Abstract
Workers at the Mayak nuclear facility in the Russian Federation offer a unique opportunity to evaluate health risks from exposure to inhaled plutonium. Risks of mortality from lung cancer, the most serious carcinogenic effect of plutonium, were evaluated in 14,621 Mayak workers who were hired in the period from 1948–1982, followed for at least 5 years, and either monitored for plutonium or never worked with plutonium. Over the follow-up period from 1953–2008, there were 486 deaths from lung cancer, 446 of them in men. In analyses that were adjusted for external radiation dose and smoking, the plutonium excess relative risk (ERR) per Gy declined with attained age and was higher for females than for males. The ERR per Gy for males at age 60 was 7.4 (95% CI: 5.0–11) while that for females was 24 (95% CI: 11–56). When analyses were restricted to plutonium doses <0.2 Gy, the ERR per Gy for males at age 60 was similar: 7.0 (95% CI: 2.5–13). Of the 486 lung cancer deaths, 105 (22%) were attributed to plutonium exposure and 29 (6%) to external exposure. Analyses of the 12,708 workers with information on smoking indicated that the relationship of plutonium exposure and smoking was likely sub-multiplicative (P = 0.011) and strongly indicated that it was super-additive (P < 0.001). Although extensive efforts have been made to improve plutonium dose estimates in this cohort, they are nevertheless subject to large uncertainties. Large bioassay measurement errors alone are likely to have resulted in serious underestimation of risks, whereas other sources of uncertainty may have biased results in ways that are difficult to predict.
INTRODUCTION
In 1948 the Mayak nuclear facility, which is located in the town of Ozyorsk in the Chelyabinsk region of the Russian Federation, began to produce plutonium for the former Soviet Union’s nuclear weapons program. Workers at the facility were exposed to radiation from both external sources and inhaled plutonium, with doses that were much higher than those from similar operations in other countries, especially during the early period of operations (1948–1958). Thus, study of these workers offers a unique opportunity to evaluate health effects from both protracted external exposure and to plutonium, which is the focus of this article. Because inhalation is the exposure route of greatest concern, lung cancer is perhaps the most serious consequence of plutonium exposure. Lung cancer risks in Mayak workers have been most recently studied by Sokolnikov et al. (1). The analyses in this study make use of five additional years of follow-up (through 2008) and improved plutonium and external dose estimates to evaluate the relative risk as a function of plutonium and external doses, as well as other factors. Special attention is given to the nature of the joint effect of plutonium exposure and smoking, to the shape of the plutonium dose-response function and to the impact of changes in the plutonium dosimetry system.
A major objective of the paper is to provide the models that are needed for plutonium risk assessment. Plutonium exposure remains of concern due to occupational exposure from plutonium production, nuclear fuel reprocessing and cleanup operations, and because exposure to the general public might occur from reactor accidents, nuclear wastes and space accidents.
MATERIALS AND METHODS
This record-based epidemiological study required no contact with the cohort members. The project was reviewed and approved by the Institutional Review Board of the Southern Urals Biophysics Institute.
The Study Population and Follow-Up
The Mayak worker cohort (MWC) was established in the 1980s and currently includes 25,757 workers hired during the period from 1948–1982 in one of the main plants (nuclear reactors, radiochemical plant, plutonium production facility) or in auxiliary plants involved with water treatment or mechanical repair. Workers in the radiochemical and plutonium production facilities had potential for plutonium exposure. Information on vital status and cause of death has been obtained from address bureaus that maintain records on current vital status, and date and place of migration and death. In the past these address bureaus, located in Ozyorsk and throughout Russia, could be used to trace workers who left Ozyorsk. Recently, however, new privacy laws in Russia have made it difficult to obtain vital status information for workers who have migrated from Ozyorsk. Thus, in our current analyses, workers known to have migrated and also known to be alive on December 31, 2003 were treated as lost-to-follow up on this date: that is, for these workers, person-years and deaths occurring after 2003 were not included in our analyses. Additional detail on the characteristics of the Mayak cohort and methods of follow-up can be found elsewhere (2, 3).
Dosimetry
Analyses are based on estimates of individual annual external and internal (plutonium) doses to the lung from the Mayak Worker Dosimetry System 2008 (MWDS-2008) that was developed as a collaborative effort of Russian and U.S. dosimetrists. Film badge dosimeter readings provide the basis for most external gamma-radiation doses with adjustments to convert the originally recorded doses to organ doses, as well as to account for limitations in the ability of dosimeters to respond accurately to all radiation energies and to radiation coming from all directions. External dose was primarily from whole-body gamma radiation and in most cases was protracted over many years. MWDS-2008 external dose estimates are similar to MWDS-2005 external doses estimates (4), although some refinements have been made.
Methods for estimating internal doses from plutonium under MWDS-2008 are described by Khokhyrakov et al.2 In short, mathematical models were developed for converting measurements of plutonium alpha activity in urine to annual doses to the lung (and other organs) taking account of workers’ occupational histories, the physiochemical form of the plutonium aerosols, body mass and whether or not workers smoked. Although many workers were monitored through urinalysis in the early 1960s, a systematic urine monitoring program aimed at covering all workers with potential for plutonium exposure did not begin until about 1970. For this reason, plutonium dose estimates are available for only about 40% of workers in the radiochemical and plutonium plants. Improvements in MWDS-2008 compared with MWDS-2005, used in reference (1), include refinement of the model for plutonium biokinetics, in particular the plutonium distribution between lung tissue and pulmonary lymph nodes. An important change in MWDS-2008 compared with MWDS-2005 is that plutonium doses based on bioassay measurements were estimated for all subjects, whereas in MWDS-2005, autopsy data were used for 507 workers who had both autopsy and bioassay measurements. This change was made so that the dosimetry approach would be consistent for all workers. To investigate the impact of this change, alternative analyses were conducted using dose estimates based on autopsy data for these workers.
Statistical Methods
The analytic approach was similar to that used in previous analyses (1, 5). Analyses were based on Poisson regression methods implemented with the AMFIT module of the software package EPICURE (6). The follow-up period began 5 years after the date of first employment in an eligible plant and ended on the date of death, date lost to follow-up or December 31, 2008, whichever occurred first. Analyses were also based on cumulative dose received as of 5 years before the time at risk. Categories for lagged cumulative external and internal doses were a zero-dose category and 14 other categories with boundaries of 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6 and 10 Gy. Person-years with potential for risk for previous plutonium exposure (defined by whether the person had worked in the radiochemical or plutonium plants 5 or more years prior to time of interest) were also classified according to plutonium monitoring status. Due to indications that some workers were monitored for plutonium as a result of suspected diseases, death rates could be artificially high in the first few years after initial monitoring. Thus, person-years were not classified as monitored until 2 years after the initial monitoring date.
Most analyses in this paper excluded unmonitored workers with potential for plutonium exposure, and also excluded person-years for monitored workers up to 2 years after the first monitoring occurred. In past analyses, the use of a categorical surrogate index of plutonium exposure allowed inclusion of unmonitored but potentially plutonium-exposed workers for the purpose of estimating the effects of external dose. Such workers have never contributed to the estimation of parameters of the plutonium dose-response except through the estimation of baseline risk parameters. Analyses including unmonitored workers and person-years by using the surrogate were conducted for the purpose of comparison with previous results, for estimating external dose effects, and for estimating the relative biological effectiveness (RBE) of plutonium dose relative to external dose.
Analyses were based on excess relative risk (ERR) models where the total age-specific risk or hazard is given by B × (1 + ERR), where B is the baseline risk, B × ERR is the excess risk due to radiation, and ERR is the excess radiation risk expressed relative to the baseline. As in previous analyses (1), the logarithm of the baseline risk was expressed as a gender-specific linear-quadratic function of log (attained age), gender, birth cohort (4 categories with cut points of 1915, 1925 and 1935), calendar period (1948–1972, 1973–2003) and gender-specific smoking status (nonsmoker, smoker, unknown smoking status). As in (5), we added a parameter that allowed differences in risk between main and auxiliary plant males since male auxiliary plant workers have been shown to have elevated baseline lung cancer risks. Auxiliary plant workers were not included in (1).
The ERR for radiation exposure was expressed as a linear function of plutonium and external dose. That is, ERR = ERRplu + ERRext.
Analyses focus on the effects of plutonium dose, with external dose included primarily to adjust for potential confounding from this variable. Previous analyses have shown that the evidence for a lung cancer dose response is much weaker for external dose than for internal dose. Although we explored modification of the external dose response by gender, age and smoking, we found no evidence for such modification and thus used the simple model ERRext = βext dext, where dext denotes external dose in Gy, and βext is the ERR per Gy for external dose.
The ERR for plutonium dose is expressed as follows:
| (1) |
where dplu denotes plutonium dose in Gy, g is gender, a is attained age in years, and βplu, γ1, and γ2 are parameters to be estimated; βplu is expressed as ERR per Gy. For some analyses, the linear function βpludplu was replaced by other functions as described in the text. These included a categorical model in which βpludplu was replaced by Σk αk Ik, where k indexes categories of plutonium dose, αk designates the excess relative risk for category k, and Ik is equal to 1 if the observation is in category k and 0 otherwise. Unless stated otherwise, analyses were based on the model in Eq. (1) and included modification of the plutonium effect by attained age and gender as indicated above. Modification by other variables such as age at first plutonium exposure and smoking status was also explored. Estimates of the expected and excess cases were calculated, as described in ref. (3).
In the above model, smoking is a part of the baseline risk and the relative risk for smoking multiplies the relative risks for radiation. With this model, referred to as a multiplicative model (with respect to radiation and smoking), the ERR for radiation is the same for smokers, nonsmokers and those with unknown smoking status. To investigate the validity of this assumption, we conducted analyses that were restricted to subjects with known smoking status, and fitted a model in which the total age-specific risk is given by the following:
| (2) |
where B0 is the baseline risk associated with factors other than smoking, s = 1 for smokers and 0 for nonsmokers, θ is the ERR for smokers relative to nonsmokers, βplu is the plutonium ERR for nonsmokers and βplu exp(ϕ) is the plutonium ERR for smokers. With ϕ = 0, the relationship of plutonium and smoking is multiplicative. If ϕ > 0, the plutonium ERR is larger for smokers than for nonsmokers, and the interaction is super-multiplicative whereas if ϕ < 0, the interaction is sub-multiplicative. With this model, referred to as a generalized multiplicative model, both the plutonium and external dose ERRs for those with smoking status s are expressed relative to the baseline risk for those with the same smoking status.
We also evaluated a generalized additive (with respect to radiation and smoking) relative risk model in which the total risk is given by:
| (3) |
With this model, the plutonium and external dose ERRs are expressed relative to the baseline risk for nonsmokers regardless of smoking status. If ϕ = 0, the relationship of plutonium and smoking is additive. If ϕ > 0, the interaction is super-additive, whereas if ϕ < 0, the interaction is sub-additive. Parameter estimates were computed with maximum likelihood methods. Hypothesis tests and confidence intervals were based on likelihood ratio tests and direct evaluation of the profile likelihood. Two-sided P values are used throughout and are referred to as significant if they are <0.05.
RESULTS
Description of the Cohort
Most analyses in this paper are based on 14,621 workers who were followed for at least 5 years and for whom internal plutonium doses could be estimated (Table 1). Analyses excluded 1,084 workers who were followed less than 5 years (no lung cancer deaths) and an additional 10,052 workers (355 lung cancer deaths) who worked in the radiochemical or plutonium plants but had not been monitored for plutonium (9,615 workers) or were first monitored within 2 years of the end of follow-up (437 workers). Among the 14,621 included workers, 6,867 workers (47%) had died by the end of follow-up, 194 (1.3%) were lost to follow-up before the end of 2003, and 1,719 (12%) had to be censored at the end of 2003 or later due to migration. There were 486 deaths from lung cancer, most of them (446) in males.
TABLE 1.
Number of Mayak Workers by Follow-Up Status and Exclusion Criteria
| Number of workers | Number of deaths (%) | Number of lung cancer deaths (%) | Number lost to follow-up before 12/31/2003 (%) | Number lost to follow-up 12/31/2003a | |
|---|---|---|---|---|---|
| Original cohort | 25,757 | 12,438 (48) | 841 (3.2) | 1263 (4.9) | 4497 (17) |
| Excluded because followed for less than 5 years | 1084b | 298 (27) | 0 (0) | 785 (72) | 0 (0) |
| Excluded because plutonium doses could not be estimatedc | 10,052d | 5273 (52) | 355 (3.5) | 284 (2.8) | 2778 (28) |
| Cohort after exclusions | |||||
| All workers | 14,621 | 6867 (47) | 486 (3.3) | 194 (1.3) | 1719 (12) |
| Males | 10,918 | 5514 (51) | 446 (4.1) | 166 (1.5) | 1292 (12) |
| Females | 3703 | 1353 (37) | 40 (1.1) | 28 (0.8) | 427 (12) |
Workers who migrated and were confirmed to be alive 12/31/2003 (see text).
Three of these workers had plutonium doses that could not be estimated and are excluded from the count below.
Worked in the radiochemical or plutonium plants and were not monitored for plutonium within two years of the end of follow-up.
Five hundred eighty-nine of these workers contributed person-years before entering the radiochemical or plutonium plants.
Of the 14,621 workers included in our analyses, 25% were female and nearly half (46%) were hired before 1959 (Table 2). About 96% of the 6,540 workers with positive plutonium doses based on bioassay monitoring had been employed in the radiochemical or plutonium plants. The mean plutonium dose among exposed workers was 0.12 Gy, with the highest doses in those exposed before 1954 (0.28 Gy) and those employed in the Plutonium-Main 1 plant (0.44 Gy). In this latter group, doses for females (0.88 Gy) were much higher than those for males (0.30 Gy). The mean external dose (among all 14,621 workers) was 0.40 Gy, with the highest doses in workers hired in the earlier years and in workers in the radiochemical plant. Smoking data, which were obtained from medical records, were available for 87% of males and 85% of females, 74% of the 9,545 males with smoking data reported smoking, whereas 3.5% of the 3,163 females with smoking data reported smoking.
TABLE 2.
Number of Mayak Workers Included in Analyses (percentage in parentheses), Mean Plutonium Lung Dose, Mean External Lung Dose and Number of Lung Cancers by Gender, Year of First Plutonium Dose, Age of First Plutonium Dose, Plant and Smoking Status
| All workers | No plutonium dose | Positive plutonium dosea | Mean plutonium dose among those with positive dosesa (Gy) | Mean external dosea (Gy) | Lung cancer deaths | |
|---|---|---|---|---|---|---|
| Total | 14,621 | 8081 | 6540 | 0.115 | 0.397 | 486 |
| By gender | ||||||
| Males | 10,918 (75) | 6349 (79) | 4569 (70) | 0.093 | 0.418 | 446 (92) |
| Females | 3703 (25) | 1732 (21) | 1971 (30) | 0.165 | 0.335 | 40 (8.2) |
| By year of first plutonium doseb | ||||||
| 1948–1953 | 4675 (32) | 2831 (35) | 1844 (28) | 0.279 | 0.813 | 270 (56) |
| 1954–1958 | 2076 (14) | 976 (12) | 1100 (17) | 0.108 | 0.459 | 88 (18) |
| 1959–1963 | 2313 (16) | 1182 (15) | 1131 (17) | 0.064 | 0.219 | 79 (16) |
| 1964–1972 | 2573 (18) | 1375 (17) | 1198 (18) | 0.025 | 0.120 | 31 (6.4) |
| 1973–1982 | 2984 (20) | 1717 (21) | 1267 (19) | 0.010 | 0.080 | 18 (3.7) |
| By age of first plutonium dosec | ||||||
| 15–19 | 4173 (29) | 2554 (32) | 1619 (25) | 0.068 | 0.312 | 74 (15) |
| 20–24 | 5009 (34) | 2828 (35) | 2181 (33) | 0.154 | 0.497 | 190 (39) |
| 25–29 | 2326 (16) | 1219 (15) | 1107 (17) | 0.118 | 0.456 | 99 (20) |
| 30+ | 3113 (21) | 1480 (18) | 1633 (25) | 0.106 | 0.308 | 123 (25) |
| By plantd | ||||||
| Auxiliary plants | 3162 (22) | 3126 (39) | 36 (0.6) | 0.013 | 0.087 | 74 (15) |
| Reactor | 5172 (35) | 4933 (61) | 239 (3.7) | 0.026 | 0.405 | 165 (34) |
| Radiochemical | 3444 (24) | 16e (0.2) | 3428 (52) | 0.057 | 0.814 | 122 (25) |
| Pu-Auxiliary | 1044 (7.1) | 5e (0.1) | 1039 (16) | 0.067 | 0.172 | 21 (4.3) |
| Pu-Main 2 | 872 (6.0) | 1e (0.0) | 871 (13) | 0.078 | 0.160 | 30 (6.2) |
| Pu-Main 1 | 927 (6.3) | 0 (0.0) | 927 (14) | 0.442 | 0.341 | 74 (15) |
| By smoking status (Males) | ||||||
| Nonsmoker | 2518 (23) | 1359 (21) | 1159 (25) | 0.086 | 0.362 | 15 (3.4) |
| Smoker | 7027 (64) | 3954 (62) | 3073 (67) | 0.101 | 0.491 | 401 (90) |
| Unknown | 1373 (13) | 1036 (16) | 337 (7.4) | 0.045 | 0.148 | 30 (6.7) |
| By smoking status (Females) | ||||||
| Nonsmoker | 3052 (82) | 1356 (78) | 1696 (86) | 0.179 | 0.367 | 28 (70) |
| Smoker | 111 (3.0) | 59 (3.4) | 52 (2.6) | 0.213 | 0.384 | 7 (18) |
| Unknown | 540 (15) | 317 (18) | 223 (11) | 0.053 | 0.145 | 5 (13) |
Based on cumulative dose up to 5 years before the end of follow-up.
Year of hire for those with no plutonium dose.
Age at hire for those with no plutonium dose.
Classified by the “most dangerous” (in the order given) plant prior to 1983.
Worked in these plants only during the last 5 years of follow-up.
Main Results
Results of analyses based on the model in Eq. (1) are shown in Table 3. Risks for smokers were clearly elevated with higher relative risks for males than for females (10 and 5.7, respectively). Risks for those with unknown smoking status were more modestly elevated (5.7 for males and 1.9 for females). Significant dose-response relationships for plutonium were observed for both genders with the ERR per Gy for females (24 at age 60) estimated to be 3.3 times that for males (7.4 at age 60). The plutonium ERR per Gy decreased with attained age and with no indication that the parameter for attained age differed by gender (P > 0.5; details not shown). There was no evidence that the ERR depended on age at first plutonium exposure regardless of whether modification by attained age was allowed for (P > 0.5; details not shown). The estimated plutonium ERR per Gy for males without allowing for modification by attained age was 6.1 (95% CI: 4.1–8.7). The estimate when smoking was not included in the baseline risk was 7.4 (95% CI: 4.9–11), nearly identical to the smoking adjusted estimate shown in Table 3. Using the multiplicative ERR model based on Eq. (1) and with results shown in Table 3 it was estimated that 104.7 lung cancer deaths (21.5%) were attributable to plutonium exposure, while 29.2 deaths (6.0%) were attributable to external exposure.
TABLE 3.
Parameter Estimates (with 95% CI) for the Multiplicative Excess Relative Risk (ERR) Model (Eq. 1) for Plutonium and External Dose by Gender
| Males | Females | |
|---|---|---|
| Baseline relative risk for smokers | 10 (6.4 to 18) | 5.7 (2.3 to 12) |
| Baseline relative risk for unknown smoking status | 5.7 (3.1 to 11) | 1.9 (0.6 to 4.7) |
| Plutonium ERR per Gy at age 60 | 7.4 (5.0 to 11) | 24 (11 to 56) |
| P for gender difference in plutonium ERR per Gy | < 0.001 | |
| Gender ratio (F:M) | 3.3 (1.4 to 7.9) | |
| Attained age (power)a | −3.1 (−5.4 to −0.8) | |
| External dose ERR per Gy | 0.13 (−0.04 to 0.38) | |
Parameter γ2 in Eq. (1).
Interaction of Plutonium and Smoking
Table 4 shows results of analyses in which the ERR was allowed to depend on smoking status. With the generalized multiplicative model, the plutonium ERR for nonsmokers was significantly greater than that for smokers (P = 0.011), suggesting a sub-multiplicative relationship. In this analysis, the plutonium ERR for nonsmokers was estimated to be 4.1 times that for smokers and the female/male ratio was estimated to be 1.0 (95% CI: 0.3–3.4). With the generalized additive model, the ERR for nonsmokers was about a quarter of that for smokers, clearly indicating that the interaction was greater than additive (P < 0.001). The generalized multiplicative and additive models resulted in very similar fits to the data.
TABLE 4.
Parameter Estimates (with 95% CI) for the Generalized Multiplicative (Eq. 2) and Generalized Additive (Eq. 3) Excess Relative Risk (ERR) Models for Plutonium and External Dose by Smoking Statusa
| Generalized multiplicative modelb
|
Generalized additive modelc
|
|||
|---|---|---|---|---|
| Smokers | Nonsmokers | Smokers | Nonsmokers | |
| Plutonium ERR per Gy at age 60 | 6.9 (4.6 to 10) | 29 (9.8 to 83) | 92 (45 to 213) | 25 (9.2 to 71) |
| P for smoking status difference in plutonium ERR per Gy | 0.011 | <0.001 | ||
| Ratio (Nonsmoker:smoker) | 4.1 (1.4 to 12) | 0.27 (0.13 to 0.54) | ||
| Gender ratio (F:M) | 0.98 (0.3 to 3.4) | 1.2 (0.3 to 4.4) | ||
| External dose ERR per Gy | 0.11 (−0.06 to 0.36) | 0d | ||
Restricted to workers with data on smoking.
With this model, the plutonium and external dose related ERRs are expressed relative to workers with the same smoking status.
With this model, the plutonium and external dose related ERRs are expressed relative to nonsmokers.
Set equal to zero since the estimate was negative; the estimated ERR per Gy was −0.26 (95% CI: <−0.26–1.30).
The estimated excess deaths attributable to smoking and radiation based on the generalized additive model are shown in Table 5. Results based on the generalized multiplicative model were similar. Overall, 68% of the deaths were attributable to smoking, 10% to plutonium exposure alone, 12% to smoking and plutonium in combination and 9% to factors other than plutonium exposure or smoking. These distributions differed markedly by gender. For example, 7% of the male lung cancer deaths were attributed to plutonium exposure alone compared to 57% in females. In contrast, 73% of male lung cancer deaths were attributed to smoking alone compared to 15% in females since few women smoked. With this model, there were no deaths attributed to external exposure, but other models such as the multiplicative model in Table 3, Eq. (1), resulted in positive estimates, as noted above.
TABLE 5.
Estimated Excess Deaths Associated with Smoking and Plutonium Exposure (percentage given in parentheses)a
| Males | Females | Total | |
|---|---|---|---|
| Observed | 416 | 35 | 451 |
| Fitted | 416 | 35 | 451 |
| Background | 32.0 (7.7) | 9.8 (28.0) | 41.8 (9.3) |
| Excess from | |||
| Smoking alone | 302.4 (72.7) | 5.1 (14.6) | 307.5 (68.2) |
| Plutonium alone | 27.9 (6.7) | 17.8 (50.9) | 45.7 (10.1) |
| Smoking plus plutonium | 53.7 (12.9) | 2.3 (6.6) | 56.0 (12.4) |
Based on generalized additive relative risk model shown in Table 4.
Shape of the Plutonium Dose-Response Function
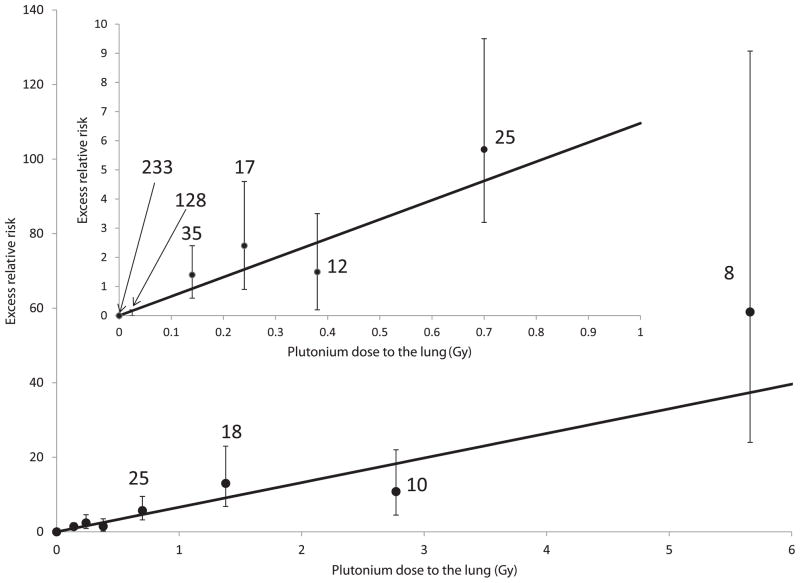
Figure 1 and Supplementary Table S1 (supplementary material: http://dx.doi.org/10.1667/RR3054.1.S1) show relative risks for each of several plutonium dose categories. The dose-response relationship is well described by a linear function, and a linear-quadratic function did not significantly improve the fit (P > 0.5). If the dose-response relationship was expressed as a power of plutonium dose (dpluα), the estimated power (α) was 1.02 (95% CI: 0.84–1.23). Table 6 shows results of analyses in which the plutonium dose range is restricted. In these analyses, the gender and attained age parameters were fixed at the values obtained from the entire data set. Highly statistically significant dose-response relationships were found even when analyses were restricted to plutonium doses less than 0.2 Gy. Estimates of the ERR per Gy were similar regardless of the dose range restriction.
FIG. 1.
Excess relative risk (with 95% CI) of lung cancer and number of lung cancer deaths by categories of plutonium dose to the lung. Shown for males at age 60. Estimated linear function also shown.
TABLE 6.
Excess Relative Risk (ERR) per Gy for Plutonium Using Various Plutonium Dose Restrictions
| Plutonium doses included | Person-yearsa (Percentage of total) | Number of lung cancer deathsa (Percentage of total) | ERRb per Gy (95% CI) | P for trend (2-sided) |
|---|---|---|---|---|
| All doses | 108,988 (100) | 253 (100) | 7.4 (5.2–10)c | <0.001 |
| Doses <1 Gy | 106,958 (98) | 217 (86) | 7.4 (4.8–11) | <0.001 |
| Doses <0.5 Gy | 104,652 (96) | 192 (76) | 6.5 (3.4–11) | <0.001 |
| Doses <0.3 Gy | 102,053 (94) | 180 (71) | 8.1 (4.1– 13) | <0.001 |
| Doses <0.2 Gy | 98,979 (91) | 163 (64) | 7.0 (2.5–13) | <0.001 |
| Doses <0.1 Gy | 90,376 (83) | 128 (51) | 1.3 (<0–9.4) | >0.5 |
Number of person-years and lung cancer deaths with positive plutonium doses in the range indicated.
Shown for males at age 60. Gender and attained age parameters set to the values obtained for the full data set; that is γ1 = 1.5; γ2 =−2.4 (see Eq. 1).
This confidence interval differs slightly from that in Table 3 because of fixing the sex and attained age parameters.
Modification of Plutonium Risks by Other Factors
In an analysis investigating doses received in different periods prior to the time at risk, the plutonium ERR per Gy was 4.0 (95% CI: <0–17) for doses received 5–15 years ago, 13 (95% CI: 2.8–26) for doses received 15–25 years ago, and 5.2 (95% CI: 0.0–13) for doses received 25 or more years ago. These ERRs did not differ significantly (P > 0.5). We also conducted an analysis in which doses received 5–15, 15–25 and 25+ years ago were given respective weights of 1.0, 0.46 and 0.21 as estimated in an analysis of French and Czech underground miners exposed to radon (7). This resulted in a similar fit to the analysis noted above with separate estimates of risks for the three times since exposure periods (P = 0.25). We also analyzed the effect of time since first exposure. There was no evidence of an effect with the model shown in Eq. (1) that included modification by attained age (P > 0.5; likelihood ratio statistic = 0.02). However, if attained age was not included as a risk modifier (γ2 = 0 in Eq. 1), a decrease in risk with time since first exposure was suggested (P = 0.065). A model including time since first exposure (without attained age) fitted the data less well than one based on attained age.
Statistically significant plutonium dose-response relationships were found for workers first exposed to plutonium in each of the periods from 1948–1953, 1954–1958, 1959–1963, but not for 1964–1972, 1973–1982 or for the combined period from 1964–1982. There was no evidence that the ERR per Gy differed between the five periods (P = 0.34).
Analyses Using Autopsy-Based Doses and Using the Full Cohort
As noted in the Dosimetry section, plutonium doses used in these analyses (MWDS-2008) were based on bioassay data and, unlike dose estimates used in previous analyses of the MWC, did not make use of autopsy data for workers who had such data. In addition, lung cancer analyses in earlier articles (1, 5) used plutonium surrogate categories for unmonitored (for plutonium) workers with potential for plutonium exposure instead of excluding these workers and person-years as in this article. To examine the impact of these changes and to facilitate comparison of current results to those reported previously, we conducted alternative analyses using autopsy doses when available and using the full cohort. Results are shown in Table 7. Of the 507 workers in the study cohort with autopsy data, only 406 were monitored more than 2 years preceding death and contributed to the plutonium dose-response analyses.
TABLE 7.
Estimates of Excess Relative Risk (ERR) per Gy for Plutonium, Attained Age Parameter, and ERR per Gy for External Dose by the Data Used and By Whether or Not Autopsy Data Used for Estimating Plutonium Doses for 406a Workers Who had Such Data
| Data used | Autopsy data used? | ERR per Gy for plutonium dose at age 60
|
Attained age parameter | ERR per Gy for external dose | |
|---|---|---|---|---|---|
| Males | Females | ||||
| Subjects and person-years with plutonium dose estimates | No | 7.4 (5.0 to 11) | 24 (11 to 56) | −3.1 (−5.4 to −0.8) | 0.13 (−0.04 to 0.38) |
| Yes | 6.5 (4.4 to 9.4) | 19 (8.2 to 43) | −3.2 (−5.5 to −0.8) | 0.21 (0.02 to 0.48) | |
| All datab | No | 8.2 (5.7 to 12) | 20 (11 to 38) | −3.6 (−5.7 to −1.4) | 0.19 (0.04 to 0.38) |
| Yes | 7.0 (4.8 to 9.9) | 17 (8.4 to 31) | −3.5 (−5.7 to −1.4) | 0.24 (0.08 to 0.44) | |
The original number was 507, but only 406 workers were monitored more than 2 years preceding death and otherwise qualified for the study cohort.
The plutonium surrogate (Sokolnikov et al.) was used for subjects whose plutonium doses could not be estimated; these subjects contributed to estimating baseline and external dose parameters, but not to estimating plutonium dose-response parameters.
Whether based on the full or restricted data, the use of autopsy-based doses slightly decreased the plutonium ERR per Gy estimates and strengthened the evidence for an external dose-response relationship, increasing the estimate based on the restricted data by 60% and changing it from a statistically nonsignificant (P = 0.25) to a statistically significant dose response (P = 0.026). Use of the full data set (keeping the dosimetry approach constant), slightly increased the estimates of the plutonium ERR for males and slightly decreased those for females. This change also slightly strengthened the effect of attained age on the plutonium ERR. Use of the full data set greatly increased the number of workers contributing to the external dose response and thus provided greater statistical power for investigating this relationship. Results based on the full data set indicated statistically significant dose-response relationships for external dose regardless of whether or not the autopsy data were used.
The full data set was used to estimate the RBE of plutonium relative to external γ radiation. To do this it was necessary to assume that modification by gender and attained age was the same for the two exposures, assumptions that were supported by the data. Using this approach the RBE was estimated to be 45 (95% CI: 21–240).
To evaluate the impact of the change from doses used in earlier analyses and MWDS-2008 (other than the use of bioassay data for all workers), we conducted analyses that restricted follow-up to the end of 2003 and excluded auxiliary plant workers, comparable to the analyses in ref. (1). The estimated plutonium ERRs per Gy using MWDS-08 with autopsy data were 7.1 (95% CI: 4.8–10) for males and 15.0 (95% CI: 7.2–29) for females, which were nearly identical to the corresponding estimates from (1): 7.1 (95% CI: 4.9–10) for males and 15.1 (95% CI: 7.6–29) for females.
Results of Parallel Analyses of Data on Mayak Workers and Japanese Atomic Bomb Survivors
To facilitate comparison of our results with lung cancer analyses based on the Life Span Study (LSS) of Japanese A-bomb survivors, we conducted analyses of both lung cancer mortality and incidence data from the LSS including only subjects exposed from age 15 to age 60, comparable to exposure ages in the Mayak worker cohort, using publicly available LSS data with dose estimates based on the DS02 dosimetry system: (http://www.rerf.jp/library/dl_e/lssinc07.html and http://www.rerf.jp/library/dl_e/ds02.html). Baseline risks were handled as in papers using these data (8–9). In contrast to Mayak data, the ERR for lung cancer did not show a statistically significant decline with attained age with the LSS data. However, a value of −1.8 for the attained age parameter was found to be compatible with all 3 data sets (P > 0.25 in each case). Using this value, the estimated ERR per Gy for males at age 60 was 7.0 (95% CI: 4.8–10) for plutonium exposure in Mayak workers, while the ERR per Gy estimates associated with acute γ ray exposures were 0.36 (95% CI: 0.04–0.78) for the LSS mortality data and 0.34 (95% CI: 0.05–0.72) for the LSS incidence data. If the Mayak estimate is expressed per Sv with a weighting factor of 20 as recommended by the ICRP (10), it becomes 0.35 (95% CI: 0.24–0.50), very similar to the LSS-based estimates. However, given the large uncertainty of the LSS-based estimates, a wide range of weighting factors would also be consistent with these data. The ratios of female and male estimates were also similar: 3.2 (95% CI: 1.4–7.7) for Mayak, 3.5 (95% CI: 1.3–32) for the LSS mortality data, and 5.3 (95% CI: 2.2–38) for the LSS incidence data.
DISCUSSION
Compared to our previous analyses (1), this paper adds 5 years of follow-up (2004–2008) and data on auxiliary plant workers, increasing the number of lung cancers with plutonium dose estimates from 354 to 486. Our new estimates are also based on improved external and internal dosimetry. The estimated ERR per Gy of 7.4 (95% CI: 5.0–11) for males at attained age of 60 was about the same as the estimate of 7.1 (95% CI: 4.9–10) as reported earlier. The comparable estimate for females of 24 (95% CI: 11–57) was about 60% higher than our earlier estimate of 15 (95% CI: 7.6–29), a difference that can be largely attributed to the considerable statistical uncertainty in risk estimates for females, although other analytic changes also contribute (see Table 7). Our earlier finding that the lung cancer ERR per Gy declines with increasing attained age was confirmed. However, the earlier suggestion of a decline with increasing age at first plutonium exposure was not confirmed. Although many others have analyzed lung cancer risks in Mayak workers (5, 11–13), these are the first to make use of the improved dose estimates (MWCDS-2008), and the first to include follow-up beyond 2003.
Unlike our earlier analyses, this paper evaluates modification of risk by smoking, an important issue in quantifying risks of radiation-related lung cancer. Our findings strongly indicate that the combined effects of plutonium dose and smoking are more than additive (P < 0.001) and also provide evidence that they are less than multiplicative (P = 0.011). These findings were also noted in analyses based on a two-stage clonal expansion model using data on a subset of the Mayak workers evaluated here (11). Based on a generalized multiplicative model, we estimated that the ERR per Gy for nonsmokers was about 4 times that for smokers, a little higher than the estimated ratio of about 3 in radon-exposed underground miners (14). When the plutonium ERR per Gy was allowed to depend on smoking, the ratio of female and male plutonium estimates was reduced from 3.3 (95% CI: 1.4–9.9) to 1.0 (0.3–3.4).
Because plutonium dose estimates make use of smoking information, dosimetry uncertainties could have affected our smoking results. Given the same occupational history and bioassay results, the estimated plutonium dose will usually be lower for a smoker than for a nonsmoker. If the smoking correction used in estimating plutonium doses was not large enough, doses for smokers would be overestimated relative to doses for nonsmokers leading to underestimation of risks per unit of dose for smokers relative to risks per unit of dose for nonsmokers. Therefore, the ratio of risk estimates per unit of dose for nonsmokers and smokers would be overestimated. The reverse would be true if the smoking correction were too large. Thus our results should be treated with caution.
Investigation of the combined effects of radiation and smoking in A-bomb survivors (15) indicated a super-multiplicative relationship for light/moderate smokers, and an additive or sub-additive relationship for heavy smokers (1+ packs per day). A weakness of our study is that the MWC smoking data did not include information on smoking rate or duration making it difficult to compare results from the two studies. A factor that might lead to differences in findings for Mayak workers and A-bomb survivors is that, unlike the acutely exposed LSS subjects, Mayak workers would often have many years of simultaneous exposure to both radiation and smoking.
In our previous article (1), we reviewed findings from other plutonium-exposed cohorts including workers at the Sellafield facility in the United Kingdom (16), and at Los Alamos (17, 18) and Rocky Flats in the U.S. (19). Plutonium doses in these studies were much lower than those of members of the MWC, and none of these studies provided clear evidence of an increase in lung cancer risk with increasing plutonium dose likely due to low statistical power.
Estimates of the ERR per Gy for external gamma dose have wide confidence intervals and depend on the analytic approach (Tables 3, 4, 7). This is especially true for estimates that are based only on subjects and person-years with plutonium dose estimates. Thus, it seems preferable to base such estimates on the full cohort using the plutonium surrogate to adjust for plutonium exposure in subjects with unknown plutonium doses. Both smoking and plutonium have strong effects on lung cancer risks, and data limitations may not have allowed adequate adjustment for these exposures. Due to the uncertainty in estimating risks from external doses, estimates of the RBE are also very uncertain.
Our estimates of risk for plutonium exposure are subject to limitations related to lack of detailed smoking information, lack of data on other lifestyle factors that might affect lung cancer risks, and reliance on death certificates as a source of information on death from lung cancer. As in any epidemiologic study, these limitations could potentially bias our findings. However, as discussed in detail in ref. (1), we have no reason to believe that any of these factors have seriously biased our results. In particular, we note that the plutonium ERR per Gy was not greatly modified in an analysis that did not control for smoking, suggesting that smoking is not a strong confounder in this study.
A particularly important limitation of our lung cancer risk estimates relates to intrinsic difficulties in estimating doses from plutonium exposure. Despite extensive efforts by Russian and non-Russian dosimetrists, these doses are subject to many uncertainties including especially those resulting from imprecision in the urine measurements. Only 33% of the 6,540 workers with estimates of positive plutonium doses had more than two measurements, although this percentage was higher (51%) for workers with estimated plutonium doses exceeding 0.2 Gy. For most workers, these measurements were taken many years after the exposure occurred, since routine plutonium monitoring did not begin until about 1970. Such measurement errors, often referred to as classical errors, are known to bias the dose-response coefficient toward zero and might also distort the shape of the dose response.
An uncertainty in estimating smaller plutonium doses is that the minimum detectable plutonium activity in urine (MDA) was relatively large, especially for measurements taken before 1970 (20). The effect of this on our results is not clear. However, since large doses are much more influential in our analyses than smaller ones, this problem may not have been as important as other sources of uncertainty.
Estimating lung doses requires knowledge of the transportability (solubility) of the inhaled plutonium, which determines how rapidly the plutonium clears the lung and moves to other organs. Information on transportability is inferred from occupational histories and from data on the chemical and physical forms of the airborne plutonium in various workplaces and time periods. Limitations in occupational histories are yet another source of uncertainty, particularly for workers who were exposed in several different workplaces.
Additional sources of uncertainty in plutonium dose estimates are uncertainties in the biokinetic models and parameter values used to estimate deposition and clearance in organs of the body and the fact that models can only approximate the behavior of plutonium in a given individual. Although dose estimates take account of smoking status, they could not take account of smoking rate or duration. Also, smoking data were not available for all workers, which particularly limits our ability to examine the nature of the joint effects of smoking and radiation exposure.
As noted above, uncertainties in the bioassay measurements could easily lead to serious underestimation of plutonium risk coefficients. The effects of other uncertainties are more difficult to predict but could include bias in overall risk coefficients, distortion of the shape of the dose response, and bias in evaluating the modifying effects of factors such as attained age and smoking. It is hoped that in the future these measurement errors will be quantified in a way that allows them to be taken into account in dose-response analyses.
Earlier Mayak dosimetry systems used autopsy data instead of bioassay data for the 406 workers included in our analyses with both types of data. This has the advantage that individual measurement errors for autopsy-based doses are likely to be much smaller than for bioassay-based doses. However, autopsy data are never available for workers who remain alive, and workers with positive plutonium doses dying from lung cancer are more likely to have autopsy data (31%) than are comparable workers dying from other causes (12%). Thus, any bias in the use of autopsy data compared with bioassay data could lead to bias in estimating the parameters of the lung cancer dose-response function, and this is the reason that the MWDS-2008 doses used in our analyses were based entirely on bioassay data. It is reassuring that results of dose-response analyses that made use of autopsy data were similar to those based entirely on bioassay data. However, among the 406 workers with autopsy data who contributed to the plutonium dose-response analyses, the correlation coefficient for the autopsy and bioassay doses was only 0.58. Large classical measurement errors for the bioassay measurements likely contributed strongly to this low correlation coefficient.
The MWC provides the only human data that are adequate for estimating lung cancer risks from plutonium exposure. However, using these data to estimate risks from plutonium exposures at low doses (<0.1 Gy) and in other populations presents challenges. A linear dose response describes these data extremely well, as evidenced by visual inspection of the plotted categorical relative risks (Fig. 1), the tight confidence interval on the power of dose (0.86–1.24), and the very similar estimates of the ERR per Gy obtained when restricting the dose range (Table 5). Ninety-eight percent (98%) of the plutonium-exposed person-years had doses that were less than 1 Gy and 91% had doses that were less than 0.2 Gy. Thus, no extrapolation is required for estimating risks in the 0.2–1 Gy. Estimating risks at doses lower than 0.2 Gy is uncertain, but these data as well as data on other α emitters such as radon (14, 21) support the use of linear extrapolation.
In contrast to other radiation worker cohorts (16–19), a substantial number of females in the MWC were exposed to plutonium. Nevertheless, the number of lung cancers in females (40) was small. Thus, the MWC provides only limited data on lung cancer risks in females and no data on exposure in childhood. Even for estimating risks from adult male exposure, there are uncertainties in transporting risks to other populations. Our data along with data from other radiation-exposed cohorts, suggest that smoking may be an important modifier of radiation-related lung cancer risks. It is therefore desirable to account for smoking in estimating risks in other populations that may have smoking habits that differ from those of Mayak workers. If this is not feasible, relative risk transport may be preferable to absolute risk transport given the strong evidence against additivity of smoking and plutonium effects.
It is desirable to consider alternative approaches for estimating risks from plutonium. One such approach is to apply an RBE derived from experimental data to lung cancer risk estimates that are derived from the LSS cohort. Our comparisons of Mayak-based ERR estimates with those from the LSS cohort support use of an RBE of 20 for this purpose, and estimates of parameters quantifying modification of risk by gender and attained age were found to be reasonably compatible. However, in an earlier analysis (5), excess absolute risks for lung cancer mortality were found to increase much more sharply with attained age in the LSS cohort than in the MWC. With an RBE of 20, the EAR for Mayak workers for attained ages under 55 was significantly larger than the comparable EAR based on the LSS mortality data. Repeating these analyses using the current data and both LSS incidence and mortality data confirmed these findings (data not shown).
The models developed in this paper can be compared with those developed from underground miners who, like Mayak workers, received protracted exposure to α-particle emitters. Both analyses of data from the MWC and of data from 11 cohorts of underground miners (14, 21) indicate declines in the ERR with attained age and sub-multiplicative interactions of radiation and smoking. In addition, data from the 11 cohorts and more recent data on French and Czech uranium miners (7) indicate a decline in the ERR with time since exposure. Although we did not find a statistically significant decline with time since exposure in the MWC (perhaps because of uncertainty in estimating the pattern of internal lung dose accumulation in individual workers), our data were compatible with the decline estimated in the French and Czech cohorts. In addition, a decline in the ERR with time since first exposure was suggested in the MWC when attained age was not included as a modifier [the analyses in ref. (7) did not evaluate attained age as a modifier]. A decline in risk with age at first exposure was identified in analyses of the French/Czech data, but was not found in the MWC or in the 11 miner cohorts.
The MWC provides the only direct human data for estimating lung cancer risks from plutonium exposure with reasonable precision. In this study, we have developed models based on improved dosimetry for accomplishing this task. Estimates based on the MWC are most appropriate for male smokers. Given the limitations of the MWC, data from other cohorts such as the LSS cohort and cohorts of radon-exposed underground miners also need to be considered in plutonium risk assessment.
Supplementary Material
Acknowledgments
We wish to acknowledge the role of the late Elaine Ron in establishing the collaborative research between the U.S. and Russia that made this article possible. We also wish to thank Alexander Efimov, Vadim Vostrotin and Alan Birchall for their work in finalizing the plutonium doses used in our analyses. Support for this study has been provided by the U.S. Department of Energy (DOE) under the auspices of the JCCRER and by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. This report makes use of data obtained from RERF in Hiroshima, Japan. RERF is a private, non-profit foundation funded equally by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the U.S. Department of Energy through the National Academy of Sciences. The data include information obtained from the Hiroshima City, Hiroshima Prefecture, Nagasaki City and Nagasaki Prefecture Tumor Registries and the Hiroshima and Nagasaki Tissue Registries. The conclusions in this report are those of the authors and do not necessarily reflect the scientific judgment of RERF or its funding agencies.
Footnotes
Khokhryakov VV, Khokhjryakov VF, Suslova KG, Vostrotin VV, Vvedensky VE, Sokolova AB, et al. Mayak Worker Dosimetry System 2008 (MWDS-2008): Internal dosimetry. Health Phys (submitted for publication).
The online version of this article (DOI: 10.1667/RR3054.1) contains supplementary information that is available to all authorized users.
References
- 1.Sokolnikov ME, Gilbert ES, Preston DL, Ron E, Shilnikova NS, Khokhryakov VV, et al. Lung, liver and bone cancer mortality in Mayak workers. Int J Cancer. 2008;123(4):905–11. doi: 10.1002/ijc.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koshurnikova NA, Shilnikova NS, Okatenko PV, Kreslov VV, Bolotnikova MG, Sokolnikov ME, et al. Characteristics of the cohort of workers at the Mayak nuclear complex. Radiat Res. 1999;152(4):352–63. [PubMed] [Google Scholar]
- 3.Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, et al. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003;159(6):787–98. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Vasilenko EK, Khokhryakov VF, Miller SC, Fix JJ, Eckerman K, Choe DO, et al. Mayak worker dosimetry study: an overview. Health Phys. 2007;93(3):190–206. doi: 10.1097/01.HP.0000266071.43137.0e. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert ES, Koshurnikova NA, Sokolnikov ME, Shilnikova NS, Preston DL, Ron E, et al. Lung cancer in Mayak workers. Radiat Res. 2004;162(5):505–16. doi: 10.1667/rr3259. [DOI] [PubMed] [Google Scholar]
- 6.Preston DL, Lubin JL, Pierce DA, McConney ME. Epicure User’s guide. Hirosoft International Corporation; Seattle, WA: 1993. [Google Scholar]
- 7.Tomasek L, Rogel A, Tirmarche M, Mitton N, Laurier D. Lung cancer in French and Czech uranium miners: Radon-associated risk at low exposure rates and modifying effects of time since exposure and age at exposure. Radiat Res. 2008;169(2):125–37. doi: 10.1667/RR0848.1. [DOI] [PubMed] [Google Scholar]
- 8.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168(1):1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 9.Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res. 2004;162(4):377–89. doi: 10.1667/rr3232. [DOI] [PubMed] [Google Scholar]
- 10.International Commission on Radiological Protection (ICRP) Relative Biological Effectiveness (RBE), Quality Factor (Q) and Radiation Weighting Factors (wR) Oxford: Pergamon Press; 2003. p. 80. (ICRP no. 92) [DOI] [PubMed] [Google Scholar]
- 11.Jacob P, Meckbach R, Sokolnikov M, Khokhryakov VV, Vasilenko E. Lung cancer risk of Mayak workers: modelling of carcinogenesis and bystander effect. Radiat Environ Biophys. 2007;46:383–94. doi: 10.1007/s00411-007-0117-0. [DOI] [PubMed] [Google Scholar]
- 12.Tokarskaya ZB, Scott BR, Zhuntova GV, Okladnikova ND, Belyaeva ZD, Khokhryakov VF, et al. Interaction of radiation and smoking in lung cancer induction among workers at the Mayak nuclear enterprise. Health Phys. 2002;83(6):833–46. doi: 10.1097/00004032-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Kreisheimer M, Sokolnikov ME, Koshurnikova NA, Khokhryakov VF, Romanow SA, Shilnikova NS, et al. Lung cancer mortality among nuclear workers of the Mayak facilities in the former Soviet Union. An updated analysis considering smoking as the main confounding factor. Radiat Environ Biophys. 2003;42(2):129–35. doi: 10.1007/s00411-003-0198-3. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council, Committee on the Biological Effects of Radiation. Health Effects of Exposure to Indoor Radon (BEIR VI) National Academy Press; Washington, D.C: 1999. p. 500. [Google Scholar]
- 15.Furukawa K, Preston DL, Lonn S, Funamoto S, Yonehara S, Matsuo T, et al. Radiation and smoking effects on lung cancer incidence among atomic bomb survivors. Radiat Res. 2010;174(1):72–82. doi: 10.1667/RR2083.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omar RZ, Barber JA, Smith PG. Cancer mortality and morbidity among plutonium workers at the Sellafield plant of British Nuclear Fuels. Br J Cancer. 1999;79(7–8):1288–301. doi: 10.1038/sj.bjc.6690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voelz GL, Lawrence JN, Johnson ER. Fifty years of plutonium exposure to the Manhattan Project plutonium workers: an update. Health Phys. 1997;73(4):611–9. doi: 10.1097/00004032-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Wiggs LD, Johnson ER, Cox-DeVore CA, Voelz GL. Mortality through 1990 among white male workers at the Los Alamos National Laboratory: Considering exposures to plutonium and external ionizing radiation. Health Phys. 1994;67(6):577–88. doi: 10.1097/00004032-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Brown SC, Schonbeck MF, McClure D, Baron AE, Navidi WC, Byers T, et al. Lung cancer and internal lung doses among plutonium workers at the Rocky Flats Plant: A case-control study. Am J Epidemiol. 2004;160(2):163–72. doi: 10.1093/aje/kwh192. [DOI] [PubMed] [Google Scholar]
- 20.Krahenbuhl MP, Bess JB, Wilde JL, Vostrotin VV, Suslova KG, Khokhryakov VF, et al. Uncertainty analysis of doses resulting from chronic inhalation of plutonium at the Mayak Production Association. Health Phys. 2005;89(1):33–45. doi: 10.1097/01.hp.0000154027.92466.97. [DOI] [PubMed] [Google Scholar]
- 21.Lubin JH, Boice JD, Jr, Edling C, Hornung RW, Howe GR, Kunz E, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Nat Cancer Inst. 1995;87(11):817–27. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.