Abstract
A central question in the study of the mind is how cognitive functions are shaped by a complex interplay of genetic and experiential processes. Recent evidence from cultural neuroscience indicates that cultural values, practices, and beliefs influence brain function across a variety of cognitive processes from vision to social cognition. This evidence extends to low-level perceptual systems comprised of domain-specific mechanisms, suggesting the importance of ecological and cultural variation in the evolutionary and developmental processes that give rise to the human mind and brain. In this article, we argue that investigating the architecture of the human mind will require understanding how the human mind and brain shape and are shaped by culture–gene coevolutionary processes.
Keywords: cultural neuroscience, modularity, domain-specificity, domain-generality
Introduction
Nearly three decades ago, Fodor (1983) introduced the theory of modularity of mind to cognitive science. A couple of years later, Boyd and Richerson (1985) introduced culture–gene coevolutionary theory, proposing for the first time that cultural and genetic selection processes may interact as forces shaping the human mind. Together, these disparate accounts launched a decades-long exploration and debate for psychologists and neuroscientists eager to understand the mind’s core architecture.
Over the years, behavioral and brain scientists alike have made much progress in understanding the mind’s architecture. However, much less empirical and theoretical attention have been paid to understanding the role of cultural and genetic processes in psychological and neural architecture, especially as the vast majority of research in this area has been conducted with Western participants living in the industrialized world (Chiao & Cheon, 2010; Henrich, Heine, & Norenzayan, 2010). Most recently, advances in cultural neuroscience have begun to provide evidence of cultural variation in the neural correlates of mental processing, amounting to greater evidence for culture–gene coevolution and launching a new approach to understanding how mental processes are organized by the interaction of cultural and biological factors across situational, developmental, and evolutionary timescales. Our goal in this article is to examine how the emerging field of cultural neuroscience can contribute to advancing cognitive theory by investigating how cultural and genetic factors shape the neural processes underlying mental functioning. Even at this early stage, we argue that findings in cultural neuroscience are already informing cognitive theory in visual and social cognition.
Cultural neuroscience is an emerging field that examines how cultural values, practices, and beliefs shape and are shaped by neurobiological processes across multiple time scales (Chiao & Ambady, 2007; Chiao, Hariri, et al., 2010; Chiao, Cheon, Pornpattananangkul, Mrazek, Blizinsky, in press). Cultural neuroscientists typically use noninvasive neuroscience methodologies, such as functional neuroimaging (fMRI) or event-related potentials (ERPs), to test hypotheses regarding cultural influences on brain functioning and relations to behavior (Chiao & Ambady, 2007; Han & Northoff, 2008; Kitayama & Uskul, 2011; Park & Gutchess, 2006). Over the past 40 years, elegant behavioral research by cultural psychologists has revealed cultural differences in how people think, feel, and behave (Kitayama & Cohen, 2007); however, in the absence of evidence that these often subtle differences reflect influences of social experience on neural functioning, rather than evolutionary factors, cultural psychological evidence could not easily be brought to bear on questions of modularity of mind. In addition, behavioral research often requires large sample sizes given the distal nature of observing effects of culture on behavior. By contrast, cultural neuroscience research has revealed cultural differences in brain structure and function with comparatively small samples and often in the absence of culture-related differences in observable behavior (Chiao et al., 2010). Together, the findings hold promise that cultural neuroscientists are in a unique position to infer cultural effects on cognitive processes from behavioral, neural, and genetic measures (Fig. 1).
Fig. 1.
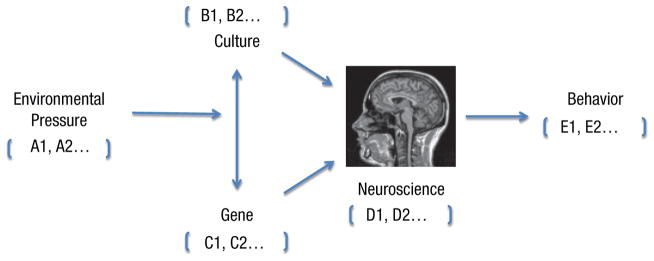
Cultural neuroscience model of human behavior. Each factor in the cultural neuroscience model may be composed of a set of variables of each type (e.g., A1, A2 refers to distinct environmental variables; B1, B2 refers to distinct cultural variables).
One of the key challenges at the frontier of cultural neuroscience is understanding the role of culture in shaping variation in functionally specialized neural mechanisms. To the extent that social transmission of cultural values, practices, and beliefs leads to cultural adaptations in low-level sensory systems, and to the extent that genetic propensities facilitate the transmission of cultural values, practices, and beliefs, the architecture of the mind may be shaped by both genetic and cultural selection in a manner akin to culture–gene coevolutionary theory (Boyd & Richerson, 1985; Chiao & Blizinsky, 2010; Chudek & Henrich, 2011). Culture–gene coevolutionary theory posits that cultural and genetic selection both affect how the mind and brain give rise to behavior. For example, recent evidence indicates culture–gene coevolution of individualism–collectivism and the serotonin transporter gene (5-HTTLR) such that cultural collectivism may have emerged in tandem with the genetic selection of the S allele of the serotonin transporter gene (5-HTTLPR) in response to environmental pressures, such as pathogen prevalence (Chiao & Blizinsky, 2010; Fig. 2). The serotonin transporter gene may also have played an important role in the emergence of cultural tightness–looseness, such as sensitivity to social norms or deviance, in response to other kinds of ecological pressures across geographic regions (Gelfand et al., 2011; Mrazek, Chiao, Blizinsky, Lun, & Gelfand, 2012). However, little is known about how cultural values of individualism–collectivism or of tightness–looseness interact with genes such as the serotonin transporter gene (5-HTTLPR) to shape psychological and neural processes that give rise to human behavior. Given the known role of the serotonin transporter gene in shaping the neural response within brain regions associated with emotion (Munafo, Brown, & Hariri, 2008) and social cognition (Canli & Lesch, 2007), investigating culture–gene coevolution of human behavior is an essential frontier in the field of cultural neuroscience (Chiao, 2011; Chiao, Hariri, et al., 2010).
Fig. 2.
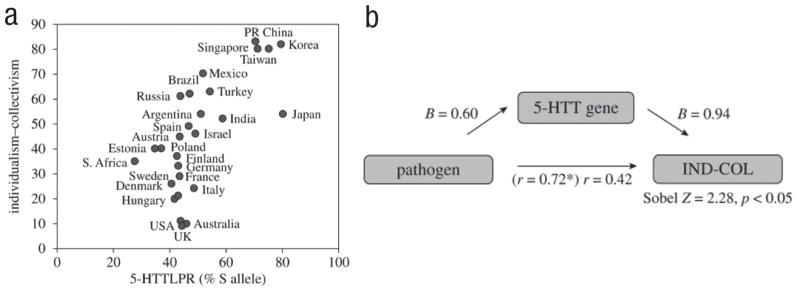
Culture–gene coevolution of individualism–collectivism and the serotonin transporter gene (adapted from Chiao & Blizinsky, 2010).
Contributions of cultural neuroscience to the study of visual cognition
As envisioned by early philosophers of mind, such as Fodor (1983), the mind consists of multiple cognitive domains or modules, each self-contained and responsible for specific cognitive operations. In this view, domain-specific psychological mechanisms are identifiable based on a set of characteristic features including automaticity, information encapsulation, innateness, and fixed neural architecture (among others). In pursuit of evidence for Fodor’s modularity of mind thesis (1983), cognitive scientists have searched for convergent evidence of domain-specific mechanisms with a variety of experimental paradigms, from looking-time studies in infants and nonhuman primates to neuropsychological and neuroimaging studies with adults. In this work, modules are specialized psychological components that perform specific cognitive processes consistently across situational, lifespan, and evolutionary timescales.
One of the major foci in this research is domain specificity in perceptual systems, such as vision. For example, within the ventral visual pathway, patches of cells within the fusiform gyrus preferentially respond to faces over other types of complex visual stimuli including objects, scenes and scrambled images (Kanwisher, McDermott, & Chun, 1997). This preferential response to faces occurs not only within fusiform regions of humans (Kanwisher et al., 1997), but also in macaques (Tsao, Freiwald, Tootell, & Livingstone, 2006), suggesting a phylogenetically shared cortical space dedicated to the processing of faces. Cortical specialization has also been observed for a variety of nonface complex visual processes, including the processing of places (Epstein, 2008) and objects (Grill-Spector, Kourtzi, & Kanwisher, 2001). The apparent robustness of specialized neural machinery for processing of specific categories of visual information in human and nonhuman primates and prosopagnosic patients who have specific deficits in face processing subsequent to damage to their fusiform region, as well as the presence of face recognition ability in early infancy (Kanwisher, 2010; Tzourio-Mazoyer et al., 2002), is thought to reflect genetic selection for psychological and neural mechanisms dedicated to face recognition.
However, despite the apparent robustness of the organization of visual processing in the brain, culture appears to shape neural processing by influencing the process by which a visual stimulus is perceived, encoded and recognized, even within domain-specific neural regions along the ventral visual pathway. One of the hallmark findings from cultural psychology is the distinction between analytic and holistic processing. When compared across various levels of visual processing (e.g., from perceiving to perspective-taking to remembering images), researchers have found that East Asians tend to process visual information in a more holistic manner, attending to the surround and the central object, whereas Westerners tend to process visual information in an analytic manner, paying attention to the central object over the surround (Nisbett & Miyamoto, 2005; Nisbett, Peng, Choi, & Norenzayan, 2001). Recently, cultural neuroscientists have found that this analytic–holistic processing distinction affects neural responses even in domain-specific visual brain regions. Goh and colleagues (2007) compared elderly Westerners and elderly East Asians and found differences in visual processing in object-specific areas of the lateral occipital cortex (LOC), which they interpreted as reflecting life-long cultural entrainment of analytic and holistic styles of perceiving the world. In addition, Goh and colleagues (2010) found that Westerners showed greater neural selectivity during face processing in the left fusiform gyrus face-processing area (FFA), whereas East Asians showed greater selectivity within this neural area on the right. This cultural difference in visual neural processing may reflect cultural differences in analytic–holistic processing style, as the right hemisphere is thought to process more holistically, whereas the left is thought to process more analytically and sequentially. This neural finding is consistent with psychological findings in which Caucasians engage in more sequential processing of facial features such as the eyebrow and mouth, whereas East Asians devote relatively more attention to processing the eye region, a central facial feature that may allow for more holistic, simultaneous processing of peripheral facial features (Jack, Caldara, & Schyns, 2011).
Fodorian notions of modularity of mind were not meant to explain the possibility of cultural diversity in core cognitive capacities like visual processing; nevertheless, culture–gene coevolution provides a plausible explanation for cultural variation within domain-specific regions of the ventral visual pathway (Boyd & Richerson, 1985) by suggesting that socially transmitted cultural traits, in this case an emphasis on analytic versus holistic processing, are selected for, and develop in tandem with, genetic traits that regulate face-recognition abilities. Together, these complementary forces provide humans the necessary cognitive capacities for social survival, while at the same time leading to cultural diversity of processing within these cognitive capacities, possibly in response to global variation in ecological demands (Chiao & Blizinsky, 2010; Fincher, Thronhill, Murray, & Schaller, 2008; Mrazek et al., 2012).
Contributions of cultural neuroscience to the study of social cognition
Another domain of cognition where cultural neuroscience may offer insights is self-construal theory. In the 1970s, cultural psychologists began to question and document ways in which people think about the “self.” Using the Twenty Statements Test, Cousins (1989) found that people from Western cultures tend to describe themselves in an individualistic fashion, using trait adjectives (e.g., “I am active”), whereas people from Eastern cultures tended to describe themselves in a more collectivistic fashion, referring to situational roles (e.g., “I am my sister’s friend”). Markus and Kitayama (1991) later introduced self-construal theory to explain cultural differences in a range of psychological processes and behaviors from emotion and cognition to motivation and decision making. Initial behavioral evidence provided strong support for this distinction; however, subsequent behavioral studies also revealed inconsistencies in empirical evidence concerning self-construal theory. For example, meta-analytic evidence by Oyserman, Coon, and Kemmelmeier (2002) showed that, among East Asians, only the Chinese were more likely to be collectivistic rather than individualistic across a variety of tasks. In addition, Westerners were not found to be more individualistic than Easterners in some studies. If indeed individualism–collectivism is a valid cultural distinction, it was apparently not one that could be reliably described, nor assumed as, a cultural difference between East and West.
Empirical advances in cultural neuroscience have resolved this debate, at least partially, by demonstrating that cultural values, practices, and beliefs are represented neurally in the individual within brain regions specialized for social processing, rather than as fixed neural differences differentiating geographic or national groups. Cultural priming studies have shown that even temporarily heightening awareness of culture affects psychological processes and behavior (Hong, Morris, Chiu, & Benet-Martinez, 2000; Oyserman & Lee, 2008). Similar to prior cultural psychological studies of the self, Zhu, Zhang, Fan, and Han (2007) found that Chinese participants showed similar neural responses within the medial prefrontal cortex (MPFC), a brain region specialized for social cognition, when encoding trait adjectives of self and mother compared with a familiar other, but Westerners living in China did not. By contrast, in a comparison of Japanese and American participants, Chiao, Harada, and colleagues (2009) showed that, irrespective of nationality, people with individualist tendencies showed greater MPFC response when thinking of themselves in a general manner, whereas people with collectivist tendencies show greater MPFC response when thinking of themselves in a relational manner. Ray and colleagues (2010) further showed that individual differences in cultural values for Caucasians living in the U.S. predicted brain activity in cortical midline regions, the MPFC, and the posterior cingulate cortex when judging trait adjectives of mother and self. Chiao and colleagues showed that temporarily heightening awareness of cultural values in bicultural and monocultural individuals is sufficient to modulate this neural response during implicit (Harada, Li, & Chiao, 2010) and explicit self-judgments (Chiao, Harada, et al., 2010). Taken together, these results illustrate that neural responses within the MPFC are heightened when people think about self and others in a culturally congruent manner. These studies further show that self-reported cultural values, not nationality (e.g., Japanese vs. American) or cultural group (e.g., Eastern vs. Western) per se, predict neural responses during self-judgments, even in the absence of measurable group differences in behavior. Hence, cultural styles of self-construal are dynamically represented within cortical midline structures involved in self-knowledge, whose activity likely reflects fluctuations related to internal awareness.
Although the initial observations discussed above pertain to the effects of culture on neural processes during self/other judgments, several studies have now shown cultural influences on the functioning of brain regions involved in other kinds of social cognitive tasks involving theory of mind (Adams et al., 2010; Kobayashi, Glover, & Temple, 2006), interpersonal perception (Freeman, Rule, Adams, & Ambady, 2009), empathy (Cheon et al., 2011; Chiao, Mathur, et al., 2009; Mathur, Harada, & Chiao, 2011; Mathur, Harada, Lipke, & Chiao, 2010; Xu, Zuo, Wang, & Han, 2009) and emotion (Chiao et al., 2008; Chiao et al., 2012; Derntl et al., 2009; Moriguchi et al., 2005). For instance, work in progress by Immordino-Yang and colleagues reveals individual and cultural differences in somatosensory activation during the processing of social emotion and that the correspondence between neural activation and subjective emotion awareness relates to individual and cultural differences in emotional expressivity (Immordino-Yang, Yang, & Damasio, 2012; Saxbe, Yang, Borofsy, & Immordino-Yang, 2012). In one experimental paradigm, participants in an open-ended interview reacted to a series of documentary-style narratives about emotion- provoking social situations and then viewed short versions of the same narratives as emotion induction stimuli in the fMRI scanner (Immordino-Yang, 2010; Immordino-Yang, McColl, Damasio, & Damasio, 2009). To test the hypothesis that individuals’ verbal descriptions of emotional feelings are associated with neural processing biases during a nonlinguistic emotion task, the ratio of cognitive to affective words used by the participants in the interview was correlated with somatosensory activation when participants subsequently reported experiencing strong emotion to the same narratives in the fMRI scanner. The results suggest that individual differences among Americans (of mixed ethnic backgrounds, including first-generation East Asian) in naturalistic word use when describing feelings correspond to differences in reliance on somatosensory neural regions during emotion experience—“feeling” in the literal, embodied sense (Saxbe et al., 2012). In a cross-cultural version of the experiment conducted with participants in Los Angeles and Beijing, there were additional cultural differences in the correspondence between visceral somatosensory neural activations and the timing and magnitude of participants’ reported real-time emotion experiences (Immordino-Yang et al., 2012). These findings further highlight that spontaneously invoked cultural strategies for emotion processing influence low-level sensory systems in the brain during emotion processing, a surprising discovery given that low-level sensory systems are often thought to serve as evidence for an evolutionarily honed, rather than variable, neural architecture (Kanwisher, 2010). Cultural values have also been shown to modulate neural responses to emotional scenes within the human amygdala (Chiao et al., 2012), an evolutionarily ancient brain region necessary for fear perception and learning (e.g., “fear module”; Adolphs, 2010; Ohman & Mineka, 2001). In sum, it is important for future cultural neuroscience research to better understand the role of cultural and individual variation in adaptive low-level sensory systems and how the architecture of the mind may be shaped by the interplay of genetic and cultural selection, possibly due in response to environmental or ecological pressures (Boyd & Richerson, 1985; Chiao & Blizinsky, 2010).
Future directions in cultural neuroscience: Contributions to understanding cognition and beyond
Much theoretical and empirical neuroimaging research shows that the mind is constructed, at least partially, from domain-specific mechanisms for processing our social and perceptual worlds. Yet, neuroimaging has recently added a new layer of complexity with the discovery that many of the psychological biases associated with developmental and genetically selected cultural influences extend to differences in neural processing, even in brain regions that are thought to support genetically determined, domain-specific mechanisms. Moving forward, behavioral and brain scientists should delve further into the mechanisms by which cultural and genetic factors shape the structure of the mind and brain and finally unite parallel strands of cultural psychological and neurobiological inquiry in pursuit of a richer scientific reality.
Acknowledgments
Funding
This work is supported in part by NIH Grants 1R21NS074017-01A1 0 and 1R13CA162843-01 to Joan Y. Chiao.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Adams RB, Rule NO, Franklin RG, Jr, Wang E, Stevenson MT, Yoshikawa S, Ambady N. Cross-cultural reading the mind in the eyes: An fMRI investigation. Journal of Cognitive Neuroscience. 2010;22:97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ. Culture and the evolutionary process. Chicago, IL: University of Chicago Press; 1985. [Google Scholar]
- Canli T, Lesch KP. Long story short: The serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Cheon BK, Im D, Harada T, Kim J, Mathur VA, Scimeca JM, Chiao JY. Cultural influences on neural basis of intergroup empathy. NeuroImage. 2011;57:642–650. doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Chiao JY. Cultural neuroscience: Visualizing culture-gene influences on brain function. In: Decety J, Cacioppo J, editors. Handbook of social neuroscience. Oxford, England: Oxford University Press; 2011. pp. 742–761. [Google Scholar]
- Chiao JY, Ambady N. Cultural neuroscience: Parsing universality and diversity across levels of analysis. In: Kitayama S, Cohen D, editors. Handbook of cultural psychology. New York, NY: Guilford Press; 2007. pp. 237–254. [Google Scholar]
- Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene (5-HTTLPR) Proceedings of the Royal Society B: Biological Sciences. 2010;277:529–537. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Cheon BK. The weirdest brains in the world. Behavioral and Brain Sciences. 2010;33:88–90. doi: 10.1017/S0140525X10000282. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Cheon BK, Pornpattananangkul N, Mrazek AJ, Blizinsky KD. Cultural neuroscience: Progress and promise. Psychological Inquiry. doi: 10.1080/1047840X.2013.752715. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito DN, Iidaka T. Neural basis of individualistic and collectivistic views of self. Human Brain Mapping. 2009;30:2813–2820. doi: 10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito DN, Iidaka T. Dynamic cultural influences on neural representations of the self. Journal of Cognitive Neuroscience. 2010;22:1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Hariri AR, Harada T, Hechtman LA, Mano Y, Komeda H, Iidaka T. Cultural influences on amygdala response to emotion. 2012 Manuscript submitted for publication. [Google Scholar]
- Chiao JY, Hariri AR, Harada T, Mano Y, Sadato N, Parrish TB, Iidaka T. Theory and methods in cultural neuroscience. Social Cognitive and Affective Neuroscience. 2010;5:356–361. doi: 10.1093/scan/nsq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Iidaka T, Gordon HL, Nogawa J, Bar M, Aminoff E, Ambady N. Cultural specificity in amygdala response to fear faces. Journal of Cognitive Neuroscience. 2008;20:2167–2174. doi: 10.1162/jocn.2008.20151. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Mathur VA, Harada T, Lipke T. Neural basis of preference for human social hierarchy versus egalitarianism. Annals of the New York Academy of Sciences. 2009;1167:174–181. doi: 10.1111/j.1749-6632.2009.04508.x. [DOI] [PubMed] [Google Scholar]
- Chudek M, Henrich J. Culture-gene coevolution, norm-psychology and the emergence of human prosociality. Trends in Cognitive Science. 2011;15:218–226. doi: 10.1016/j.tics.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Cousins S. Culture and self-perception in Japan and the United States. Journal of Personality and Social Psychology. 1989;56:124–131. [Google Scholar]
- Derntl B, Habel U, Robinson S, Windischberger C, Kryspin-Exner I, Gur RC, Moser E. Amygdala activation during recognition of emotions in a foreign ethnic group is associated with duration of stay. Social Neuroscience. 2009;4:294–307. doi: 10.1080/17470910802571633. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher CL, Thronhill R, Murray DR, Schaller M. Pathogen prevalence predicts human cross-cultural variability in individualism/collectivism. Proceedings of the Royal Society B: Biological Sciences. 2008;275:1279–1285. doi: 10.1098/rspb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor JA. The modularity of mind. Cambridge, MA: MIT Press; 1983. [Google Scholar]
- Freeman JB, Rule NO, Adams RB, Jr, Ambady N. Culture shapes mesolimbic response to signals of dominance and subordination that associates with behavior. NeuroImage. 2009;47:353–359. doi: 10.1016/j.neuroimage.2009.04.038. [DOI] [PubMed] [Google Scholar]
- Gelfand MJ, Raver JL, Nishii L, Leslie LM, Lun J, Lim BC, Yamaguchi S. Differences between tight and loose cultures: A 33-nation study. Science. 2011;332:1100–1104. doi: 10.1126/science.1197754. [DOI] [PubMed] [Google Scholar]
- Goh JO, Chee MW, Tan JC, Venkatraman V, Hebrank A, Leshikar ED, Park DC. Age and culture modulate object processing and object-scene binding in the ventral visual area. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Goh JO, Leshikar ED, Sutton BP, Tan JC, Sim SK, Hebrank AC, Park DC. Culture differences in neural processing of faces and houses in the ventral visual cortex. Social, Cognitive, and Affective Neuroscience. 2010;5:227–235. doi: 10.1093/scan/nsq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Research. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–654. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Harada T, Li Z, Chiao JY. Differential dorsal and ventral medial prefrontal representations of the implicit self modulated by individualism and collectivism: An fMRI study. Social Neuroscience. 2010;22:1–15. doi: 10.1080/17470910903374895. [DOI] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A. The weirdest people in the world. Behavioral and Brain Sciences. 2010;33:61–83. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- Hong Y, Morris MW, Chiu C, Benet-Martinez V. Multicultural minds: A dynamic constructivist approach to culture and cognition. American Psychologist. 2000;55:709–720. doi: 10.1037//0003-066x.55.7.709. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang MH. Toward a microdevelopmental, interdisciplinary approach to social emotion. Emotion Review. 2010;2:217–220. [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences, USA. 2009;106:8021–8026. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang MH, Yang X, Damasio H. Neurobiological correlates of experiencing emotion in Beijing and Los Angeles. 2012. Manuscript submitted for publication. [Google Scholar]
- Jack RE, Caldara R, Schyns PG. Internal representations reveal cultural diversity in expectations of facial expressions of emotion. Journal of Experimental Psychology: General. 2011;141:19–25. doi: 10.1037/a0023463. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proceedings of the National Academy of Sciences, USA. 2010;107:11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun M. The fusiform face area: A module in human extrastriate cortex specialized for the perception of faces. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Cohen D. Handbook of cultural psychology. New York, NY: Guilford Press; 2007. [Google Scholar]
- Kitayama S, Uskul AK. Culture, mind, and the brain: Current evidence and future directions. Annual Review of Psychology. 2011;62:419–449. doi: 10.1146/annurev-psych-120709-145357. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Glover G, Temple E. Cultural and linguistic influence on neural bases of “theory of mind”: An fMRI study with Japanese bilinguals. Brain & Language. 2006;98:210–220. doi: 10.1016/j.bandl.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion and motivation. Psychological Review. 1991;98:224–253. [Google Scholar]
- Mathur VA, Harada T, Chiao JY. Racial identification modulates default network activity for same- and other-races. Human Brain Mapping. 2011;33:1883–1893. doi: 10.1002/hbm.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. Neuro-Image. 2010;51:1468–1475. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Kawachi T, Mori T, Hirakata M, Yamada M, Komaki G. Specific brain activation in Japanese and Caucasian people to fearful faces. NeuroReport. 2005;16:133–136. doi: 10.1097/00001756-200502080-00012. [DOI] [PubMed] [Google Scholar]
- Mrazek AJ, Chiao JY, Blizinsky KD, Lun J, Gelfand MJ. Culture-gene coevolution of tightness-looseness and the serotonin transporter gene. 2012 doi: 10.1007/s40167-013-0009-x. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: >A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett RE, Miyamoto Y. The influence of culture: Holistic versus analytic perception. Trends in Cognitive Science. 2005;9:467–473. doi: 10.1016/j.tics.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Peng K, Choi I, Norenzayan A. Culture and systems of thought: Holistic vs. analytic cognition. Psychological Review. 2001;108:291–310. doi: 10.1037/0033-295x.108.2.291. [DOI] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Oyserman D, Coon HM, Kemmelmeier M. Rethinking individualism and collectivism: Evaluation of theoretical assumptions and meta-analyses. Psychological Bulletin. 2002;128:3–72. [PubMed] [Google Scholar]
- Oyserman D, Lee S. Does culture influence what and how we think? Effects of priming individualism and collectivism. Psychological Bulletin. 2008;134:311–342. doi: 10.1037/0033-2909.134.2.311. [DOI] [PubMed] [Google Scholar]
- Park D, Gutchess A. The cognitive neuroscience of aging and culture. Current Directions in Psychological Science. 2006;15:105–108. [Google Scholar]
- Ray RD, Shelton AL, Hollon NG, Matsumoto D, Frankel CB, Gross JJ, Gabrieli JD. Interdependent self-construal and neural representations of self and mother. Social Cognitive and Affective Neuroscience. 2010;5:318–323. doi: 10.1093/scan/nsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D, Yang X, Borofsky L, Immordino-Yang MH. The embodiment of emotion: Language use during the feeling of social emotions predicts cortical somatosensory activity. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss075. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, De Schonen S, Crivello F, Reutter B, Aujard Y, Mazoyer B. Neural correlates of woman face processing by 2-month-old infants. NeuroImage. 2002;15:454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience. 2009;29:8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self representation. NeuroImage. 2007;34:1310–1317. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]


