Abstract
Hypoxia Inducible Factor (HIF) is a master heterodimeric transcriptional regulator of oxygen (O2) homeostasis critical to proper angiogenic responses. Due to the distinctive coexpression of HIF-1α and HIF-2α subunits in endothelial cells, our goal was to examine the genetic elimination of HIF transcriptional activity in response to physiological hypoxic conditions by using a genetic model in which the required HIF-β subunit (ARNT, Aryl hydrocarbon Receptor Nuclear Translocator) to HIF transcriptional responses was depleted. Endothelial cells (ECs) and aortic explants were isolated from ArntloxP/loxP mice and infected with Adenovirus -Cre/GFP or control -GFP. We observed that moderate levels of 2.5% O2 promoted vessel sprouting, growth, and branching in control aortic ring assays while growth from Adenovirus -Cre infected explants was compromised. Primary Adenovirus -Cre infected EC cultures featured adverse migration and tube formation phenotypes. Primary pulmonary or cardiac ARNT-deleted ECs also failed to proliferate and survive in response to 8 or 2.5% O2 and hydrogen peroxide treatment. Our data demonstrates that ARNT promotes EC migration and vessel outgrowth and indispensible for the proliferation and preservation of ECs in response to the physiological environmental cue of hypoxia. Thus, these results demonstrate that ARNT plays a critical intrinsic role in ECs and support a critical role for the collaboration of HIF-1 and HIF-2 transcriptional activity in these cells.
Keywords: Angiogenesis, ARNT, HIF, physiological hypoxia, endothelium
Introduction
Hypoxia is known to contribute to the regulation of the growth of new blood vessels in ischemic diseases. Hypoxia Inducible Factor (HIF) -1 and -2 are the predominant transcriptional regulators of multiple genes involved in adaptation to hypoxic stress. In the presence of oxygen (O2), HIF-α protein subunits are hydroxylated at specific proline residues by prolyl hydroxylases (PHDs) protein (pVHL)- mediated ubiquitin-proteasome pathway [1–3]. As O2 becomes limiting, HIF-α subunits bind and dimerize to the ubiquitously, constitutively expressed β-subunit, ARNT [4]. The resulting heterodimers transactivate multiple genes containing hypoxia response elements (HREs) that encode for proteins involved in the delivery and use of O2 and nutrients as well as genes that mediate vascular responses to hypoxia [5].
Despite a high degree of sequence similarities between HIF-1α and HIF-2α proteins, they appear to have highly independent functions [6, 7]. First, both α-subunits activate common genetic targets but each also coordinate cell specific genetic transcriptional profiles [8–12]. Second, each HIF-α subunit can also be uniquely regulated [13–15]. Third, different HIF-α subunit genes are differentially transcribed in different cell types and while HIF-1α is broadly expressed in most cells, HIF-2α expression is more restricted to endothelial, hepatic, kidney intestinal, and lung epithelial cells [6]. Last, the exact contribution of each α-subunit appears to also differ between cell types[16–21].
EC-specific disruption of either HIF-1α or HIF-2α results in no apparent biological insult under a quiescent state [7, 22, 23]. However, each of these conditional knock-out mice displays deficient angiogenic responses suggesting independent requirements for each α-subunit. In concert, both may be necessary in establishing appropriate responses to hypoxia when the vasculature is disturbed. Similar deletion of endothelial-ARNT results in mostly embryonic lethality with impaired hepatic vasculature [24]. However, endothelial cells function and biology was not examined any further. Our efforts in this study were to use a genetic approach to delete ARNT and thereby entirely inactivate HIF-transcriptional responses to examine the cell intrinsic requirements of the HIF-canonical pathway in endothelial cells.
Methods
Cell culture
All the experiments that used animals were performed in accordance with approved animal protocols from the Case Western Reserve University Animal Care and Use Committee and National Institutes of Health guidelines. Mouse primary pulmonary and cardiac endothelial cells (ECs) were isolated from ArntloxP/loxP pups and cultured as previously described [25, 26]. Briefly, lungs and hearts were digested with collagenase, sorted for CD31 and then ICAM-1 (Pharmingen) using coated Goat anti-Rat magnetic beads (Invitrogen), and maintained in complete VascuLife medium (Lifeline Cell Technology) for one additional passage [25]. Experimental cells were infected with Adenovirus-Cre/GFP or Adenovirus-GFP (1.1 x1012 pfu/ml, Vector Development Lab). Where indicated, ArntloxP/loxP ECs were further immortalized with a retrovirus expressing the polyoma virus middle T antigen [27]. Surviving cells were then infected with Adenovirus-Cre/GFP or Adenovirus-GFP and FACS sorted to create ArntloxP/loxP EC4 control and ArntΔ/Δ (Arnt-null) EC4 lines. Culture experiments were done at 37°C with 5% CO2 under normoxic (21% O2) or physiological hypoxic (2.5% O2) conditions.
Aortic ring angiogenesis assay
Ex vivo aortic ring angiogenesis assays were carried out as previously described with the following condition modifications [28]. Thoracic aortas were isolated from 8 week-old ArntloxP/loxP mice, sectioned into 1mm rings, and infected overnight with Adenovirus-Cre/GFP or Adenovirus-GFP. Rings were cultured in rat tail interstitial collagen gel (1.25 mg/ml collagen, 1x MEM, and NaHCO3 (15.6mg/ml) to pH 7.4) polymerized at 37°C. Assay conditions included 100 µl of MCDB131 (Invitrogen) media supplemented with human recombinant VEGF-A (20 ng/ml), 1% glutamine, and 100 U/ml penicillin/streptomycin, and cultures maintained at 37°C in a humidified incubator under normoxia (21% O2) or physiological hypoxia (2.5% O2). EC sprouting and neovessel formation was assessed at day 10 using an inverted microscope platform with bright-field optics (Leica DMI6000B). Vessel numbers and branch numbers were quantified within corresponding regions of interest and using image J software (n=10). Neovessels were stained with CD31 (1:50, BD Pharmingen) and Rhodamine labeled anti-Rat IgG (1:200, Jackson Immuno. Res.)
Cell proliferation
Cell proliferation of mouse primary lung and heart endothelial cells was measured by MTT assay and Bromodeoxyuridine (BrdU) incorporation at 0, 24, 48 and 72 hours according to manufacturer’s instruction (Chemicon). Briefly, isolated primary ECs were removed from complete VascuLife medium, plated (2×104/ml) and cultured in 2% FBS DMEM (Sigma) for 48 or 72 hours under normoxia (21% O2) or physiological hypoxia (8% or 2.5% O2) conditions. BrdU incorporation and MTT reduction was detected every 24 hours. Data are shown is the mean±SEM of triplicates representative of three independent experiments with similar results.
Cell apoptosis
Isolated primary lung ECs were removed from complete VascuLife medium and cultured in 0.5% FBS DMEM (Sigma) for 12 hours and apoptosis was first assessed by examination of Lyso Tracker Red (LTR) accumulation [29]. Primary ECs were washed with PBS and incubated in diluted 2.5 µM LTR (Molecular Probes, Eugene, OR) solution for 15 minutes at room temperature, then washed, and LTR+ cells were quantified with an inverted fluorescent microscope (Leica DMI6000B). Further apoptotic analysis was carried out on fixed primary ECs by TUNEL staining using the manufacturer’s instruction (Roche). Briefly, primary ECs were cultured as described above and incubated in 0.5% FBS DMEM (Sigma) for 12 hours under normoxia (21% O2) or physiological hypoxia (2.5% O2). Then cells were fixed with 4% paraformaldehyde and incubated with TUNEL reaction mixture for 1 hour at 37°C. Positive apoptotic cells staining brown following treatment with DAB were counted from triplicates.
Caspase3 staining
Isolated primary lung and heart ECs were removed from complete VascuLife medium and cultured in 0.5% FBS DMEM (Sigma) for 24 hours under normoxia (21% O2), hypoxia (8% or 2.5% O2), or treated with 300uM H2O2 for 2 hours. Then cells were fixed with 4% paraformaldehyde and incubated with cleaved caspase3 antibody (Cell Signaling) overnight at 4°C. Positive apoptotic cells staining brown following treatment with DAB were counted from triplicates.
In vitro endothelial cell wound healing assay
Isolated mouse primary lung ECS were plated at 1 × 104 cells/well in six-well-plate and allowed to adhere and become confluent monolayers after overnight culturing in complete VascuLife media. A wound line was created with a 200-µl standard pipette tip, washed to removed cell debris, and incubated in 2% FBS DMEM under normoxia (21% O2) or physiological hypoxia (2.5% O2). Migration distance was quantified from phase contrast images taken at 0 and 16 hours (n=10). ECs and distance travel were analyzed using NIH Image J software. Data are shown by a mean of triplicates from one of three independent experiments with similar results.
Cell migration
Migration (chemotaxis) assays were performed using 8 µm pore transwell plates (Costar) coated with fibronectin (2.5 µg/cm2). Following 18 h of serum starvation, Control and Arnt-null EC4 cells were plated (4x105 /ml) in triplicate and permitted to migrate for 6 hours under normoxia (21% O2) or physiological hypoxia (2.5% O2) in the presence of 15 ng/ml VEGF in migration medium (2% FBS DMEM) in the lower chamber compartment. The cells on the upper side were removed with a cotton tip before fixing the insert membrane with 4% paraformaldehyde and the remaining cells were stained with with 2.3% crystal violet (Sigma) for 5 min. Relative cell numbers were quantified by washing the filter with water, solubilizing the crystal violet with methanol, and measuring at an OD of 560. These experiments were repeated three times.
Matrigel tube formation
The tube formation assay was performed on growth factor-reduced Matrigel (Becton Dickinson, Bedford, Massachusetts). 96-well plates were coated with 100 µl of Matrigel (10 mg/ml) and incubated at 37°C for 30 min to promote solidification. EC4 control and EC4 Arnt-null cells were seeded on coated plates at 5000 cells/well in 10% FBS VascuLife medium and incubated at 37°C for 24 hours under normoxia (21% O2) or moderate hypoxia (2.5% O2). Tube formation was examined using phase contrast microscopy and photographed. A connecting branch of consistent thickness between two ECs was counted as one tube. The degree of tube formation was quantified by measuring the branches of tubes in 5 random fields from each of 3 wells.
RT-PCR analysis
Total RNA was isolated from ECs using Trizol reagent (Invitrogen) and reverse transcribed into cDNA with random hexamers and Superscript Reverse Transcriptase (Invitrogen). Gene expression of the target sequence were analyzed by real-time quantitative RT-PCR using the TaqMan system (Roche, designed in Universal Probe Library Assay Design Center from Roche Applied Science) or Syber Green (Roche) and normalized in relation to the expression of an endogenous control, 18S ribosomal RNA (rRNA). Oligonucleotide pairs:
TaqMan: forward primer 5’-AAATCAGTTATGGTTCCTTTGGTC-3’ and reverse primer 5’-GCTCTAGAATTACCACAGTTATCCAA-3’ for 18S; forward primer 5’-GCAGCTTGAGTTAAACGAACG-3’ and reverse primer 5’-GGTTCCCGAAACCCTGAG-3’ for VEGF; forward primer 5’-CAGTGGTACTGGCAGCTAGAAG-3’ and reverse primer 5’-ACAAGCATACGGGCTTGTTT-3’ for Flk-1; forward primer 5’-GGCCCGGGATATTTATAAGAAC-3’ and reverse primer 5’-CCATCCATTTTAGGGGAAGTC-3’ for Flt-1; forward primer 5’-TTCGCAGTTCCGAAAGAAGT-3’ and reverse primer 5’-GCTCTAGAATTACCACAGTTATCCAA-3’ for ADM; forward primer 5’- GGTCCTCACGCAGAGTTCC -3’ and reverse primer 5’-TCACCAAGCCATTGTACCG-3’ for Adora2a; forward primer 5’-TACCTGCTGGCTGGATGG-3’ and reverse primer 5’-CACAGCCTCGGCATATTTCT-3’ for PGK.
Syber Green were: forward primer 5’-GCCTGATGCTCTCACTCTGCT-3’ and reverse primer 5’-CAGAGAGATGATGGTGTCGCC-3’ for Hif-1α; forward primer 5’-GCTACTTGGACGCTCTGCCTA-3’ and reverse primer 5’-TTCTCCGAATCCAGGGCAT-3’ for Hif-2α.
Statistical Analysis
Statistics were performed by Student t test and all data is expressed as the mean±SEM.
Results
Loss of ARNT inhibits hypoxia enhanced vessel outgrowth and branching in aortic explants
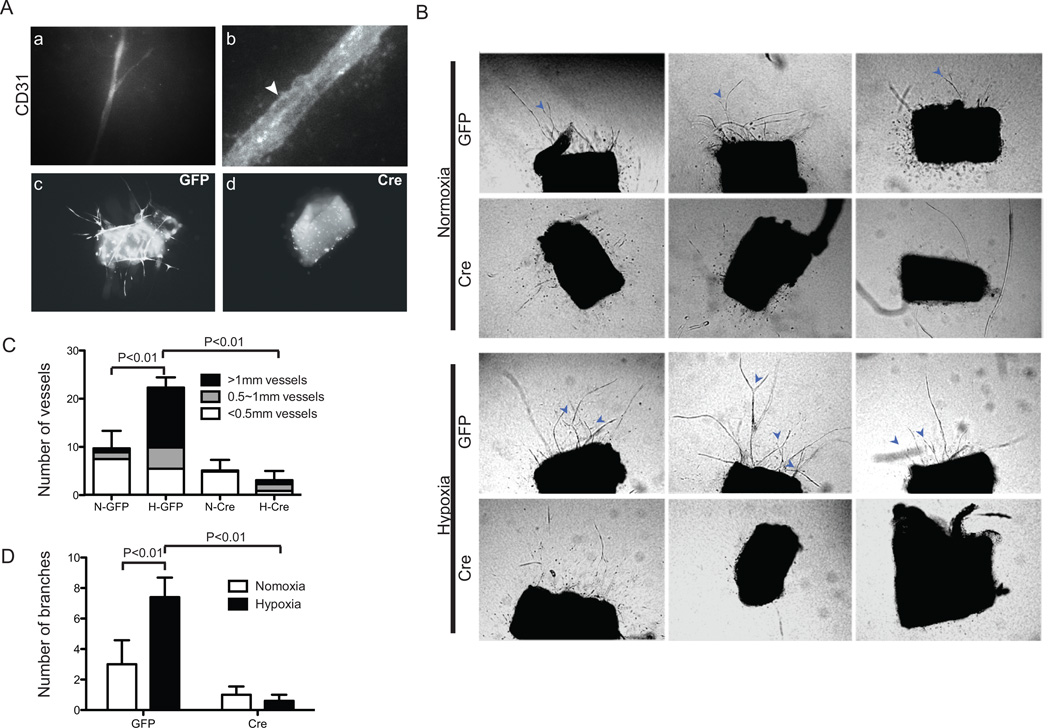
Angiogenesis is defined by various vessel-remodeling parameters that include the growth and sprouting of endothelial cells [30]. To carefully investigate the effects of hypoxia in sprouting angiogenesis, we utilized an ex vivo three-dimensional aortic ring assay in which ring fragments from descending aortas of ArntloxP/loxP adult mice were cultured for 10 days under normoxic (21% O2) or moderate hypoxic (2.5% O2) conditions in a defined microenvironment of rat type I collagen supplemented with 20 ng/ml of recombinant VEGF. In control explants exposed to hypoxic conditions, endothelial tube staining for CD31 was observed as early as day 5 (Fig. 1A a and b). Further hypoxia treatment for 10 days statistically enhanced the number of sprouts from control aortic ring explants of which ~50% of sprouts were longer than 1mm (Fig. 1B and C). Moreover, the numbers of branching vessels doubled in control rings exposed to hypoxia (Fig. 1B and D). These findings indicate that hypoxia greatly enhances microvessel outgrowth, sprouting, and branching in this ex vivo angiogenic explant assay.
Fig. 1.
Loss of ARNT inhibits hypoxia enhanced vessel outgrowth and branching in aortic-ring explants. (A) Magnification of vessel outgrowth from rings stained for Rhodamine-CD31 (a, b). Note the vacuolated vessels in b (Arrowhead). Fluorescent micrographs of rings dissected from aortas of ArntloxP/loxP adult mice were infected with Ade -GFP (c) or -Cre/GFP (d). (B) Phase-contrast micrographs of representative rings infected with Ade -GFP or -Cre/GFP after culturing for 10 days under normoxic or hypoxic (2.5% O2) conditions. Arrows indicate vessel branches. Quantification of vascular outgrowth assessed by the number of vessels, length of vessels (C) and branches (D). Data shown are the mean ± SEM, n=10 samples. Statistical analysis was performed using an unpaired Student’s t-test.
To examine the cell intrinsic requirements of endothelial-HIF for sprouting and growth of vessels, the delivery of Adenovirus–GFP/Cre recombinase enzyme to intact vascular explants from ArntloxP/loxP adult mice permitted inactivation of ARNT, and therefore canonical HIF transcriptional activity (Fig. 1A c and d). In contrast to control Adeno-GFP infected cultures, we observed minimal sprouting in Cre-recombinase infected aortic rings and lengths of the vessels were significantly reduced (Fig. 1B and C). Furthermore, we failed to see any induction by hypoxia treatment of vessel numbers, lengths, or branches in these mutant cultures (Fig. 1B–D and Supplemental Fig. 1). Thus, the absence of ARNT in aortas results in an impaired angiogenic response.
Arnt-null endothelial cells fail to upregulate known HIF gene targets in response to hypoxia
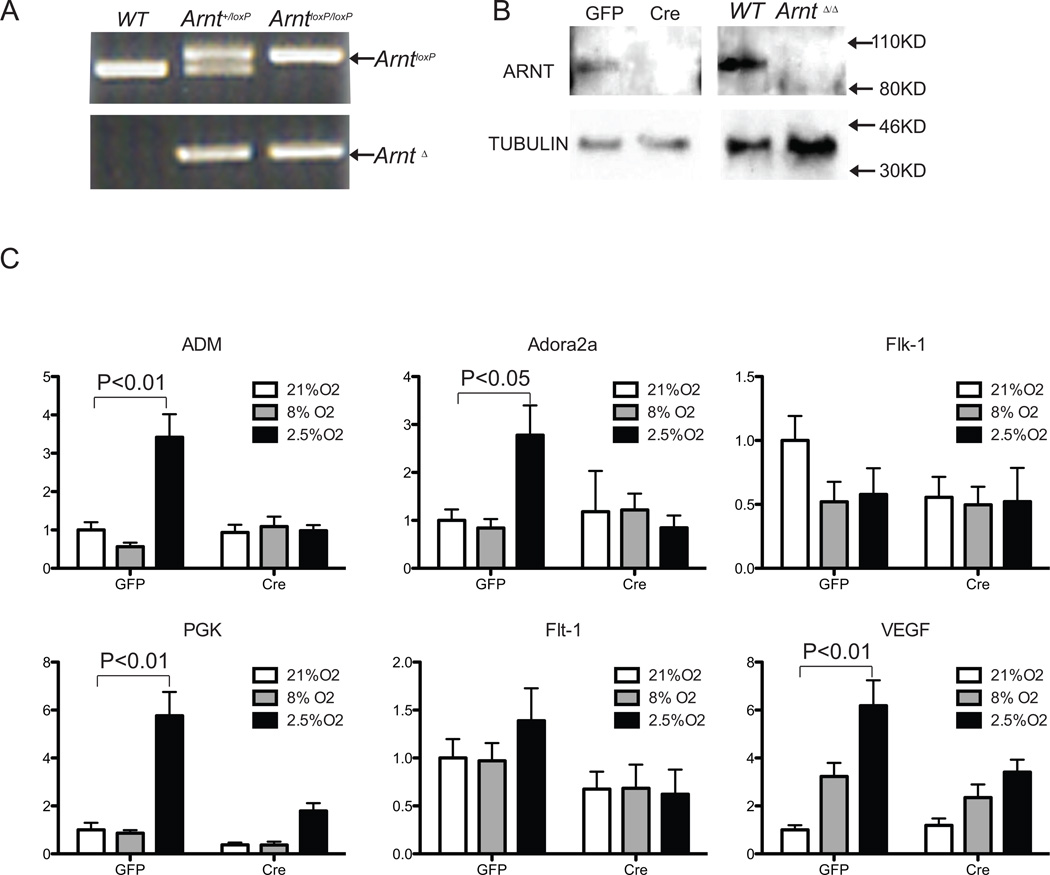
To investigate the cell intrinsic requirements of HIF in endothelial cells, CD31+ICAM1+ lung ECs isolated from ArntloxP/loxP neonates were immortalized with a polyoma middle T antigen (EC4 Cells) [27]. Cells were infected with Adenovirus -GFP or -Cre/GFP and FACS sorted to generate ArntloxP/loxP and Arnt-null cell lines. ARNT deletion was examined by genomic-PCR amplification and its absence of ARNT protein expression confirmed by Western-blot (Fig. 2A and B). These data indicated efficient abolishment of ARNT expression in vitro.
Fig. 2.
Arnt-null endothelial cells fail to upregulate HIF target genes in response to hypoxia. Generation of Arnt-null endothelial cells (A and B). (A) PCR amplification of genomic DNA detecting ARNT deleted band. (B) Western blot analysis of ARNT protein from endothelial cells (EC4) cells derived from CD31+ICAM+ ECs isolated from lungs of ArntloxP/loxP mice which were then immortalized, infected with Adenovirus -GFP and -Cre/GFP, and FACS sorted. Proteins isolated from WT and Arnt-null (ArntΔ/Δ) embryonic stem cells were used as controls. (C) Real-time PCR analysis of HIF-target gene expression in control or Arnt-null EC4s and exposed to normoxic (21% O2) or hypoxic (8% or 2.5% O2) conditions for 16 hours. Relative transcript levels were all normalized to 18S RNA and compared to Ade-GFP normoxic EC4 cultures. Phosphoglycerate kinase 1 (PGK) is considered HIF-1α specific target; adenosine A2A receptor (Adora2a) and VEGF receptor -2 and -1 (Flk-1, and Flt-1) are considered HIF-2α specific targets; adrenomedullin (ADM) and vascular endothelial growth factor (VEGF), are regulated by both α subunits.
In response to hypoxia, HIF-1α overexpression, or expression of a stable form of HIF-1α, endothelial cells are known to upregulate numerous transcripts [8, 9]. To determine the molecular consequences to the loss of ARNT, we screened for the expression of targets considered to be unique targets to each of the α-subunits. ArntloxP/loxP and Arnt-null EC4 cells. Cells were cultured in normoxic (21% O2) or physiological levels of 8% O2 for lung ECs or moderate 2.5% O2 conditions for 16 hours and total RNA was isolated, reversed transcribed and the expression of various HIF targets was quantitatively analyzed by RTQ-PCR [31]. Under either condition, we observed no difference in the expression of Hif-1α or Hif-2α transcripts in either control or Arnt-null EC4 cells (Supplemental Fig. 2A). While 8% O2 treatment upregulated only VEGF transcript levels in EC4 cells, 2.5% O2 treatment promoted the expression HIF-1α- (PGK, ADM), HIF-2α- (Flt-1, Adora2a), or common- (Vegf) responsive gene targets. Instead, exclusive to minimal induction of VEGF transcript levels (P<0.05), loss of ARNT was associated with a failure to induce these targets in response to either 8 or 2.5% O2 (Figure 2C). In a closer examination of the VEGF pathway in primary lung ECs, only VEGF levels were induced by hypoxia in control cells albeit neither receptor genes were induced (Figure S2B). Our results indicate that ARNT deletion in endothelial cells dampens the overall transcriptional responses to physiological hypoxia, indicative of ARNT-dependent endothelial cell functions.
Loss of ARNT disrupts endothelial cell functions
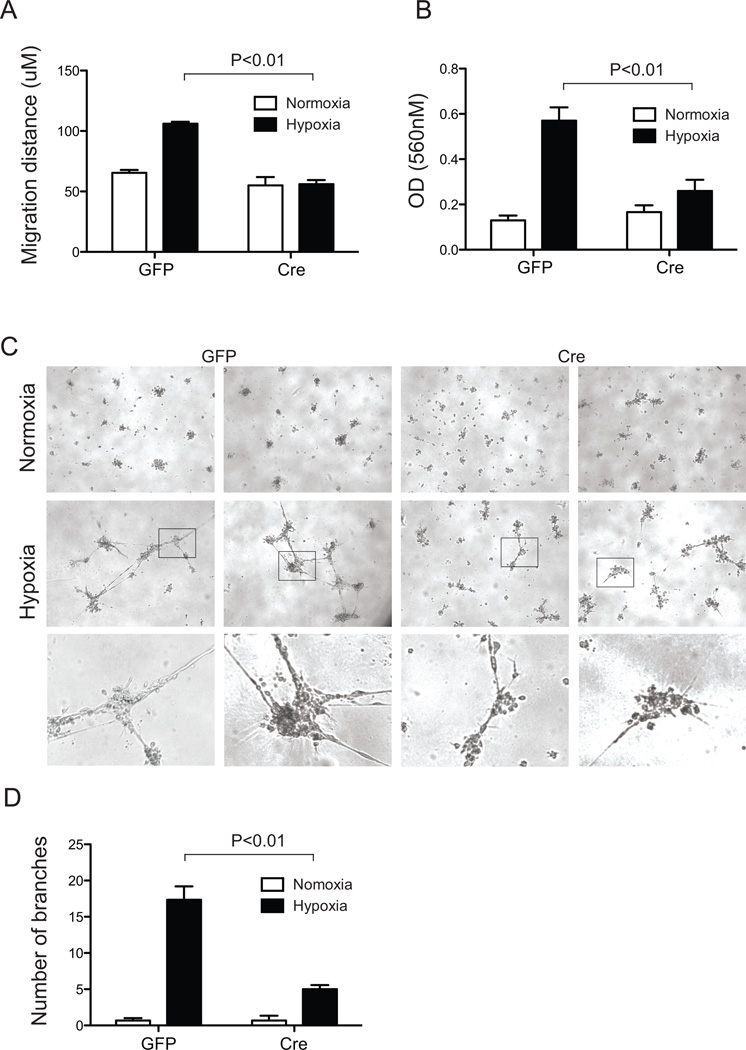
The failure to observe significant neovessel growth from Ade-Cre/GFP infected aortic rings suggested that ARNT could normally promote the maintenance, proliferation, survival and/or migration of ECs. To test the intrinsic requirements of ARNT in ECs, we first examined cell migration using an in vitro scratch wound assay. After16 hours, no significant difference between the distances migrated by Adeno-GFP and -Cre infected primary ArntloxP/loxP EC cultures were recorded for normoxic conditions (Fig. 3A, S3). While the migration of control ECs doubled in hypoxic treatment (2.5% O2), primary Adeno-Cre infected ECs failed to respond to the same hypoxic exposure (Fig. 3A).
Fig. 3.
Loss of ARNT disrupts endothelial cell migration and tube formation. (A) Migration was detected in a 2-dimensional scratch wound assay of primary CD31+ICAM-2+ lung endothelial cells isolated from ArntloxP/loxP mice after 48 hours of infection with Adenovirus -GFP or -Cre/GFP. Migration was measured after 16 hours of normoxic and hypoxic (2.5% O2) treatment. Data are mean±SEM, n=3. (B) Cell migration capacity of immortalized EC4s examined in response to 15 ng/ml of VEGF using Boyden chamber cultures. The relative cell numbers remaining were quantified by measuring solubilized crystal violet uptake by cells remaining after removal of cells from upper side of chamber. Data are expressed as migrated cells and correspond to mean±SEM of 3 experiments performed in duplicate. (C) Representative pictures of control ArntloxP/loxP EC4 and Arnt-null EC4 cells plated on Matrigel for 24 hours. Panels: Upper, normoxic cultures; Middle, hypoxic cultures; Lower, higher magnification of the hypoxia panels. (D) Quantitative data of tube branch formation after 24 hours.
Next, since the survival or proliferation of Ade-Cre infected ArntloxP/loxP ECs may contribute to the observed decrease in vascular sprouts in aortic rings or migration of primary EC, we made use of our immortalized EC4 lines to elucidate further angiogenic deficiencies. In Transwell migration assays, control and Arnt-null EC4 cells display indistinguishable responses to VEGF (Fig. 3B). Unlike control EC4 cells, however, mobilization of Arnt-null ECs did not increase in response to hypoxia (Fig. 3B).
Finally, we examined the requirements of ARNT on the ability of ECs to form capillary tubes, another important angiogenic process, in 2-D Matrigel cultures (Fig. 3C). The tube-like structures and vascular networks were significantly more extensive in control cultures exposed to hypoxia compared to time-matched normoxia conditions (Fig. 3C). In contrast, while Arnt-null EC4 cells formed few tube structures in response to hypoxia, branch networks were consistently reduced (17.3±1.9 versus 5.0±0.6, Fig. 3C and D).
Loss of ARNT results in reduced survival and proliferation in primary endothelial cells
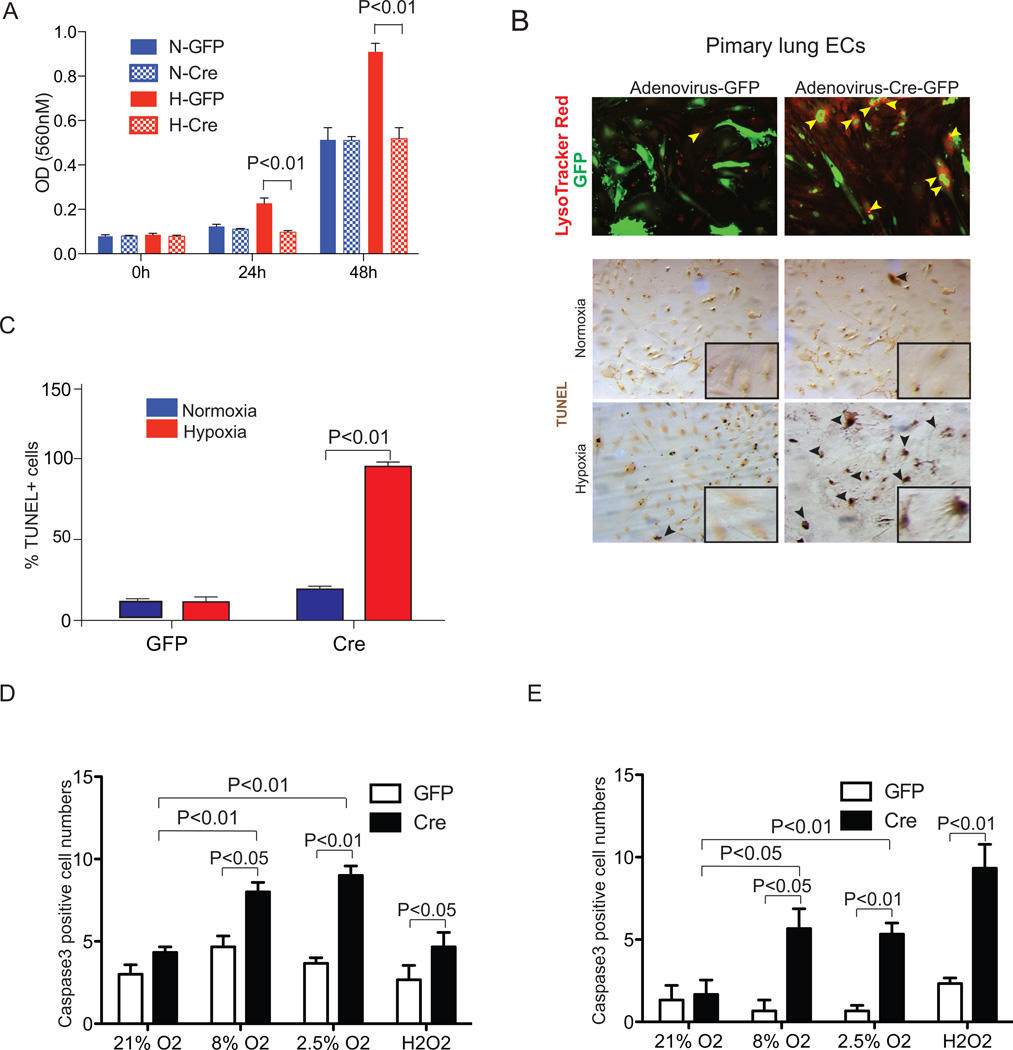
Since we failed to see proper vessel sprouting or cell migration from either Ade-Cre infected ex vivo aortic explants or in vitro EC cultures, we hypothesized that loss of ARNT, and therefore HIF transcriptional activity, affects the maintenance and proliferation of endothelial cells. To test the intrinsic requirements of ARNT in ECs, we examined cell proliferation and viability in primary CD31+ICAM-1+ lung or heart ECs. Under normoxic conditions, the proliferation of Adenovirus-GFP or Adenovirus-Cre/GFP infected ArntloxP/loxP ECs were indistinguishable (blue bars, Fig. 4A, S4A). Using LysoTracker- red uptake to label apoptotic cells, we observed an increased number of LTR+ Adenovirus-Cre/GFP+ cells relative to control Adenovirus-GFP+ ArntloxpP/loxP primary lung ECs (37% and 1%, respectively) in conditions of reduced serum (0.5% FBS, Fig. S4B). Therefore, while under normoxic conditions loss of ARNT does not affect the proliferation of primary endothelial cells, it alters their viability.
Figure 4.
Arnt-null endothelial cells display proliferation and survival defects. ArntloxP/loxP primary lung and heart endothelial cells (ECs) were infected with Adenovirus -GFP or -Cre/GFP. (A) BrdU incorporation to assess lung EC proliferation under normoxic or hypoxic (2.5% O2) conditions. (B) Upper panels, Lysotracker Red (LTR) staining detecting cellular death in primary lung ECs. Arrows, LTR+/Cre+ cells. Middle/lower panels, TUNEL staining (brown) and quantification (C) of primary lung ECs cultures 24 hours following serum withdrawal in 2.5% or 21% O2 conditions. High magnification images are shown in the bottom right corner of each panel. (D,E) Quantification of Caspase-3 from primary lung (D) and heart (E) endothelial cells in response to 21, 8, or 2.5% O2for 24 hours or 300uM H2O2 for 2 hours.
Next, we examined how moderate hypoxia affects the maintenance of ECs. In these experiments, we included environmental O2 levels for each lung and cardiac ECs of 8 and 2.5%, respectively [31, 32]. When exposed to 2.5% O2there was an overall increased rate of proliferation in control lung ECs over normoxic conditions in BrDU incorporation experiments (Fig. 4A). Further proliferation experiments show that, compared to normoxia, the proliferation rate of control lung ECs to 8% O2 was higher exclusively at 24 hours (1.4X, P<0.05) but significantly induced at throughout 72 hours of 2.5% O2 exposure (1.6X, 1.4X and 1.6X at 24, 48, and 72 hours, respectively) (open bars, Fig. S4A). To verify the requirement of ARNT in ECs, we isolated cardiac ECs which are considered to reside in a lower gradients of O2 [32]. Cultures of control cardiac ECs also show that both 2.5 and 8% O2 induce their proliferation (Figure S4A). In contrast, either O2 treatment failed to induce the proliferation of Cre-recombinase infected lung or heart EC cultures over control 21% O2 conditions (striped bars, Figure S4A). Instead, we observed that Adenovirus-Cre infected ArntloxP/loxP primary lung EC cultures appear to die more rapidly as detected by the uptake of lysozomal dye (Figure 4B). TUNEL staining after 24 hours of 2.5% O2 and 0.5% serum treatment further confirmed their refraction to survival as a consequence of Arnt deletion (~90 % of death, Figure 4C). Similar results were obtained from cardiac ECs whereby Caspase-3 proteolytic activity from Cre-infected primary ECs increased 1.85- and 2.1-fold in pulmonary and 3.5- and 3.3-fold in cardiac derived cells relative to control Ade-GFP injected cells following either 8% or 2.5% O2 treatment, respectively (Figure 4D, E and S4B).
Since oxidative stress, defined as excess production of reactive oxygen species (ROS), often leads to EC damage we examined whether Cre-infects primary ECs were susceptible to apoptosis in response to high concentration of hydrogen peroxide (H2O2) [33]. Casapase-3 levels were higher in both Adenovirus-Cre infected ArntloxP/loxP primary lung and heart ECs over control cultures (P < 0.05 and 0.01, respectively, Figure 4D,E and S4B). These results demonstrate that loss of ARNT significantly hinders endothelial cell proliferation and viability, particularly in response to hypoxia. Indeed, we unsuccessfully maintained Arnt-null primary endothelial cell cultures over two passage numbers.
Discussion
The biological significance of the canonical-HIF pathway is supported by the in vivo requirements requirements of ARNT. Arnt-null mice are embryonic lethal with severe neural, cardiac, hematopoietic, placental, and vascular phenotypes and tissue specific deletion of Arnt in mice that include epidermis, β cells, adipocytes, T-cells, and liver result in compromised phenotypes [34–41], Mice with EC-specific deletion of Hif-1a or Hif-2a survive into adulthood without gross vascular abnormalities, but endothelial cell expression of a dominant-negative transgene for either HIF-canonical transcription or loss of endothelial-ARNT resulted in embryonic or postnatal lethality, respectively [22–24, 42]. Ultimately, these genetic studies strongly suggests that Hif-1a and Hif-2a have important nonredundant roles in endothelial cells and are concomitantly required for the maintenance of endothelial cells. As such in this study we examined, at the cellular level, the biological consequences from inactivating all HIF transcriptional activity in endothelial cells by deleting ARNT and describe pronounced proliferation, survival, and angiogenic defects.
We had previously identified the requirements of ARNT during vasculogenic and angiogenic phases of vessel differentiation and maturation in embryonic stem cell differentiation experiments noting that hypoxia promoted vessel growth and sprouting [43]. Our present manuscript further demonstrates that hypoxic treatment promotes vessel outgrowth and branching in aortic ring explants and Arnt-null aortic rings fail to generate any vessels supporting our model that a heightened deregulation of the HIF-canonical pathway affects endothelial sprouting. Importantly, we show that ARNT inactivation significantly suppresses EC capacity to form tubes or to migrate in response to VEGF, inflecting the requirement for the heterodimerization of HIF in this process. Previous experiments have shown that in vitro exposure of ECs to hypoxia or to a constitutively active form of HIF-1α induce tube formation, which is otherwise abrogated by loss of HIF-1α expression [8, 9, 22]. In vitro over expression of HIF-1α or HIF-2α have also indicated additive effects on the adhesion, migration, and tube formation in ECs, perhaps due to unique as well as synergistic transcriptional effects between HIF-1α and HIF-2α on multiple angiogenic growth factors [44]. Data suggests that HIF-1α may promote cellular sprouting by promoting cell proliferation, migration, and tube formation, whereas HIF-2α may be involved in promoting differentiation of endothelial cells and in guiding their maintenance thereby promoting vessel quiescence, presumably in a dose-dependent manner [23, 44].
At the organismal level, dynamic changes in response to hypoxia include the overall production of VEGF to promote vessel growth, for example. In adult mice, at baseline conditions of normoxia HIF-1α protein is present in various tissues while HIF-2α is absent. However, systemic exposure to hypoxia results in increased levels of both subunits in multiple organs, albeit with differences in amount, timing, and duration [21, 45]. In addition, HIF subunits may also play opposing effects in mice in response to intermittent hypoxia (IH), in which tissues incur cycling between normoxic and hypoxic O2 levels [46]. Thus, each subunit may have unique response to levels and duration of hypoxia. At the cellular level, previous studies have suggested that HIF-2α is critical for prolonged hypoxia. In a human epithelial cell line HIF-1α and HIF-2α proteins can be induced by 4 hours in acute hypoxia (0.5% O2,) but by 12 hours HIF-1α transcription is turned off while HIF-2α expression remains stable [47]. In endothelial cells, levels of hypoxia have distinct effects whereby 5% O2 promotes their survival and proliferation but more severe hypoxia may cause increased apoptosis (reviewed in [48]). It is also important to consider that while VEGF can upregulate HIF-1α, it was recently shown to decrease the expression of HIF-2α in endothelial cells [49, 50]. Furthermore, opposing effects for these subunits have been reported in monocytes and prostate tumor cells [51, 52].
The role of HIF in EC cell maintenance remains elusive. Our in vitro data shows that ARNT is critical for the proliferation and survival of endothelial cells and that loss of HIF-transcriptional activity is detrimental to EC viability. Indeed, under physiological levels of hypoxia, while the numbers of proliferating primary Arnt-null cells decrease, apoptotic numbers increase suggesting that ARNT is not only critical for circumstances requiring EC activation, but is particularly important for the preservation of a proper vasculature. In VHL loss in renal cell carcinomas HIF subunits are reported to have distinct effects on c-Myc proto-oncogene whereby HIF-1α antagonizes c-Myc’s function restricting cell cycle progression, and HIF-2α promotes c-Myc signaling supporting cell proliferation and tumor growth [53, 54]. Specific to endothelial cells, this differential effect on c-Myc expression by either HIF subunit consequently affects the expression of IL-8 [55]. However, in our control or Arnt-null EC4 cells, no differences were observed for c-Myc expression for either 21% or 2.5% treatment (data not shown) suggesting that other pathways are involved. We recognize that ARNT could also be regulated by other pathways as, for example, has been recently shown that ARNT mRNA and protein expression directly regulated by NF-κB [56]. Moreover, ARNT is also known to dimerize with aryl hydrocarbon receptor (AHR), essential for adaptive xenobiotic metabolism. Indeed AHR inactivation in endothelial cells led to reduced basal endothelin-1 mRNA but did not alter hypoxia-induced expression [57]. Still, we consider that the coregulation of and by HIF-1 and HIF-2 involving ARNT is necessary to refine the adaptation of endothelial cells to physiological hypoxia.
In response to physiological hypoxia, we failed to see induction of HIF target genes (including Vegf and those unique to either HIF-1α or HIF-2α) that are important for angiogenic processes in Arnt-null endothelial cells. Interestingly, under identical conditions, we observe hypoxic induction of Flt-1 but repressed Flk-1 transcript levels in control EC4 cells suggesting a potential feedback downregulation of VEGF signaling [58]. This suggests that an alternate non-HIF canonical pathway may be involved, including potentially unique endothelial cell cooperation between HIF-2α/Ets-1 transcription factors for the regulation of Flk-1 whereby Ets-1 is downregulated by the hypoxic induction of miR-200b [59, 60]. Alternatively, we propose that the down regulation of Flk-1 is dependent on HIF activity in activated endothelial cells, albeit indirectly.
Importantly, our study demonstrates that intrinsic ARNT in endothelial cells is necessary for their cellular adaptation to moderate levels of hypoxia normally found within their microenvironment in order to activate the expression of angiogenic factors and other proteins important for their proliferation, survival, and migration [61]. HIF regulation is vital in various human vascular pathologies including cancer, preeclampsia, peripheral arterial disease, pulmonary hypertension, and myocardial, retinal, and cerebral ischemia [62]. While inhibition of angiogenesis is a promising strategy for treatment of cancer and other disorders, these results warrant the use of anti-HIF therapies that may hinder vessel integrity and maintenance of the vasculature as warranted by the discovery of increasing number of differences existing between these two HIF-subunits and the severity associated with loss of ARNT in endothelial cells.
Supplementary Material
Acknowledgements
We thank Alla Gomer for her technical assistance. This work was supported by the National Heart, Lung, and Blood Institute R01-HL096597 (D.R.B) and R01-HL096603 (A. P.).
Footnotes
NOTE: Original publication is available at link.springer.com
Literature Cited
- 1.Lonergan KM, Iliopoulos O, Ohh M, Kamura T, Conaway RC, Conaway JW, Kaelin WG., Jr Regulation of hypoxia-inducible mrnas by the von hippel-lindau tumor suppressor protein requires binding to complexes containing elongins b/c and cul2. Mol Cell Biol. 1998;18(2):732–741. doi: 10.1128/mcb.18.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH. Hypoxia inducible factor-alpha binding and ubiquitylation by the von hippel-lindau tumor suppressor protein. J Biol Chem. 2000;275(33):25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JM, Gleadle AC, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. Elegans egl-9 and mammalian homologs define a family of dioxygenases that regulate hif by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang BH, Jiang GL, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-pas heterodimer regulated by cellular o2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, Simon MC. Hif-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev Cell. 2006;11(1):81–92. doi: 10.1016/j.devcel.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15(4):628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sowter RR, Raval HM, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (hif)-1alpha versus hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63(19):6130–6134. [PubMed] [Google Scholar]
- 8.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by hif-1. Blood. 2005;105(2):659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 9.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93(7):664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 10.Nagao K, Oka K. Hif-2 directly activates cd82 gene expression in endothelial cells. Biochem Biophys Res Commun. 2011;407(1):260–265. doi: 10.1016/j.bbrc.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (hif-1alpha) and hif-2alpha in stem cells. Mol Cell Biol. 2006;26(9):3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordan JD, Lal P, Dondeti VR, Letrero R, Parekh KN, Oquendo CE, Greenberg RA, Flaherty KT, Rathmell WK, Keith B, Simon MC, Nathanson KL. Hif-alpha effects on c-myc distinguish two subtypes of sporadic vhl-deficient clear cell renal carcinoma. Cancer Cell. 2008;14(6):435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park AM, Dadak SK, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1alpha (hif-1alpha): Role of cytoplasmic trapping of hif-2alpha. Mol Cell Biol. 2003;23(14):4959–4971. doi: 10.1128/MCB.23.14.4959-4971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmquist L, Jogi A, Pahlman S. Phenotypic persistence after reoxygenation of hypoxic neuroblastoma cells. Int J Cancer. 2005;116(2):218–225. doi: 10.1002/ijc.21024. [DOI] [PubMed] [Google Scholar]
- 15.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324(5932):1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 16.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bhlh-pas factor with close sequence similarity to hypoxia- inducible factor 1alpha regulates the vegf expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94(9):4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flamme I, Frohlich T, von Reutern M, Kappel A, Damert A, Risau W. Hrf, a putative basic helix-loop-helix-pas-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech Dev. 1997;63(1):51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- 18.Tian H, McKnight SL, Russell DW. Endothelial pas domain protein 1 (epas1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11(1):72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 19.Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc Res. 2003;60(3):569–579. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Wiesener M, Turley H, Allen W, William C, Eckardt K, Talks K, Wood S, Gatter K, Harris A, Pugh C, Ratcliffe P, Maxwell P. Induction of endothelial pas domain protein-1 by hypoxia: Characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92(7):2260–2268. [PubMed] [Google Scholar]
- 21.Wiesener JS, Jurgensen MS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of hif-2alpha in distinct cell populations of different organs. FASEB J. 2003;17(2):271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 22.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of hif-1alpha in endothelial cells disrupts a hypoxia-driven vegf autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (hif-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114(2):469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yim SH, Shah Y, Tomita S, Morris HD, Gavrilova O, Lambert G, Ward JM, Gonzalez FJ. Disruption of the arnt gene in endothelial cells causes hepatic vascular defects and partial embryonic lethality in mice. Hepatology. 2006;44(3):550–560. doi: 10.1002/hep.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobczak M, Dargatz J, Chrzanowska-Wodnicka M. Isolation and culture of pulmonary endothelial cells from neonatal mice. J Vis Exp. 2010;46 doi: 10.3791/2316. 2010/12/24:[Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21178973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha. Mol Endocrinol. 2000;14(10):1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the ve-cadherin gene in mice impairs vegf-mediated endothelial survival and angiogenesis. Cell. 1999;98(2):147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 28.Yang K, Proweller A. Vascular smooth muscle notch signals regulate endothelial cell sensitivity to angiogenic stimulation. J Biol Chem. 2011;286(15):13741–13753. doi: 10.1074/jbc.M110.181842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan XM, Li W, Dalen H, Lotem J, Kama R, Sachs L, Brunk UT. Lysosomal destabilization in p53-induced apoptosis. Proc Natl Acad Sci U S A. 2002;99(9):6286–6291. doi: 10.1073/pnas.092135599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folkman J. What is the role of endothelial cells in angiogenesis? Lab Invest. 1984;51(6):601–604. [PubMed] [Google Scholar]
- 31.Hoffstein V, Duguid N, Zamel N, Rebuck AS. Estimation of changes in alveolar-arterial oxygen gradient induced by hypoxia. J Lab Clin Med. 1984;104(5):685–692. [PubMed] [Google Scholar]
- 32.Whalen WJ, Savoca J, Nair P. Oxygen tension measurements in carotid body of the cat. Am J Physiol. 1973;225(4):986–991. doi: 10.1152/ajplegacy.1973.225.4.986. [DOI] [PubMed] [Google Scholar]
- 33.Cai H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc Res. 2005;68(1):26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Lee KY, Gesta S, Boucher J, Wang XL, Kahn CR. The differential role of hif1beta/arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 14(4):491–503. doi: 10.1016/j.cmet.2011.08.006. (epub 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wondimu A, Weir L, Robertson D, Mezentsev A, Kalachikov S, Panteleyev AA. Loss of arnt (hif1beta) in mouse epidermis triggers dermal angiogenesis, blood vessel dilation and clotting defects. Lab Invest. 92(1):110–124. doi: 10.1038/labinvest.2011.134. (epub 2011) [DOI] [PubMed] [Google Scholar]
- 36.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 60(10):2484–2495. doi: 10.2337/db11-0174. (epub 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, Xu CX, Krager SL, Bottum KM, Liao DF, Tischkau SA. Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces ppar-alpha pathway activity in mice. Environ Health Perspect. 119(12):1739–1744. doi: 10.1289/ehp.1103593. (epub 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XL, Suzuki R, Lee K, Tran T, Gunton JE, Saha AK, Patti ME, Goldfine A, Ruderman NB, Gonzalez FJ, Kahn CR. Ablation of arnt/hif1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9(5):428–439. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng S, Mezentsev A, Kalachikov S, Raith K, Roop DR, Panteleyev AA. Targeted ablation of arnt in mouse epidermis results in profound defects in desquamation and epidermal barrier function. J Cell Sci. 2006;119(Pt 23):4901–4912. doi: 10.1242/jcs.03282. [DOI] [PubMed] [Google Scholar]
- 40.Gunton RN, Kulkarni JE, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, Kahn CR. Loss of arnt/hif1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Tomita S, Jiang HB, Ueno T, Takagi S, Tohi K, Maekawa S, Miyatake A, Furukawa A, Gonzalez FJ, Takeda J, Ichikawa Y, Takahama Y. T cell-specific disruption of arylhydrocarbon receptor nuclear translocator (arnt) gene causes resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced thymic involution. J Immunol. 2003;171(8):4113–4120. doi: 10.4049/jimmunol.171.8.4113. [DOI] [PubMed] [Google Scholar]
- 42.Licht AH, Muller-Holtkamp F, Flamme I, Breier G. Inhibition of hypoxia-inducible factor activity in endothelial cells disrupts embryonic cardiovascular development. Blood. 2006;107(2):584–590. doi: 10.1182/blood-2005-07-3033. [DOI] [PubMed] [Google Scholar]
- 43.Han Y, Kuang SZ, Gomer A, Ramirez-Bergeron DL. Hypoxia influences the vascular expansion and differentiation of embryonic stem cell cultures through the temporal expression of vascular endothelial growth factor receptors in an arnt-dependent manner. Stem Cells. 2010;28(4):799–809. doi: 10.1002/stem.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Shoshan J, Maysel-Auslender S, Luboshits G, Barshack I, Polak-Charcon S, Tzahor E, Keren G, George J. Hypoxia-inducible factor-1alpha and -2alpha additively promote endothelial vasculogenic properties. J Vasc Res. 2009;46(4):299–310. doi: 10.1159/000181546. [DOI] [PubMed] [Google Scholar]
- 45.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. Hif-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15(13):2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 46.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades hif-2alpha via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106(4):1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchida T, Rossignol F, Matthay MA, Mounier R, Couette S, Clottes E, Clerici C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (hif)-1alpha and hif-2alpha expression in lung epithelial cells: Implication of natural antisense hif-1alpha. J Biol Chem. 2004;279(15):14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 48.Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med (Berl) 2009;87(6):549–560. doi: 10.1007/s00109-009-0458-z. [DOI] [PubMed] [Google Scholar]
- 49.Rivera CG, Mellberg S, Claesson-Welsh L, Bader JS, Popel AS. Analysis of vegf--a regulated gene expression in endothelial cells to identify genes linked to angiogenesis. PLoS ONE. 2011;6(9):e24887. doi: 10.1371/journal.pone.0024887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deudero JJ, Caramelo C, Castellanos MC, Neria F, Fernandez-Sanchez R, Calabia O, Penate S, Gonzalez-Pacheco FR. Induction of hypoxia-inducible factor 1alpha gene expression by vascular endothelial growth factor. J Biol Chem. 2008;283(17):11435–11444. doi: 10.1074/jbc.M703875200. [DOI] [PubMed] [Google Scholar]
- 51.Chae MJ, Kang KS, Lee JH, Ryu BK, Lee MG, Her NG, Ha TK, Han J, Kim YK, Chi SG. Opposite functions of hif-alpha isoforms in vegf induction by tgf-beta1 under non-hypoxic conditions. Oncogene. 2010;30(10):1213–1228. doi: 10.1038/onc.2010.498. [DOI] [PubMed] [Google Scholar]
- 52.Eubank JM, Roda TD, Liu H, O'Neil T, Marsh CB. Opposing roles for hif-1alpha and hif-2alpha in the regulation of angiogenesis by mononuclear phagocytes. Blood. 2011;117(1):323–332. doi: 10.1182/blood-2010-01-261792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordan JA, Bertout JD, Hu CJ, Diehl JA, Simon MC. Hif-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11(4):335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz L, Chaux A, Albadine R, Hicks J, Kim JJ, De Marzo AM, Allaf ME, Carducci MA, Rodriguez R, Hammers HJ, Argani P, Reuter VE, Netto GJ. Immunoexpression status and prognostic value of mtor and hypoxia-induced pathway members in primary and metastatic clear cell renal cell carcinomas. Am J Surg Pathol. 35(10):1549–1556. doi: 10.1097/PAS.0b013e31822895e5. (epub 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Florczyk U, Czauderna S, Stachurska A, Tertil M, Nowak W, Kozakowska M, Poellinger L, Jozkowicz A, Loboda A, Dulak J. Opposite effects of hif-1alpha and hif-2alpha on the regulation of il-8 expression in endothelial cells. Free Radic Biol Med. 51(10):1882–1892. doi: 10.1016/j.freeradbiomed.2011.08.023. (epub 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Uden P, Kenneth NS, Webster R, Muller HA, Mudie S, Rocha S. Evolutionary conserved regulation of hif-1beta by nf-kappab. PLoS Genet. 2011;7(1):e1001285. doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lund LN, Agbor AK, Zhang N, Baker A, Zhao H, Fink GD, Kanagy NL, Walker MK. Loss of the aryl hydrocarbon receptor induces hypoxemia, endothelin-1, and systemic hypertension at modest altitude. Hypertension. 2008;51(3):803–809. doi: 10.1161/HYPERTENSIONAHA.107.100586. [DOI] [PubMed] [Google Scholar]
- 58.Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH. Chronic hypoxia attenuates vegf signaling and angiogenic responses by downregulation of kdr in human endothelial cells. Am J Physiol Cell Physiol. 2009;296(5):C1162–C1170. doi: 10.1152/ajpcell.00533.2008. [DOI] [PubMed] [Google Scholar]
- 59.Chan YC, Khanna S, Roy S, Sen CK. Mir-200b targets ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem. 2011;286(3):2047–2056. doi: 10.1074/jbc.M110.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2alpha (hif-2alpha) and ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (flk-1) J Biol Chem. 2003;278(9):7520–7530. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- 61.Hogenesch WK, Chan JB, Jackiw VH, Brown RC, Gu YZ, Pray-Grant M, Perdew GH, Bradfield CA. Characterization of a subset of the basic-helix-loop-helix-pas superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272(13):8581–8593. doi: 10.1074/jbc.272.13.8581. [DOI] [PubMed] [Google Scholar]
- 62.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.