightfield microscopy and histological analysis.
Results
Mini-laparotomic implantation resulted in microcapusle aggregation in both baboons. In contrast, laparoscopic implantation resulted in even distribution of microcapsules throughout the peritoneum without sedimentation to the Douglas space in all animals. In 8 out of 9 animals, retrieved microcapsules were evenly distributed in the peritoneal cavity and presented with no pericapsular overgrowth and easily washed out during laparoscopic procedure. The one exception was attributed to microcapsule contamination with blood from the abdominal wall following trocar insertion.
Conclusions
Laparoscopic implantation of microcapsules in non-human primates can be successfully performed and prevents microcapsule aggregation. Given the current widespread clinical application of laparoscopy, we propose that this presented laparoscopy technique could be applied in future clinical trials of microencapsulated islet transplantation.
Keywords: islets, microcapsules, laparoscopy, implantation, non-human primate (NHP), pouch of Douglas, microcapsule delivery device (MDD)
Introduction
While microencapsulated islet transplantation has been extensively investigated in rodents [1-4] and has been conducted intraportally in pigs [5], research in clinically relevant non-human primate (NHP) models remains limited. In particular, relatively little work has been devoted to optimizing the transplantation site and investigating the biocompatibility of implanted microcapsules in NHP models. Intraperitoneal transplant of encapsulated porcine islets in monkeys, via a catheter, has been documented [6, 7] in the past. More advanced surgical approaches, such as laparoscopy, have not yet been explored in NHP models.
Due to the significantly larger transplant volume in the case of microencapsulated islets, in comparison to free islets, the implantation site is restricted to the domain of the peritoneal cavity [8]. Within this site, encapsulated islets rely solely on diffusion to obtain oxygen and nutrients, as well as to remove wastes, that are essential for graft survival and function. This dependence on diffusion associated with the implantation site is exacerbated by properties of the microcapsule itself, which change the nutrient/waste exchange kinetics of the islets. The biopolymer composition of the microcapsule extends the biotransport time and, therefore, microencapsulated islets experience greater difficulty in the uptake of essential nutrients in comparison to non-encapsulated islets. Moreover, oxygen supply to the islets is further hampered in the case of 1) microcapsular clumping and 2) cellular overgrowth on the microcapsule surface [9].
Non-human primates are highly relevant preclinical models because of the close phylogenic relationship to humans [10]; specific for this study, the observation of microcapsule clumping and sedimentation in context of the bipedal feature of the NHP model holds particular clinical relevance. In order to maximize the surface area available for the diffusion of oxygen and critical nutrients, as well as for the removal of metabolic wastes, it is vital that transplanted microcapsules are 1) distributed evenly throughout the spaces of the peritoneal cavity, and 2) highly biocompatible.
Laparoscopic surgery represents a safe, minimally invasive procedure that is widely applied in patients for a variety of surgical procedures [11-15]. One advantage of laparoscopic technique is its ease of monitoring and recording. This facilitates the determination of graft status and the progression of graft assimilation or rejection post-transplantation, which may provide important information for later troubleshooting. Herein, we report the first successful performance of empty microcapsule implantation in a NHP model using a laparoscopic technique. The purpose of the present study was to evaluate this new laparoscopic method for implantation in a NHP model, with the ultimate goal of creating a clinically relevant platform for microencapsulated islet transplantation and biocompatibility screening of different microcapsule types.
Materials and Methods
Non-human primates
A total of 11 Papio anubis baboons (2 male, 9 female; weight: 9.9-15.3 kg) were purchased from various commercial sources for the implantation study. All the baboons were housed at the University of Illinois at Chicago (UIC), in the Biologic Resources Laboratory (BRL). Procedures involving these animals were conducted in accordance with the guidelines of the National Institute of Health (NIH) and the Animal Care Committee (ACC) at UIC.
Microcapsules
Empty PMCG microcapsules, synthesized by the polyelectrolyte complexation between sodium alginate (SA), cellulose sulfate (CS) and polymethylene-co-guanidine (PMCG), first developed as described in [16], were optimized for the pre-clinical validation at the Polymer Institute of the Slovak Academy of Sciences (Bratislava, Slovakia) and produced either at the Polymer Institute in Bratislava or at the University of Illinois at Chicago (Chicago, USA) by the same group . The microcapsules produced in Slovakia were shipped to the US, in 50 ml conical tubes containing CMRL 1066 culture medium, by a commercial courier (World Courier, Inc.). The empty microcapsules were stored in Hank’s Buffered Salt Solution (HBSS, Mediatech) at room temperature until implantation. At the day of implantation, the empty microcapsules were collected and washed five times with 250 ml of HBSS. In each experiment, 80,000 empty microcapsules (30 ml of volume) were implanted into each baboon.
Microcapsule Delivery Device (MDD) and validation experiment
In order to transfer the microcapsules effectively and aseptically into the peritoneal cavity of baboons, we developed a simple Microcapsule Delivery Device (MDD) and adapted it to the laparoscopic procedure. This MDD apparatus consists of one 1 ml-pipette (Fisher) and one 60 ml syringe (Becton Dickinson and Company) connected by a 15-inch long sterile silicon tube (96400-16, MASTERFLEX) (Fig. 1, Fig. 2A).
Figure 1.
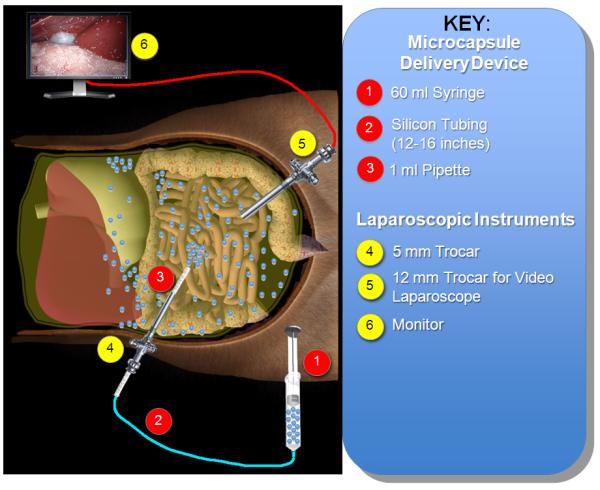
Schematic representation of the laparoscopic implantation procedure for empty PMCG microcapsules.
Figure 2.
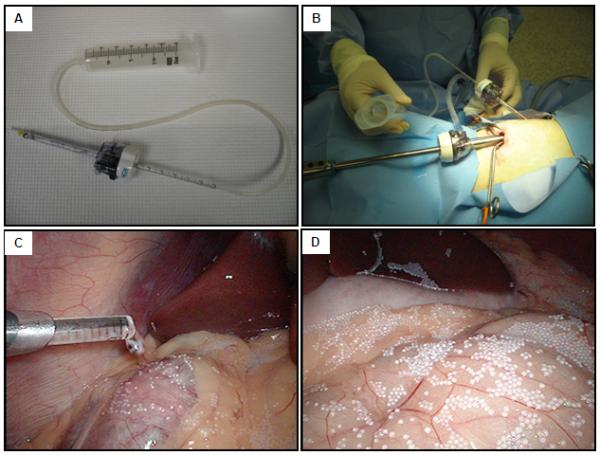
Laparoscopic approach. (A): The Microcapsule Delivery Device (MDD). (B): Overview of the laparoscopy and MDD set up. (C): Inner end of the MDD, observed from peritoneal cavity. (D): Overview of the peritoneal cavity seeded with PMCG microcapsules.
13,000 empty microcapsules (approximate 5 ml in volume) from the first batch of implantation were preserved in order to validate this MDD. The empty microcapsules were divided equally into three groups, each supplemented in 10 ml of HBSS (concentration of 400 microcapsules/ml), and three independent experiments were conducted. For each experiment, the microcapsules were transferred into a 60 ml syringe and infused through MDD via a syringe pump (Harvard Apparatus) at rate of 30 ml/min into a 500 ml glass beaker. Throughout this experiment, microcapsules were evaluated for changes in size and morphology. Before and after being infused through the MDD device, 25 microcapsules were randomly selected for microscopic analysis. For each microcapsule, the vertical and horizontal diameters were measured using Leica Application Suit V3 imaging system (Leica Microsystems Inc.). The values of 50 measurements from 25 microcapsules at each condition were averaged. Shape and integrity were examined and served as the indicators for morphological changes.
Implantation of microencapsulated islets by mini-laparotomy
This initial study was conducted to examine the anatomical spatial distribution and functional capacity of microencapsulated islets in the peritoneal cavity following random implantation via mini-laparotomy in two baboons. Briefly, recipient animals were fasted for 12 hours prior to surgery. On the day of the surgery, recipient animals were sedated with ketamine (10 mg/kg, im), induced with propofol (3-5 mg/kg, iv), and anesthetized using continuous isoflurane gas infusion. Additionally, buprenorphine (0.01-0.03 mg/kg, im), cefazolin (25 mg/kg, im), and bupivicaine (1 mg/kg) were given preoperatively. A midline incision (4cm) was made and the microencapsulated islets (30 ml of volume contained in a 250 ml conical tube) were infused randomly into peritoneal cavity of the baboons.
Implantation of the microcapsules by laparoscopy
Nine recipient baboons were implanted with microcapsules by laparoscopy. Laparoscopic equipment (Olympus 10 mm videolaparoscopy; Olympus Visera digital video processor, OTV-S7; light source, CLV-S40; high flow insufflations unit, UHI-3; color video monitor, OEV 203) was applied for intraperitoneal microcapsule implantation in all animals. The baboon was placed in the supine position and, with the surgeon on the right side of the baboon and the assistant on the left side, a small (2 cm) supraumbilical incision was made and a 12 mm trocar (Endopath Xcel F4N315, ETHICON ENDO-SURGERY, USA) was inserted. Pneumoperitoneum was created by insufflating CO2 gas and the pressure was kept at 12 mmHg. The videolaparoscopy was warmed by 70°C saline and inserted into peritoneal cavity through the 12 mm trocar. Another small incision (1 cm) was created and a 5 mm trocar (Endopath Xcel F4N315, ETHICON ENDO-SURGERY, USA) was inserted into peritoneal cavity under the view of videolaparoscopy. The peritoneal cavity was viewed and the MDD was inserted, as shown in Fig. 2B. The microcapsules were transferred from the 250 ml conical tube to the 60-ml syringe and infused into peritoneal cavity at a rate of 30 ml/min. 80,000 empty PMCG microcapsules (approximate 30 ml in volume), supplemented with 200 ml HBSS, were implanted into peritoneal cavity of each baboon. Even distribution of microcapsules was accomplished through adjusting the 1ml-pipette of the MDD in the following spaces: perihepatic, retrogastric, perisplenic, omentum, and behind small bowel (Fig. 2C, D). The total infusion time was approximately 6 minutes.
In vivo observation of distribution and subsequent retrieval of microcapsules
The implantation procedure was filmed using a video recorder (Panasonic VHS, PV-9400). Pictures were taken by the videolaparoscopy and stored in an Olympus xD-picture card. The peritoneal cavity was viewed for complications and unusual events during the implantation procedure. The implanted microcapsules were retrieved at 1, 2, 4 weeks (n=8 for each time point), and 4 months (n=1) after infusion. The surgical set up for microcapsule retrieval was similar to the implantation procedure. In brief, after anesthesia, pneumoperitoneum was created and two trocars were inserted into the peritoneal cavity of recipient baboons. Before harvesting the implanted microcapsules, the peritoneal cavity was viewed and screened for microcapsule clumping, inflammatory adhesion, and organ abnormalities. Pictures were taken using the camera attached to laparoscopy. In order to harvest the microcapsules, a 60 ml of HBSS was injected through MDD to gently flush the microcapsules. The floating microcapsules were aspirated back into the 60 ml syringe and collected for histological analysis.
Histological evaluation of the retrieved microcapsules
Upon retrieval, the microcapsules were rinsed with HBSS and fixed in 10% formalin overnight. On day 2, microcapsules were rinsed twice with Phosphate-Buffered Saline (PBS) (Mediatch, Inc.), for 15 minutes each, and dehydrated in ascending ethanol solutions (30%, 50%, 60%, 70%, 90%, 95%, and 100% EtOH), for twenty minutes each. Microcapsules were then incubated in xylene for 10 minutes and finally in a 50/50 solution of xylene and paraffin over night at 57°C. On day 3, microcapsules were incubated twice in paraffin (Fisherbrand, Paraplast plus catalogue No.: 23-021-400), for 1 hour each, at 57°C and then embedded in a paraffin mold. Subsequently, embedded microcapsules were sectioned onto positively charged lysine microscope slides. Consecutive slides were then stained for Hematoxylin/Eosin (H&E).
Statistical analysis
The collected data values are presented as mean values ± standard deviation. A statistical comparison of capsule size between the pre- and post-infusion groups was performed by Student’s t-test, and a value of p < 0.05 was considered statistically significant.
Results
The validation experiment for the MDD demonstrated no significant change between pre-infusion microcapsule size (875 ± 13 μm) and post-infusion microcapsule size (876 ± 18 μm) (p=0.55) (Fig. 3). Microscopic analysis demonstrated that microcapsules remain intact and maintain their spherical shape during this infusion procedure.
Figure 3.
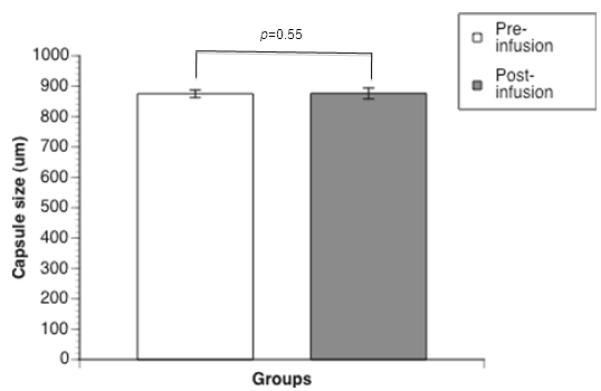
Validation of the Microcapsule Delivery Device (MDD): microcapsule size. Microcapsule size quantification, via Leica Application Suite V3 imaging system, pre-infusion and post-infusion through the MDD. The values are expressed by mean ± SD from three individual experiments. There was no significant difference between the two groups (p=0.55).
At 76 days after mini-laparotomic implantation, the two baboons were euthanized and the peritoneal cavity was inspected. In both animals, microcapsules sedimented to the pouch of Douglas, formed large aggregates, elicited a foreign body reaction (Fig. 4).
Figure 4.
View of the peritoneal cavity at 76 days following random implantation of microencapsulated islets via mini-laparotomy. Images reveal clumping of alginate bead microcapsules on the abdominal wall (A) and the colon (B).
A total of 8 implantations were successfully conducted using laparoscopic technique. Within this group, the microcapsules were safely infused and no complications were reported during the observation period. Microcapsules were seeded evenly across the peritoneal spaces (Fig. 2D) and no aggregation or sedimentation of the implanted microcapsules was observed for up to 4 weeks post implantation in all animals (Fig. 5). In one animal, the microcapsules were retrieved after 4 months of implantation and they were also found to be clean and intact. At each time point of microcapsule retrieval, the microcapsules were evenly distributed in the peritoneal cavity and easily washed out during laparoscopic procedure (Fig. 6A). Histological analysis revealed that the PMCG microcapsules were intact and free of cellular adhesion until end of the experiment at 4 months after implantation (Fig. 6B).
Figure 5.
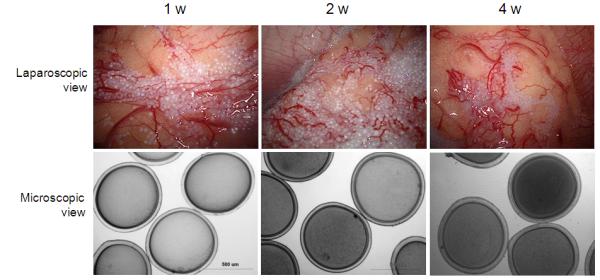
Laparoscopic and microscopic images of retrieved microcapsules at 1, 2, and 4 weeks post-implantation in successfully implanted baboons (n=8).
Figure 6.
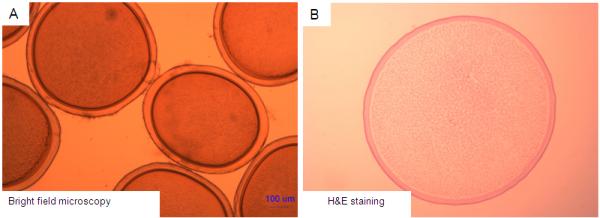
Retrieved microcapsules at 4 months post-implantation in a successfully implanted baboon. (A): Optical microscopic view. (B): Histologic view. The microcapsules are intact and present with no cellular overgrowth around the microcapsules.
During the implantation process in one animal, trocar insertion caused abdominal wall bleeding and, subsequently, microcapsule-blood contact. One week post-implantation, most of the microcapsules adhered to surrounding tissue in this animal. Brightfield optical microscopy (Fig. 7A) and HE staining (Fig. 7B) revealed pericapsular cell overgrowth on the surface of the microcapsules.
Figure 7.
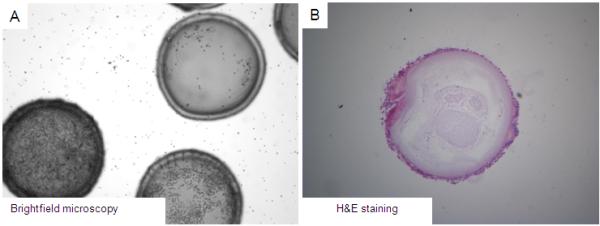
Retrieved microcapsules at 1 week post-implantation in the baboon that experienced abdominal bleeding. (A): Optical microscopic view. (B): Histologic view. Histological analysis demonstrates 2-3 layers of cellular overgrowth.
Discussion
In this presented study, we demonstrate for the first time that laparoscopic microcapsule implantation in non-human primates can be successfully performed. The main benefit of laparoscopy, in this application, is that enables even distribution of the microcapsules. Our results demonstrated that random microcapsule implantation via mini-laparotomy resulted in microcapsule aggregation and clumping in the lower part of the abdominal space (Douglas space) (Fig. 4). However, laparoscopic implantation led to even distribution of transplanted microcapsules throughout the peritoneal cavity, preventing microcapsule sedimentation and clumping (Fig. 2D).
In order to implement this technique, it is of primary concern that microcapsules can be safely delivered through the laparoscopic device into the peritoneal cavity without changing the physical properties of the microcapsules. This is especially important in the case of microencapsulated islet transplantation, since a rupture of the microcapsule after implantation can result in islet tissue release and, consequently, cause the mounting of an immune response that could ultimately lead to sensitization, transplant rejection and graft failure [21]. As demonstrated in this study, our developed laparoscopic infusion procedure did not compromise the physical properties of the microcapsules. There were no significant changes in microcapsule size and morphology (Fig. 3) when the microcapsules were infused at the speed of 30 ml/min. This same speed was used to infuse the microcapsules into the peritoneal cavity of baboon implant recipients.
In addition to safe delivery of microcapsules with this device, the described laparoscopic setup, allows precise delivery to desired anatomical locations by the operator. The minimally invasive nature of this procedure also enables the application of this same implantation technique for later capsule retrieval. As presented in this study, microcapsules can be easily harvested at later time points without distressing the other implanted microcapsules. Retrieved PMCG microcapsules were found intact and free of cellular overgrowth after 4 weeks of implantation in 8 animals (Fig. 5). In the one animal that was followed for 4 months, intact PMCG microcapsules were found free-floating in the abdominal cavity (Fig. 6). Out of the 9 animals that were implanted with empty microcapsules by laparoscopy, some bleeding occurred during trocar insertion in one animal, which lead to microcapsule adhesion one week after implantation (Fig. 7). This finding, indicating a non-specific systemic immune reaction from host initiated promptly following direct contact with the blood stream, is consistent with a previous study by Toso et al., in which implantation of empty microcapsules similar to our PMCG microcapsules into the portal vein triggers a foreign body reaction [22]. The finding also implies that mini-laparotomy, as a more invasive procedure than laparoscopy, might lead to more bleeding and thus more adhesions. Certainly, this is an unexpected result from only one animal within 9 animals that received microcapsule implantation. Further experiment may be necessary to reproduce and confirm this single observed reaction.
In this study, the described laparoscopic approach (laparoscopy + MDD) is considered as a whole procedure since laparoscopy helps visualize the field in the peritoneal cavity and the MDD delivers the capsules to the expected location. The MDD is a device designed specifically for laparoscopic technique and it would not function properly without the guide of laparoscopic camera during implantation and retrieval. Therefore, we have not conducted the experiment where laparotomy and MDD are used together.
In conclusion, this study demonstrates that laparoscopic implantation is an effective and simple approach for microcapsule implantation and subsequent evaluation in the preclinical non-human primate model. By using this implantation protocol in combination with the employed microcapsule delivery device, microcapsules can be implanted easily, in the absence of a long and stressful surgical operation, and retrieved repeatedly if required. We propose that this technique can be utilized in the preclinical evaluation of encapsulated islet transplantation in the future. Additionally, the laparoscopic MDD device developed in this study may be beneficial for application in other cellular therapies.
Acknowledgments
This study was conducted as part of the Chicago Diabetes Project, an international collaboration to find a functional cure for diabetes. The authors gratefully thank the CDP for financial support and AQUA+TECH for the time and advice to this work.
This study was also sponsored by the Christopher Family Foundation, the Efroymson Fund, the Wirtz Family, and the College of Medicine at UIC. J.O. is supported by NIH (ICR Grant 1 U42 RR023245-01) and JDRF (#5-2006-398 and #5-2006-424 and #5-2007-773), as well as by the Washington Square Health Foundation and the Grant Health Care Foundation. IL and GK were also supported by the Slovak Research and Development Agency (contract No. APVV-51-033205).
References
- 1.Meyer T, Hocht B, Ulrichs K. Xenogeneic islet transplantation of microencapsulated porcine islets for therapy of type I diabetes: long-term normoglycemia in STZ-diabetic rats without immunosuppression. Pediatr Surg Int. 2008 Dec;24(12):1375–1378. doi: 10.1007/s00383-008-2267-9. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T, Harb G, Rajotte RV, Korbutt GS, Mallett AG, Arefanian H, et al. Immune mechanisms associated with the rejection of encapsulated neonatal porcine islet xenografts. Xenotransplantation. 2006 Nov;13(6):547–559. doi: 10.1111/j.1399-3089.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 3.Foster JL, Williams G, Williams LJ, Tuch BE. Differentiation of transplanted microencapsulated fetal pancreatic cells. Transplantation. 2007 Jun 15;83(11):1440–1448. doi: 10.1097/01.tp.0000264555.46417.7d. [DOI] [PubMed] [Google Scholar]
- 4.Qi M, Strand BL, Morch Y, Lacik I, Wang Y, Salehi P, et al. Encapsulation of human islets in novel inhomogeneous alginate-ca2+/ba2+ microbeads: in vitro and in vivo function. Artif Cells Blood Substit Immobil Biotechnol. 2008;36(5):403–420. doi: 10.1080/10731190802369755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toso C, Oberholzer J, Ceausoglu I, Ris F, Rochat B, Rehor A, et al. Intra-portal injection of 400-mu m microcapsules in a large-animal model. Transpl Int. 2003;16(6):405–410. doi: 10.1007/s00147-003-0555-9. [DOI] [PubMed] [Google Scholar]
- 6.Sun YL, Ma XJ, Zhou DB, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98(6):1417–1422. doi: 10.1172/JCI118929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, et al. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005 Jan-Feb;37(1):466–469. doi: 10.1016/j.transproceed.2004.12.198. [DOI] [PubMed] [Google Scholar]
- 8.King A, Andersson A, Strand BL, Lau J, Skjak-Braek G, Sandler S. The role of capsule composition and biologic responses in the function of transplanted microencapsulated islets of Langerhans. Transplantation. 2003 Jul 27;76(2):275–279. doi: 10.1097/01.TP.0000078625.29988.0A. [DOI] [PubMed] [Google Scholar]
- 9.Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann N Y Acad Sci. 1997 Dec 31;831:145–167. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94(16):8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoikes N, Mangiante E, Voeller G. Laparoscopic repair of a man with massive bilateral femoral hernias. Am Surg. 2009 Dec;75(12):1189–1192. [PubMed] [Google Scholar]
- 12.Chen WS, Tzeng KH, Leu SY, Hsu H. The application of laparoscopy in colorectal surgery: a preliminary report of twelve cases. Zhonghua Yi Xue Za Zhi (Taipei) 1994 Jun;53(6):357–362. [PubMed] [Google Scholar]
- 13.Soper NJ. Effect of nonbiliary problems on laparoscopic cholecystectomy. Am J Surg. 1993 Apr;165(4):522–526. doi: 10.1016/s0002-9610(05)80954-7. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga Y, Higashino M, Tanimura S, Takemura M, Fujiwara Y, Osugi H. Laparoscopic surgery for stage IV colorectal cancer. Surg Endosc. 2009 Dec 24; doi: 10.1007/s00464-009-0778-7. [DOI] [PubMed] [Google Scholar]
- 15.E CHL, Ngai TC, G PCY, Li MK. Laparoscopic approach of surgical treatment for primary hepatolithiasis: a cohort study. Am J Surg. 2009 Dec 1; doi: 10.1016/j.amjsurg.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Lacik I, Brissova M, Anilkumar AV, Powers AC, Wang T. New capsule with tailored properties for the encapsulation of living cells. J Biomed Mater Res. 1998;39(1):52–60. doi: 10.1002/(sici)1097-4636(199801)39:1<52::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Hirshberg B, Montgomery S, Wysoki MG, Xu H, Tadaki D, Lee J, et al. Pancreatic islet transplantation using the nonhuman primate (rhesus) model predicts that the portal vein is superior to the celiac artery as the islet infusion site. Diabetes. 2002 Jul;51(7):2135–2140. doi: 10.2337/diabetes.51.7.2135. [DOI] [PubMed] [Google Scholar]
- 18.Thomas FT, Ricordi C, Contreras JL, Hubbard WJ, Jiang XL, Eckhoff DE, et al. Reversal of naturally occuring diabetes in primates by unmodified islet xenografts without chronic immunosuppression. Transplantation. 1999;67(6):846–854. doi: 10.1097/00007890-199903270-00011. [DOI] [PubMed] [Google Scholar]
- 19.Kenyon NS, Chatzipetrou M, Tzakis A, Miller J, Alejandro R, Ricordi C. Allogeneic hematopoietic stem cell transplantation in recipients of cellular or solid organ allografts. Cancer Treat Res. 1999;101:109–132. doi: 10.1007/978-1-4615-4987-1_6. [DOI] [PubMed] [Google Scholar]
- 20.Chabot JA, Stegall MD, Weber C, Reemtsma K, Hardy MA. Pancreatic islet allo- and xenotransplantation in cynomolgus monkeys. Transplant Proc. 1989 Feb;21(1 Pt 3):2739–2740. [PubMed] [Google Scholar]
- 21.Chang TM. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005 Mar;4(3):221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 22.Toso C, Mathe Z, Morel P, Oberholzer J, Bosco D, Sainz-Vidal D, et al. Effect of microcapsule composition and short-term immunosuppression on intraportal biocompatibility. Cell Transplant. 2005;14(2-3):159–167. doi: 10.3727/000000005783983223. [DOI] [PubMed] [Google Scholar]


