Abstract
Cognitive efficiency decreases with age, and advancing age is the leading risk factor for most neurodegenerative disorders that result in dementia. In HIV infection, risk for cognitive impairment is consistently linked to advancing chronological age. As the HIV epidemic enters its fourth decade in the USA, extended life expectancy will likely result in an increased prevalence of cognitive disorders by virtue of these factors. However, it is less clear if HIV potentiates or accelerates the risk for cognitive impairment given that most reports are mixed or demonstrate only a small interaction effect. More critically, it is unclear if HIV will modulate the neuropathology associated with non-HIV cognitive disorders in a manner that will increase risk for diseases such as cerebrovascular and Alzheimer’s disease. In the coming years, with increasing numbers of HIV+ patients entering their 60s and 70s, background risk for neurodegenerative disorders will be sufficiently high as to inform this issue on clinical grounds. This review summarizes knowledge of cognition in HIV as it relates to age and presents some emerging controversies.
Keywords: HIV, Dementia, Cognition, Aging
Introduction
In the last 30 years, the human immunodeficiency virus-1 (HIV) has evolved from a subacute and ultimately terminal disease to one characterized by long-term chronic illness. Important changes are occurring in the frequency of HIV in older adults, driven in large part by extended survival due to effective combination antiretroviral therapies (cART) (Manfredi 2004; Palella et al. 1998). In the years since the advent of cART, the population has shifted from a predominantly young demographic (91 % below age 45 years in 1996) to an older, aging population, with approximately one half of HIV+ individuals currently over 45 years of age. It is estimated that by 2015, half of all people living with HIV/AIDS will be over 50 years of age (Stoff et al. 2004). Critical to the issue of neurodegeneration, the CDC estimated that over 50,000 of 663,084 HIV+ adults in the USA were over age 60 in 2008 (CDC 2009). Most older HIV patients acquired infection as young adults and aged with HIV; however, the frequency of becoming infected in older age may also be rising (Zablotsky and Kennedy 2003). Epidemiological trends indicate greater risk behaviors in the contemporary population over 50 with insufficient public health emphasis on safe sexual practices (Zablotsky and Kennedy 2003).
Despite advancements in HIV treatments, a sizable proportion of the HIV+ population continues to suffer from HIV-associated neurocognitive disorders (HAND). Patients with HAND may report that they have trouble completing tasks that involve a series of steps, or they may be more easily distracted. They may also report problems with balance and motor coordination, and often evidence difficulty with learning, such as memorizing lists of words (Ances and Ellis 2007). Early signs of CNS involvement often include mental slowing with impaired retrieval of information, gait disturbances with reports of falling or tripping, decreased manual dexterity, and general signs of apathy or depression (McArthur et al. 2003). On cognitive tests, HAND generally manifests as psychomotor slowing, problems with attention and concentration, executive dysfunction, and impairment of learning and recall, with sparing of semantic and visuo-spatial abilities (Ances and Ellis 2007; Heaton et al. 2011; McArthur et al. 2003). While this review focuses on the neuropsychological aspects of HIV, it is well recognized that the clinical manifestations of HIV CNS disease involve a triad of cognitive, behavioral, and motor findings.
In the USA, the current trend toward growing numbers of aged HIV+ patients has led to concern for increased risk for age-related neurodegenerative diseases, principally Alzheimer’s disease (AD) and Parkinson’s disease (PD), as well as concern for the effects of other comorbidities, such as cerebrovascular disease. Should overlap exist, greater heterogeneity in presenting symptoms would be expected and may confuse presenting neuropsychological testing deficits due to a mixture of mechanisms. These considerations will impact diagnosis, screening approaches, and treatment options.
Clinical and potentially confounding factors associated with aging of HIV+ individuals
The clinical features of older HIV+ individuals differ from those of younger populations in a manner that could be expected to impact vulnerability to cognitive impairment (Fig. 1). Older adults tend to have longer overall duration of infection and possibly a longer duration of exposure to antiretroviral medications (Cherner et al. 2004; Valcour et al. 2004a). These factors may contribute to unique cohort phenomena such as longer duration of viremia or exposure to treatments and dosing that are no longer common. Many older individuals survived a period of time when cART was not yet conceived. Thus, survivorship tendencies may exist, whereby unrecognized host or viral factors associated with long-term survival in the absence of effective treatment may also modulate risk for cognitive problems. Older patients are more likely to have been exposed to more toxic antiretroviral medications, such as dideoxynucleoside reverse transcriptase inhibitors, which are now less commonly used. Limited data from a magnetic resonance spectroscopy study suggest that such treatment could impact brain health (Schweinsburg et al. 2005). In this work, authors compared individuals taking stavudine or didanosine, two medications known to be linked to mitochondrial toxicity, with those taking medications less toxic to mitochondria (zidovudine or lamivudine) and identified a dose-dependent decreased concentration of brain n-acetyl aspartate in frontal white matter, suggesting decreased mitochondrial integrity. Prior to availability of cART, many individuals were treated with mono or dual therapy, increasing the likelihood of periods of incomplete viral suppression and viral resistance. By virtue of age, older subjects are more likely to have experienced this treatment era. While speculative, these factors should be considered when investigating potential contributions to cognitive impairment in the oldest demographic.
Fig. 1.
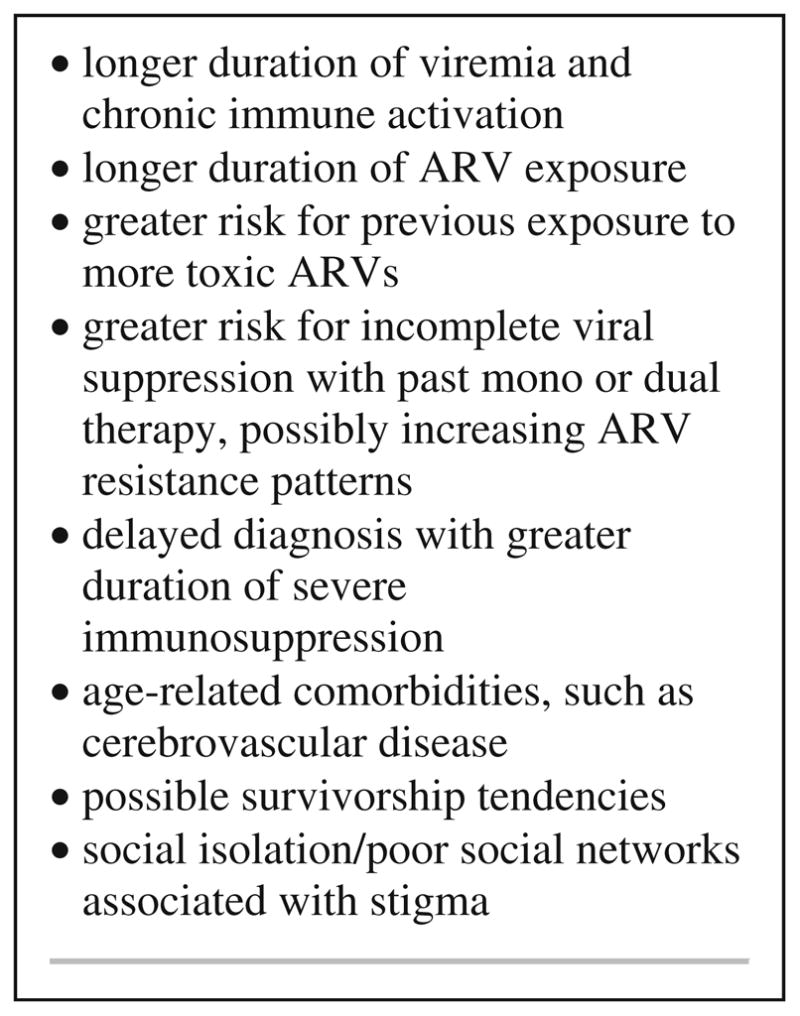
Clinical factors to consider when investigating cognitive disorders in aged HIV patients
Individuals who become infected at older ages may have unique cognitive risk factors in part due to delayed diagnosis, which is more frequent in older adults (Castilla et al. 2002; Ferro and Salit 1992; Mugavero et al. 2007; Nogueras et al. 2006). Delayed diagnosis translates to longer periods of time with HIV viremia and immunosuppression contributing to risk for HIV-associated dementia (HAD) (Heaton et al. 2010; McArthur et al. 1993). A diagnosis of dementia concurrent with first identification of HIV infection was noted to occur more frequently with advanced age during the pre-cART era (Janssen et al. 1992).
Higher frequency of HIV cognitive disorders with aging
Research-based HAND diagnoses were redefined in 2007 and range in increasing severity from asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND) to HAD (Table 1) (Antinori et al. 2007). Although there is a stepwise increase in severity from ANI or MND to HAD, there are insufficient data to conclude that HAD is the final outcome, with significant bidirectional changes in cognition noted in several cohorts (Antinori et al. 2007; McArthur et al. 2003). The distinction of ANI from MND and HAD is based on patient report of symptoms or poor performance on objective measures of everyday functioning, whereas among patients diagnosed as ANI, the severity of neuropsychological testing abnormalities can vary greatly. Our experience and that from others suggest that ANI may represent our inability to identify functional deficits due to reliance on patient or proxy reporting with little differences in objective measures of functional compromise when comparing ANI to MND (Gandhi et al. 2011; Valcour et al. 2012). Moreover, past work demonstrates functional consequences associated with neuropsychological deficits among individuals not stratified by reports of functional limitations (Heaton et al. 2004; Thames et al. 2011). Social isolation is frequent in aging HIV patients (Grov et al. 2010; Vance et al. 2009), and information obtained from proxies who are acquaintances rather than family members could hinder the identification of functional symptoms. The variability of cognitive impairment within ANI and the questionable reliability of self-report have proved problematic in the clinical setting, which highlights the need to consider objective measures to assess everyday function (Blackstone et al. 2012; Thames et al. 2011).
Table 1.
Diagnostic criteria for HAND
| Diagnosis | Criteria |
|---|---|
| Asymptomatic cognitive impairment (ANI) | Impairment in at least two cognitive domains by NP testing (at least 1 SD); no known functional impairment |
| Mild neurocognitive disorder (MND) | Impairment in at least two cognitive domains by NP testing (at least 1 SD); mild interference with daily functioning |
| HIV-associated dementia (HAD) | Severe impairment in at least two domains by NP testing (at least 2 SD); marked impact on daily functioning |
Since the introduction of cART, several groups have reported a reduction in the overall incidence of the most severe forms of cognitive impairment. Duration of HIV has not emerged as a risk factor; however, low CD4 nadir is consistently identified as a risk factor (Heaton et al. 2010). In the Multicenter AIDS Cohort Study, researchers observed a 50 % reduction in HAD incidence between 1990 and 1998, from 21.1 % in 1990 to 10.5 % in 1998 (Sacktor et al. 2001). In the cART-era CHARTER study, researchers reported HAD in only 2 % of their cohort, but 44 % demonstrated milder forms of cognitive impairment, over two thirds of which was ANI (Heaton et al. 2010). Data from 15,380 HIV+ patients followed through the Concerted Action on Seroconversion to AIDS and Death in Europe project showed a decrease in incidence of HAD with the introduction of cART, down from 6.49 per 1,000 persons prior to 1997 to 0.66 between 2003 and 2006, with older age at infection increasing risk of cognitive impairment (Bhaskaran et al. 2008). Researchers estimate the cART-era prevalence of all forms of HAND at 50 % (Heaton et al. 2010; Woods et al. 2009).
During the pre-cART era, and prior to the introduction of current diagnostic criteria, several epidemiological studies identified age as a robust risk factor for HIV-associated cognitive disorders (Chiesi et al. 1996; Janssen et al. 1992). In studies with HIV+ cohorts over 50 years of age, researchers have found both age and HIV effects (Becker et al. 2004; Cherner et al. 2004). In the Hawaii Aging with HIV cohort, increasing age was shown to be a robust risk factor for cognitive impairment (Valcour et al. 2004a). Comorbid illnesses such as cardiovascular disease, cerebrovascular disease, and metabolic dysfunction (i.e., high cholesterol and diabetes) may also affect cognitive functioning, and are more likely to be present in older individuals (Becker et al. 2009; Valcour et al. 2005).
Neuropsychological testing performance associated with HIV and aging
The literature offers a broad consensus that older HIV+ patients are at greater risk to meet research criteria for HIV-related cognitive disorders when cognitive test scores are corrected for normal aging and/or when age-matched, uninfected controls are included in the study. The relationship between HIV and aging on neuropsychological testing performance, however, is less clear. Some authors describe domain-specific overlap of performance deficits with both age and HIV preferentially affecting psychomotor speed and cognitive flexibility (Hardy et al. 1999; Wilkie et al. 2003). However, conflicting findings are noted in other studies and, if evidence for deficits is present, the age at which these findings would translate into clinically relevant outcomes remains to be defined. Becker et al. reported higher rates of neuropsychological testing abnormalities among older compared to younger individuals in the Allegheny County Neuropsychological Survey (Becker et al. 2004). This study included 290 HIV+ and 114 HIV-negative individuals. They identified 37 % of older (n=22, age >50 years) compared to 31 % of younger individuals (age <50 years) testing in an impaired range, with most of the older patients having greater degrees of impairment (23 % defined as “dementia” compared to 9 % in the younger group). In this work, the investigators defined dementia based on neuropsychological performance alone rather than clinical diagnostic characterization and, notably, 40 % of impaired subjects were clinically asymptomatic. Other methodological limitations were apparent, including a control group that was statistically younger than the HIV+ group and methodology that allowed designation of impairment with only minimal standard deviation abnormality on individual tests.
The HIV Neurobehavioral Research Program Group recently reported an effect of age and serostatus on individual variability in performance (i.e., dispersion) across multiple domains on neuropsychological testing, such that HIV+ individuals over the age of 50 years demonstrated greater dispersion than younger (<40 years) HIV+ subjects and older (>50 years) HIV-negative controls, which may indicate enhanced compromise of cognitive integrity (Morgan et al. 2011). Hardy et al. identified differences by age in performance among HIV+ patients; however, they were unable to evaluate HIV–age interaction as they did not have a comparative HIV-negative group (Hardy et al. 1999). This study identified age effects on most neuropsychological tests. Kessel et al. compared performance among 25 individuals over 45 years of age to 155 HIV+ individuals who were less than 35 years of age. They identified HIV effects and age effects but failed to identify age–HIV interaction effects (Kissel et al. 2005). Their sample included only six HIV-negative individuals over age 45 years. Goodkin et al. identified higher rates of Minor Cognitive Motor Disorder symptom reporting among older patients (Goodkin et al. 2001). Since the reporting of symptoms is central to the diagnosis of MND or HAD, this finding would support a higher ratio of symptomatic to asymptomatic impairment in older subjects; but this finding in relation to neuropsychological testing abnormalities should be interpreted with caution given that symptom reporting is often more reflective of affective state rather than objective cognitive functioning, even though the authors carefully adjusted for such factors in their analyses (van Gorp et al. 1994, 1991; Wilkins et al. 1991).
The Hawaii Aging with HIV Cohort was designed to address the issue of interaction effects between HIV and aging on cognition. Here, investigators carefully enrolled HIV-negative groups from similar social venues, often enrolling partners and friends of HIV+ subjects. Compared to published normative data, researchers found that HIV+ status negatively affected neuropsychological performance in both younger (<40 years) and older (>50 years) patients. However, within the cohort, when using co-enrolled matched HIV-negative control cases rather than published normative data, they failed to identify clinically important interaction effect between age and HIV status (Valcour et al. 2011). Marginal and isolated effects were identified in three tests associated with visual memory, attention, and motor speed, but the actual differences were small and were not reflected in other tests within the battery that were also referable to these cognitive domains. In a separate evaluation, older subjects in this cohort appeared to have heightened impairment in tests of executive functioning when employing published normative data (Sacktor et al. 2007). Older patients in this cohort were also noted to have more motor findings on neurological examination using the United Parkinson’s Disease Rating Scale, with even higher rates noted among those with cognitive impairment, highlighting the critical need to include neurological exam findings when clinically evaluating central nervous system disorders in HIV (Valcour et al. 2008). A pre-cART analysis within the Multicenter AIDS Cohort Study suggested an impact of age on tests of manual dexterity, adding the possibility that including more subjects with advanced disease may augment the interactions on neuropsychological testing performance, and a cART era study within this cohort investigating longitudinal performance on tests of psychomotor speed reported an interaction effect of age and serostatus on a single test: Trail Making Test Part B (Sacktor et al. 2010; van Gorp et al. 1994). Table 2 summarizes available articles addressing this issue.
Table 2.
Selected reports of neuropsychological outcomes associated with aging in HIV infection (arranged alphabetically by first author)
| Author and Reference | Patient population | Outcome measure | Major findings | Limitations |
|---|---|---|---|---|
| Becker et al., AIDS 2004 | 22 HIV+ age ≥50 vs. 100 age <50 | Dementia and cognitive impairment not dementia (CIND) defined by neuropsychological measures | 22 % of older compared to 9 % of younger patients with “dementia” at baseline; 14 % of older compared to 22 % of younger with CIND; 1-year incidence of dementia at 7 % older vs. 4 % younger | Limited age matching to controls; NP abnormality requirements for mild impairment were mild (0 to −1 SD abnormality); 40 % classified as “dementia” denied symptoms |
| Cherner et al., AIDS 2004 | 67 HIV+ age ≥50 vs. 52 <35 years | Cognitive impairment rating scales based on neuropsychological performance | Trends for age-associated differences were noted on most ability domains. Age–CSF HIV RNA interaction effects identified | Preliminary report, unclear if sample size was adequate |
| Cysique et al., J Neuropsychiatry Clin Neurosci 2011 | 116 HIV+ vs. 30 HIV-negative, stratified into younger (<49 years) and older (≥49) age groups | Neuropsychological summary scores | Negative effects of HIV+ serostatus and increasing age on NP performance, but no effect of age × HIV | Small HIV-negative comparison group, and relatively young cohort; no analysis of domain-specific NP performance; possible survivor bias |
| Hardy et al., New Zealand J Psychology 1999 | 257 HIV+ between 1989 and 1995. Divided into 2 groups (older/younger) at the median of 36 years | Neuropsychological testing battery | Age effects were seen on most neurocognitive measures in HIV+ patients | No seronegative control group to determine HIV × age effects |
| Kissel et al., J Neuropsychiatry Clin Neurosci 2005 | 66 HIV-negative (10 over 45 years old) compared to 188 HIV+ (25 over 45 years) | Neuropsychological testing battery | Both age and HIV effects noted on summary deficit score; interaction effects not identified | Limited number of HIV-negative individuals over 45 years of age |
| Scott et al., AIDS Behavior 2011 | Younger groups (age ≤40 years): HIV-neg (n=24), HIV+ (n=24); Older groups (age ≥50) HIV-neg (n=20), HIV+ (n=48) | Neuropsychological test scores | Effects of age on learning, memory, executive function, and visuoconstruction; effect of HIVon learning and memory; no effect of age × HIV | Study focused on “cortical” domains, excluding psychomotor and processing speed; relatively young sample of “older” individuals (mean=55.4 years); possible survivor bias |
| van Gorp et al., Neurology 1994 | Study 1: 1,066 HIV+ (<1 % over 55 years old) and 1,004 HIV-neg; Study 2: 76 HIV+ (age 29–55, 41 of whom had symptomatic HIV disease) and 47 age-matched HIV-neg controls | Neuropsychological testing battery | Study 1: age (but not serostatus) was associated with poorer performance on most tests; age × serostatus effects not detected. Study 2: age × serostatus interaction effects seen only in grooved pegboard test on nondominant hand | Patients in study one were relatively young (5 greater than 55 years old) |
| Valcour et al., JINS 2011 | Younger groups (avg age, 35): HIV-neg (n=98), HIV+ (n=108); Older groups (avg age, 55.5): HIV-neg (n=106), HIV+ (n=121) | Neuropsychological testing scores | Age × HIV effects were not identified; CD4 nadir had mild interaction effect with NP score | Older groups contained very few individuals >60 years of age |
| Wilkie et al., J Acquir Immune Defic Syndr 2003 | Younger groups (age 19–39): HIV-neg (n=30), HIV+ (n=56); Older groups (age 50+): HIV-neg (n=29), HIV+ (n=36) | Neuropsychological testing scores | Age × HIV effects were not identified | Preliminary report, small sample limiting power |
There are numerous methodological issues noted in these heterogeneous papers. The age at which individuals are considered “old” varies in these studies and appears to be related to availability of cohorts rather than physiological criteria. Previously, it was suggested that 50 years of age was appropriate. This age represented an approximately two standard deviation cut point from the mean for age of HIV+ patients in the mid-1990s (Linsk 2000). However, this distinction has very little pathological or physiological significance and has likely been altered with the changing demographics. Early epidemiological data hinted at an age threshold effect, whereby risk increased to a greater degree above age 65 years (Janssen et al. 1992; van Gorp et al. 1994). This cut point needs to be considered cautiously as this study relied on physician reporting of diagnoses, a factor that may be influenced by the patient’s age. To date, all studies lack appreciable numbers of individuals over age 60. An alternative approach is to consider age as a continuous variable in regression analyses, but this strategy risks obscuring thresholds where brain vulnerability may sharply increase. Dynamic methodologies designed to consider nonlinear associations may be useful. One study employed these approaches among 116 HIV+ subjects and 30 controls and similarly failed to identify clinically important interaction effects (Cysique et al. 2011).
Clinical factors impacting cognition in HIV and aging
Studies suggest various factors which might contribute to enhanced cognitive vulnerability in the older HIV+ population. For example, researchers at the HIV Neurobehavioral Research Center reported an interaction between age and CSF HIV levels, where older individuals with detectable CSF HIV RNA had twice the rate of impairment than did their older counterparts with undetectable CSF HIV RNA, while this relationship was absent in the younger cohort (Cherner et al. 2004). In addition, they found that 76 % of older patients not receiving cART compared to 57 % of older patients on cART met impairment criteria, while the rates in younger patients were unaffected by cART status (54 vs. 52 %). Furthermore, in the Hawaii Aging with HIV cohort, cognitive vulnerability associated with having at least one apolipoprotein epsilon 4 allele was identified in older, but not younger, adults (Valcour et al. 2004b).
There is significant concern that, even in the absence of HAND, the inflammatory processes implicated in HIV CNS pathology might lead to increased vulnerability to other age-related dementias such as AD and PD (Alisky 2007; Anthony et al. 2006). Researchers have pointed to reports of increased amyloid precursor protein expression in HIV brains as evidence for a potential interaction with AD pathology (Alisky 2007; Green et al. 2005), and more importantly, as a possible indication of increased susceptibility to early-onset AD. Anthony et al. presented evidence for increased tau deposition in cART-treated HIV+ patients relative to age-matched controls, although they reported no correlation between tau levels and cognitive status (Anthony et al. 2006). This group also demonstrated monocyte-based brain parenchymal immune activation in these individuals, further worrisome for continued inflammatory mediators despite suppressed plasma virus.
As yet, there is no direct evidence for a neuropathological link between HIV and AD, and no evidence of higher rates of early-onset AD in the HIV+ population. It is likely that current published reports contain too few sufficiently old HIV+ patients in the range where AD would be common enough to detect increased rates. Currently, this issue may be better informed by neuropathological findings and imaging as clinical parameters may be insufficient. Even in the absence of a direct link to neurodegenerative disorders among older patients with HIV infection, we must consider the possibility of concurrent neuropathology related to other age-associated processes including cerebrovascular disease. It is possible that complicated interaction effects exist among comorbid illness, chronic antiretroviral treatment, and age-related brain changes related to cerebrovascular disease (Valcour et al. 2004c). Cerebrovascular disease either alone or in combination with other neurodegenerative disorders is a common cause of cognitive impairment in the general population (McMurtray et al. 2006). In HIV, cerebrovascular disease can be caused by both HIV and non-HIV-related factors (reviewed in Valcour et al. 2004c). Risk factors for cerebrovascular disease that are not directly related to HIV infection will likely play an increasing role in HIV+ patients as they age since many of the risks increase with age. Such factors include hypertension, diabetes mellitus, cardiac disease, and dyslipidemia. Smoking may be of particular interest for older HIV+ patients where this behavior is more frequent, reported to be as high as 54 % among individuals living with HIV in San Francisco, and 55 % among 881 HIV+ Veterans in the Veterans Aging Cohort Three-Site Study (Mamary et al. 2002; Smola et al. 2001).
Summary
Optimal care of an aging HIV+ population has emerged as an important issue in the past 10 years. It is postulated that individuals infected with HIV now will have a life expectancy that approaches the eighth decade of life (The Antiretroviral Therapy Cohort Collaboration 2008). Cognitive health is a pivotal quality of life and mortality predictor associated with aging and, separately, HIV; however, the degree to which these two factors interact to accelerate cognitive deterioration is incompletely understood. Among HIV+ individuals under age 60 years, interaction effects are not likely to be clinically important. Among individuals over the age of 60, there is insufficient data to accurately inform definitive conclusions.
Large challenges will emerge related to diagnostic certainty of cognitive impairment among the oldest old. HIV providers have previously been spared this difficulty given that old age and HIV infection were largely mutually exclusive. These changes raise important areas of research exploration with the continued overarching goal of optimizing cognitive health and with it overall quality of life in this emerging aged HIV+ population.
Acknowledgments
Funding This work was funded by NIH grants: K23-AG032872 and 3-P50 AG023501.
Footnotes
Conflict of interest None.
Contributor Information
Lauren A. Wendelken, Email: lwendelken@memory.ucsf.edu, Memory and Aging Center, Department of Neurology, UCSF, Suite 905, 350 Parnassus Avenue, San Francisco, CA 94143-1207, USA
Victor Valcour, Memory and Aging Center, Department of Neurology, UCSF, Suite 905, 350 Parnassus Avenue, San Francisco, CA 94143-1207, USA. Division of Geriatric Medicine, Department of Medicine, UCSF, San Francisco, CA, USA.
References
- Alisky JM. The coming problem of HIV-associated Alzheimer’s disease. Med Hypotheses. 2007;69:1140–1143. doi: 10.1016/j.mehy.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(Suppl 1):S11–S18. [PubMed] [Google Scholar]
- Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, Ragin A, Sacktor N, Selnes OA, Visscher BR. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, Phillips A, Porter K. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Jr, Woods SP, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Rivera-Mindt M, Deutsch R, Ellis RJ, Hampton Atkinson J, Grant I. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla J, Sobrino P, De La Fuente L, Noguer I, Guerra L, Parras F. Late diagnosis of HIV infection in the era of highly active antiretroviral therapy: consequences for AIDS incidence. AIDS. 2002;16:1945–1951. doi: 10.1097/00002030-200209270-00012. [DOI] [PubMed] [Google Scholar]
- CDC. Centers for Disease Control and Prevention. [Accessed Aug 2011];HIV Surveillance Report. 2009 21 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/#surveillance; Published February 2011. [Google Scholar]
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, Grant I, Heaton RK. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18 (Suppl 1):S27–S34. [PubMed] [Google Scholar]
- Chiesi A, Vella S, Dally LG, Pedersen C, Danner S, Johnson AM, Schwander S, Goebel FD, Glauser M, Antunes F, et al. Epidemiology of AIDS dementia complex in Europe. AIDS in Europe Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Bain MP, Wright E, Brew BJ. HIV and age do not substantially interact in HIV-associated neurocognitive impairment. J Neuropsychiatry Clin Neurosci. 2011;23:83–89. doi: 10.1176/jnp.23.1.jnp83. [DOI] [PubMed] [Google Scholar]
- Ferro S, Salit IE. HIV infection in patients over 55 years of age. J Acquir Immune Defic Syndr. 1992;5:348–353. [PubMed] [Google Scholar]
- Gandhi NS, Skolasky RL, Peters KB, Moxley RTT, Creighton J, Roosa HV, Selnes OA, McArthur J, Sacktor N. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol. 2011;17:159–165. doi: 10.1007/s13365-011-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, Asthana D, Fujimura RK, Lee D, van Zuilen MH, Khamis I, Shapshak P, Eisdorfer C. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. J Clin Epidemiol. 2001;54(Suppl 1):S35–S43. doi: 10.1016/s0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22:630–639. doi: 10.1080/09540120903280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Hinkin C, Satz P, Stenquist P, van Gorp W, Moore L. Age differences and neurocognitive performance in HIV-infected adults. N Z J Psychol. 1999;28:94–101. [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42:1472–1476. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- Kissel EC, Pukay-Martin ND, Bornstein RA. The relationship between age and cognitive function in HIV-infected men. J Neuropsychiatry Clin Neurosci. 2005;17:180–184. doi: 10.1176/jnp.17.2.180. [DOI] [PubMed] [Google Scholar]
- Linsk NL. HIV among older adults: age-specific issues in prevention and treatment. AIDS Read. 2000;10:430–440. [PubMed] [Google Scholar]
- Mamary EM, Bahrs D, Martinez S. Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDS. 2002;16:39–42. doi: 10.1089/108729102753429389. [DOI] [PubMed] [Google Scholar]
- Manfredi R. HIV infection and advanced age emerging epidemiological, clinical, and management issues. Ageing Res Rev. 2004;3:31–54. doi: 10.1016/j.arr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McMurtray A, Clark DG, Christine D, Mendez MF. Early-onset dementia: frequency and causes compared to late-onset dementia. Dement Geriatr Cogn Disord. 2006;21:59–64. doi: 10.1159/000089546. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I. Intraindividual variability in HIV infection: evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25:645–654. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Castellano C, Edelman D, Hicks C. Late diagnosis of HIV infection: the role of age and sex. Am J Med. 2007;120:370–373. doi: 10.1016/j.amjmed.2006.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueras M, Navarro G, Anton E, Sala M, Cervantes M, Amengual M, Segura F. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis. 2006;6:159. doi: 10.1186/1471-2334-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizu B, Shikuma C, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. J Neurovirol. 2007;13:203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Cox C, Selnes O, Becker JT, Cohen B, Martin E, Miller EN. Longitudinal psychomotor speed performance in human immunodeficiency virus-seropositive individuals: impact of age and serostatus. J Neurovirol. 2010;16:335–341. doi: 10.3109/13550284.2010.504249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Woods S, Carey C, Weber E, Bondi M, Grant I The HIV Neurobehavioral Research Center (HNRC) Group. Neurocognitive Consequences of HIV Infection in Older Adults: An Evaluation of the “Cortical” Hypothesis. AIDS Behavior. 2011;15(6):1187–1196. doi: 10.1007/s10461-010-9815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, Letendre S, Videen JS, McCutchan JA, Patterson TL, Grant I. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11:356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- Smola S, Justice AC, Wagner J, Rabeneck L, Weissman S, Rodriguez-Barradas M. Veterans aging cohort three-site study (VACS 3): overview and description. J Clin Epidemiol. 2001;54 (Suppl 1):S61–S76. doi: 10.1016/s0895-4356(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Stoff DM, Khalsa JH, Monjan A, Portegies P. Introduction: HIV/AIDS and aging. AIDS. 2004;18(Suppl 1):S1–S2. [PubMed] [Google Scholar]
- Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, Heaton RK, Castellon SA, Hinkin CH. Medication and finance management among HIV-infected adults: the impact of age and cognition. J Clin Exp Neuropsychol. 2011;33:200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004a;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes OA, Grove J, Liu Y, Abdul-Majid KB, Gartner S, Sacktor N. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004b;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Watters M, Sacktor N. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004c;18(Suppl 1):S79–S86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Shikuma CM, Shiramizu BT, Williams AE, Watters MR, Poff PW, Grove JS, Selnes OA, Sacktor NC. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;38:31–36. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol. 2008;14:362–367. doi: 10.1080/13550280802216494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Paul R, Neuhaus J, Shikuma C. The effects of age and HIV on neuropsychological performance. J Int Neuropsychol Soc. 2011;17:190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, Nir TM, Thompson PM, Miller B. Functional deficits identified in patients with asymptomatic neurocognitive impairment (ANI) track to changes in brain integrity and size. Presented at the 19th Conference on Retro-viruses and Opportunistic Infections (CROI); Seattle. 5–8 March 2012.2012. [Google Scholar]
- van Gorp WG, Satz P, Hinkin C, Selnes O, Miller EN, McArthur J, Cohen B, Paz D. Metacognition in HIV-1 seropositive asymptomatic individuals: self-ratings versus objective neuropsychological performance. Multicenter AIDS Cohort Study (MACS) J Clin Exp Neuropsychol. 1991;13:812–819. doi: 10.1080/01688639108401091. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Miller EN, Marcotte TD, Dixon W, Paz D, Selnes O, Wesch J, Becker JT, Hinkin CH, Mitrushina M, et al. The relationship between age and cognitive impairment in HIV-1 infection: findings from the Multicenter AIDS Cohort Study and a clinical cohort. Neurology. 1994;44:929–935. doi: 10.1212/wnl.44.5.929. [DOI] [PubMed] [Google Scholar]
- Vance DE, Childs G, Moneyham L, McKie-Bell P. Successful aging with HIV: a brief overview for nursing. J Gerontol Nurs. 2009;35:19–25. doi: 10.3928/00989134-20090731-04. quiz 26–7. [DOI] [PubMed] [Google Scholar]
- Wilkie FL, Goodkin K, Khamis I, van Zuilen MH, Lee D, Lecusay R, Concha M, Symes S, Suarez P, Eisdorfer C. Cognitive functioning in younger and older HIV-1-infected adults. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S93–S105. doi: 10.1097/00126334-200306012-00006. [DOI] [PubMed] [Google Scholar]
- Wilkins JW, Robertson KR, Snyder CR, Robertson WK, van der Horst C, Hall CD. Implications of self-reported cognitive and motor dysfunction in HIV-positive patients. Am J Psychiatry. 1991;148:641–643. doi: 10.1176/ajp.148.5.641. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotsky D, Kennedy M. Risk factors and HIV transmission to midlife and older women: knowledge, options, and the initiation of safer sexual practices. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S122–S130. doi: 10.1097/00126334-200306012-00009. [DOI] [PubMed] [Google Scholar]


