Abstract
Background
Heart failure (HF) can occur in patients with preserved (HFpEF, EF 50%) or reduced (HFrEF, EF<50%) ejection fraction (EF), but changes in EF after HF diagnosis are not well described.
Methods and Results
Among a community cohort of incident HF patients diagnosed from 1984–2009 in Olmsted County, Minnesota, we obtained all EFs assessed by echocardiography from initial HF diagnosis until death or last follow-up through March 2010. Mixed effects models fit a unique linear regression line for each person using serial EF data. Compiled results allowed estimates of the change in EF over time in HFpEF and HFrEF. Among 1233 HF patients (48.3% male, mean age 75.0 years, mean follow-up 5.1 years), 559 (45.3%) had HFpEF at diagnosis. In HFpEF, on average, EF decreased by 5.8% over 5 years (p<0.001) with greater declines in older individuals and those with coronary disease. Conversely, EF increased in HFrEF (average increase 6.9% over 5 years, p<0.001). Greater increases were noted in women, younger patients, individuals without coronary disease, and those treated with evidence-based medications. Overall, 39% of HFpEF patients had an EF<50% and 39% of HFrEF patients had an EF≥50% at some point after diagnosis. Decreases in EF over time were associated with reduced survival while increases in EF were associated with improved survival.
Conclusions
These data suggest that progressive contractile dysfunction may contribute to the pathophysiology of HFpEF. Prospective longitudinal studies are needed to confirm these observations and establish the mechanism and clinical relevance of decline in EF over time in HFpEF.
Keywords: heart failure, echocardiography, ejection fraction, community, longitudinal
Heart failure (HF) is a major worldwide public health problem, and a substantial driver of hospital admissions and resource utilization in the United States. It is a clinically-defined syndrome, and can occur in patients with preserved (HFpEF) or reduced (HFrEF) left ventricular ejection fraction (EF).
While patients with HFpEF and HFrEF can experience similar clinical signs and symptoms, there is ongoing debate as to whether these are separate pathophysiological entities. There is mounting evidence to support that they are distinct diseases. HFpEF and HFrEF tend to occur in different patient populations, as HFpEF patients are more likely to be older and female1, 2. Furthermore, they respond differently to therapies such as angiotensin receptor blockers (ARB), which have been proven to improve outcomes in HFrEF but not HFpEF3, 4. However, further information about the natural history and progression of these diseases, particularly HFpEF, is needed to better understand their pathophysiology and potential therapeutic approach.
While patients are often classified as HFpEF or HFrEF based on an EF assessment at HF diagnosis, little is known about changes in EF that occur over time in patients with HF. Clinical trials have demonstrated an improvement in EF in some patients with HFrEF in response to medical therapies such as beta blockers5, 6. However, follow-up in these patients is often limited, and there have been no data on the change in EF over time in patients with HFpEF.
In order to address these gaps in knowledge, we examined patterns of longitudinal change in EF among a cohort of incident, community HF patients from Olmsted County, MN. Further, we sought to determine if changes in EF had prognostic implications in HFpEF and HFrEF.
Methods
Study Design
This is a cohort study conducted in Olmsted County, Minnesota. The population in the county was estimated at 145,769 (2011 U.S. Census); 51% were female and 87% Caucasian. Population-based research is possible in Olmsted County as the county is relatively isolated from other centers, and there are few providers, the largest of which is the Mayo Clinic. Medical records from all sources of care for Olmsted County residents are extensively indexed and linked via the Rochester Epidemiology Project7. This framework allows patients to be followed passively using their medical record, provided they have provided Minnesota Research Authorization (>97% of residents historically provide).
Patient Identification
Olmsted County residents with a potential HF diagnosis from 1984–2009 were identified by International Classification of Diseases, Ninth Revision (ICD9) code 428 (HF). Codes are assigned based on physician diagnoses during outpatient visits or at hospital discharge. The index date was defined as the first evidence of HF in the medical record. Patients with a diagnosis of HF prior to the study period were excluded. From all patients with ICD9 code 428, a random subset was selected to undergo validation and data abstraction. Experienced nurse abstractors reviewed records to ensure each met Framingham criteria8 and had a physician’s diagnosis of HF. When this method was utilized previously, the inter-abstractor agreement was 100%9.
Echocardiography
All echocardiograms in Olmsted County through March 2010 were performed at the Mayo Clinic; no other providers offered these services. For each patient, all echocardiograms obtained from the time of HF diagnosis until death or last follow-up were obtained from the Mayo Clinic database. Patients were included in the analysis if they had an echocardiogram with EF measurement within 60 days pre- and 90 days post-HF diagnosis. Left ventricular end diastolic dimension (LVEDD) was measured by 2-dimensional echocardiography or M-mode. The Mayo approach to EF assessment is based on the echocardiographer’s collation of multiple methods of EF measurement (M-mode or 2-dimensional echocardiography using the Quinones formula from the parasternal views or by the quantitative 2-dimensional biplane volumetric Simpson method from 4- and 2-chamber views10–12) into an EF assessment quoted in the final impressions. The final EF assessment is rendered by the echocardiologist and may be based on any one of these methods or on a “visual estimate” which incorporates individual methods and any limitations which alters their validity. The mean EF in a population was similar whether obtained by M mode, biplane Simpsons or visual estimation methods13. Preserved systolic function was defined as an EF≥50%2.
Patient Baseline Characteristics
Baseline characteristics were abstracted from the medical record. Physician’s diagnosis was used to define hyperlipidemia, chronic obstructive pulmonary disease (COPD), and cerebrovascular disease. Smoking status was classified as ‘current’ or ‘prior/never’. Hypertension was defined by a physician diagnosis of hypertension or systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg. Diabetes mellitus was defined by fasting blood glucose levels or use of insulin and/or oral hypoglycemic medications14. Myocardial infarction (MI) prior to HF diagnosis was defined using standard epidemiological criteria15. MI occurring after HF diagnosis was identified using hospitalization ICD9 code 410. Coronary artery disease (CAD) was defined as a prior history of MI, percutaneous coronary intervention or coronary artery bypass grafting. Body mass index (BMI) was calculated using the first outpatient height recorded and weight at HF diagnosis. Laboratory values closest to the time of HF diagnosis (and within 1 year) including hemoglobin and creatinine were obtained. Anemia was defined as hemoglobin <12mg/dL in women and <13mg/dL in men. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease equation16. The degree of comorbidity was assessed using the Charlson Comorbidity Index17. Whether patients were prescribed beta blockers, angiotensin converting enzyme inhibitors (ACE-I), or ARB at the time of initial HF diagnosis was recorded.
Death
Mortality follow-up was via the medical record through March 2010. In addition to deaths noted in clinical care, the Mayo Clinic registration office records obituaries and local death notices. Death data are also obtained quarterly from the State of Minnesota Department of Vital and Health Statistics.
Statistical Analysis
Baseline clinical variables are presented as means with standard deviations, medians with 25th and 75th percentiles (if distribution skewed), or frequencies. Characteristics between groups were compared using t tests for continuous variables and χ2 for categorical variables. Linear mixed effects regression models that fit a linear regression line for each person were used to assess the longitudinal change of EF. Results were compiled for the cohort to obtain estimates of the change in EF over time. Please see Supplementary Methods for further details of the modeling approach used. All results are presented categorically for ease of interpretation, though age and LVEDD were included as continuous variables in the model. To evaluate the prognostic significance of changes in EF over time, EF was evaluated as a time-dependent covariate using Cox proportional hazard regression models. Separate models were analyzed for patients with HFpEF and HFrEF. Missing data were minimal (≥3% per variable) with the exception of LVEDD (32% missing). Analysis was performed using SAS Version 9.2.1 (Cary, North Carolina). A p value <0.05 was used as the level of significance.
Results
In total, 1233 incident HF patients had EF measured at diagnosis and were included in the analysis. An additional 606 patients had incident HF but did not have an echocardiogram within the specified window surrounding HF diagnosis and thus were excluded. Patients without an echocardiogram were older (79.0 vs. 75.0 years, p<0.001) and more frequently female (59.9% vs. 51.7%, p=0.001) but had similar frequencies of hypertension, CAD, and diabetes. The EF quoted in the final impressions of the report was obtained by visual estimate (62%), parasternal 2D (21%), or M-mode (15%) measurements using Quiñones formula and Simpson’s biplane (2%).
EF at initial diagnosis followed a bimodal distribution, with a predominance of preserved EF in women (Figure 1). In total, 559 patients (45.3%) had HFpEF at diagnosis (Table 1). Patients with HFpEF were older and more frequently anemic but had a lower prevalence of prior smoking, MI, and diabetes compared with HFrEF patients. During a mean follow-up duration of 5.1 years, 935 (75.8%) patients died, and thus had their entire lifespan after diagnosis captured. Less than 2% of the cohort emigrated away from the community during follow-up.
Figure 1. Distribution of EF at Baseline.
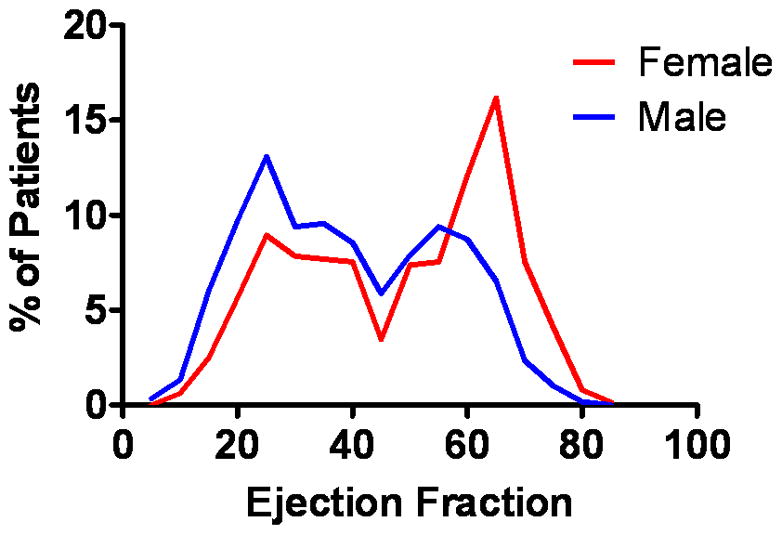
The distribution of EF (%) at incident HF diagnosis is shown for the 1233 HF patients.
Table 1.
Baseline Patient Characteristics
| N missing | Overall (n=1233) | HFpEF (n=559) | HFrEF (n=674) | |
|---|---|---|---|---|
|
|
||||
| Age (yrs) | -- | 75.0 (13.03) | 77.2 (12.28) | 73.2 (13.36)* |
| Male | -- | 596 (48.3) | 209 (37.4) | 387 (57.4)* |
| LVEDD (mm)‡ | 398 | 53 (47–59) | 48 (44–53) | 57 (52–64)* |
| Risk Factors and Comorbidities | ||||
| Hypertension | -- | 910 (73.8) | 426 (76.2) | 484 (71.8) |
| Current smoker | 7 | 189 (15.4) | 57 (10.2) | 132 (19.7)* |
| Hyperlipidemia | -- | 615 (49.9) | 276 (49.4) | 339 (50.3) |
| Diabetes mellitus | 1 | 273 (22.2) | 108 (19.4) | 165 (24.5)† |
| Body mass index (kg/m2) | -- | 27.7 (7.11) | 27.8 (7.35) | 27.5 (6.91) |
| Prior MI | 1 | 251 (20.4) | 78 (14.0) | 173 (25.7)* |
| CAD | 1 | 361 (29.3) | 128 (22.9) | 233 (34.6)* |
| COPD | -- | 274 (22.2) | 133 (23.8) | 141 (20.9) |
| Cerebrovascular disease | -- | 277 (22.5) | 117 (20.9) | 160 (23.7) |
| Anemia | 38 | 530 (44.4) | 261 (48.2) | 269 (41.2)† |
| Estimated GFR, mL/min | 17 | 54.2 (20.05) | 54.2 (20.03) | 54.2 (20.08) |
| Charlson Index ≥3 | 2 | 504 (40.9) | 219 (39.3) | 285 (42.3) |
| Number of echocardiograms | -- | 3.5 (3.3) | 3.3 (3.0) | 3.6 (3.6) |
p<0.001 compared with HFpEF,
p<0.05 compared with HFpEF;
LVEDD is listed as median (25th–75th percentile) while all others are listed as N(%) or mean (standard deviation).
The number of echocardiograms per person after HF diagnosis ranged from 1 to 30, with a median of 2 (Figure 2). As compared to patients with ≥3 echocardiograms, those with 1 or 2 echocardiograms were older (79.3 vs. 70.4 years, p<0.001), more frequently female (56.8% vs. 46.1%, p<0.001), equally likely to have hypertension and CAD but less likely to have diabetes (19.2% vs. 25.3%, p=0.011). The number of subsequent echocardiograms was similar in patients with an initial diagnosis of HFpEF or HFrEF. The mean time from initial to final EF measurement was 3.1 years, and was similar in those with HFpEF (3.0 years) and HFrEF (3.2 years, p=0.39).
Figure 2. Number of Echocardiograms Per Person After HF Diagnosis.
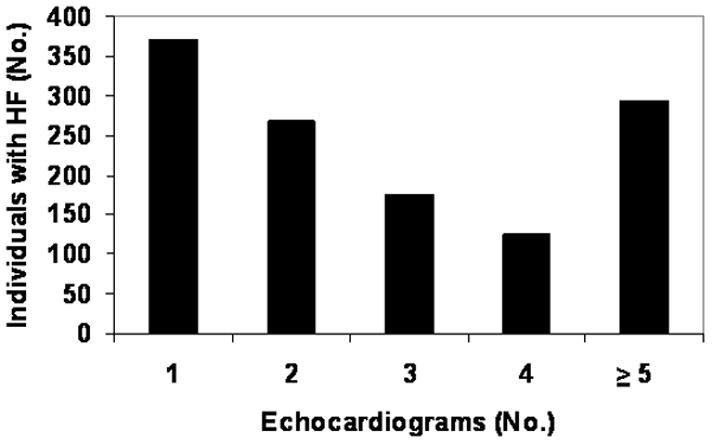
The number of echocardiograms per individual from HF diagnosis until death or last follow-up are shown.
Temporal Change in EF: HFpEF
In HFpEF, EF averaged 59.4% at diagnosis and was higher in women, elderly patients, and those with small LVEDD (Table 2). EF decreased over time with greater decreases noted in those who were older at diagnosis and those who had CAD (Table 2, Figure 3, Supplementary Results Figure). The pattern of change in EF over time was statistically different in HFpEF vs. HFrEF (p value for interaction <0.001). In HFpEF, the change in EF over time did not differ by sex, according to baseline LVEDD, or use of medications at diagnosis. Only a small number of patients (n=36) had an MI after HF diagnosis. Excluding these patients from analysis, the decline in EF for the HFpEF population was similar (estimated 5.5% decline over 5 years). The decline in EF over time was tatistically significant both in patients with an EF of 50–69% and ≥70% at diagnosis. To ensure that changes in the method used to measure EF over time did not impact results, a sensitivity analysis was performed restricting to patients who had EF assessments using methods other than visual estimation and the results were similar for both HFpEF and HFrEF (data not shown).
Table 2.
Change in Ejection Fraction Over Time: HFpEF
| Estimated Mean EF at HF Diagnosis (95% CI) | Estimated Change in EF Over 5 years (95% CI) | |
|---|---|---|
| Overall (n=559) | 59.4 (58.6, 60.2) | −5.8 (−7.3, −4.2) |
| p<0.001 | ||
| Sex | ||
| Men (n=209) | 56.7 (55.5, 57.9) | −6.0 (−8.2, −3.7) |
| Women (n=350) | 61.1 ( 60.1, 62.0) | −5.6 (−7.7, −3.5) |
| * p<0.001 | * p=0.79 | |
| Age | ||
| < 70 (n=131) | 58.7 (57.2, 60.3) | −4.0 (−6.7, −1.4) |
| 70–79 (n=141) | 58.7 (57.2, 60.3) | −6.0 (−8.9, −3.1) |
| ≥ 80 (n=287) | 60.2 (59.1, 61.3) | −7.4 (−10.1, −4.7) |
| * p= 0.04 | * p=0.02 | |
| Coronary artery disease | ||
| No (n=431) | 59.7 (58.9, 60.6) | −4.6 (−6.4, −2.8) |
| Yes (n=128) | 58.3 (56.7, 59.9) | −9.1 (−12.2, −6.0) |
| * p=0.11 | * p<.001 | |
| LVEDD | ||
| < Median (48mm) (n=172) | 63.0 (61.7, 64.4) | −5.1 (−8.2, −1.9) |
| ≥ Median (n=223) | 57.5 (56.4, 58.7) | −4.7 (−6.9, −2.5) |
| * p<0.001 | * p=0.62 | |
| Medication Use at Baseline | ||
| None (n=232) | 59.1 (57.8, 60.3) | −6.5 (−8.9, −4.1) |
| Beta blocker, ACE-I, ARB (n=327) | 59.6 (58.6, 60.6) | −5.3 (−7.3, −3.4) |
| * p=0.49 | * p=0.44 | |
CI=confidence interval,
p indicates significance of difference in EF or change in EF according to indicated variables
Figure 3. Change in Ejection Fraction for Patients with Preserved and Reduced Ejection Fraction.
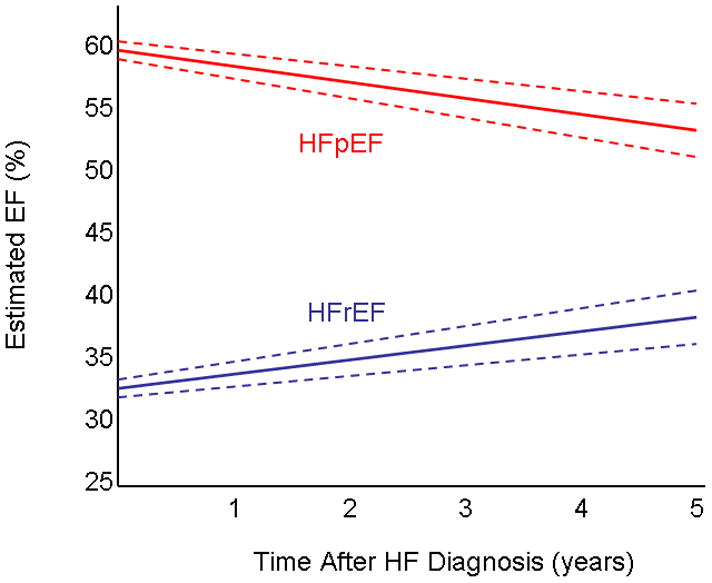
The estimated EF (solid line) and 95% CI (dashed lines) for patients who initially had HFpEF and HFrEF are shown.
In total, 38.5% of HFpEF patients had a decline in EF to <50% (range of HFrEF) during follow-up and 25.1% had a decline in EF to <40%. Among HFpEF patients with an echo around 1 year after diagnosis (n=95), 21.1% had an EF<50%. For those with an echo performed from 4–6 years after diagnosis (n=117), 32.5% had an EF<50%.
Temporal Change in EF: HFrEF
In HFrEF, EF averaged 31.7% at diagnosis, and was lower in younger men, and those with larger LVEDD (Table 3). On average, EF increased in HFrEF (Figure 3), but this differed by sex, age, CAD status, and medication use (Table 3 and Supplementary Results Figure). EF increases were greater in those who were younger at and in women but there was a significant interaction between age and sex (interaction term age*sex*time p= 0.003), so results were stratified by sex and age (Table 3). Those treated with evidence-based medications at diagnosis had a greater improvement in EF. Only a small number of patients had an MI after HF diagnosis (n=39), and excluding those patients from analysis, the increase in EF was similar (estimated 7.3% increase over 5 years). The estimated change in EF did not differ by baseline LVEDD. Sensitivity analyses performed using an EF<40% to define HFrEF yielded similar results.
Table 3.
Change in Ejection Fraction Over Time: HFrEF
| Estimated Mean EF at HF Diagnosis (95% CI) | Estimated Change in EF Over 5 years (95% CI) | |
|---|---|---|
| Overall (n=674) | 31.7 (30.9, 32.5) | 6.9 (5.4, 8.4) |
| p<0.001 | ||
| Men | ||
| < 70 (n=142) | 29.0 (27.4, 30.6) | 6.5 (4.2, 8.8) |
| 70–79 (n=128) | 30.9 (29.1, 32.6) | 4.5 (1.8, 7.1) |
| ≥ 80 (n=117) | 32.2 (30.2, 34.1) | 1.8 (−2.3, 5.9) |
| * p=0.0015 | * p=0.006 | |
| Women | ||
| < 70 (n=68) | 34.1 (31.8, 36.4) | 10.9 (8.2, 13.5) |
| 70–79 (n=89) | 33.1 (31.1, 35.2) | 8.8 (6.1, 11.6) |
| ≥ 80 (n=130) | 33.2 (31.4, 35.0) | 6.2 (2.1, 10.3) |
| * p=0.002 | * p=0.006 | |
| Coronary artery disease | ||
| No (n=441) | 31.5 (30.5, 32.5) | 9.4 (7.7, 11.1) |
| Yes (n=233) | 32.2 (30.9, 33.0) | 0.3 (−2.5, 3.1) |
| * p=0.42 | * p=0.02 | |
| LVEDD | ||
| < Median (57mm) (n=210) | 36.8 (35.5, 38.1) | 7.6 (4.8, 10.4) |
| ≥ Median (n=230) | 28.8 (27.5, 30.0) | 7.1 (4.6, 9.6) |
| * p<0.001 | * p= 0.93 | |
| Medication Use at Baseline | ||
| None (n=197) | 31.9 (30.4, 33.4) | 4.0 (1.2, 6.9) |
| Beta blocker, ACE-I, ARB (n=477) | 31.6 (30.7, 32.5) | 7.8 (6.1, 9.5) |
| * p=0.71 | * p=0.02 | |
CI=confidence interval,
p indicates significance of difference in EF or change in EF according to indicated variables
In total, 38.8% of HFrEF patients had an increase in EF to ≥50% (normal) during follow-up. Among HFrEF patients with an echocardiogram around 1 year after diagnosis (n=105), 27.6% had an EF ≥50%. For those with an echocardiogram from 4–6 years after diagnosis (n=158), 33.5% had an EF ≥50%.
Prognostic Value of Change in EF
In HFpEF, survival was better in patients with less decline in EF over time while in HFrEF, survival was better in patients with greater improvements in EF (Table 4). Among patients with HFpEF, a decline in EF of 5% was associated with a 7% increase in mortality. In patients with HFrEF, a 5% increase in EF was associated with a 12% reduction in mortality.
Table 4.
Prognostic Value of Change in Ejection Fraction Over Time
| Unadjusted HR (95% CI) for 5% Decrease EF | P value | a Adjusted HR (95% CI) for 5% Decrease EF | P value | |
|---|---|---|---|---|
| HFpEF | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.03–1.12) | <0.001 |
| HFrEF | 1.12 (1.08–1.16) | <0.001 | 1.12 (1.07–1.16) | <0.001 |
Adjusted for age, sex, and Charlson comorbidity index
Discussion
In this community population of incident HF patients, EF at HF diagnosis displayed a bimodal distribution in both sexes but with a higher prevalence of HFpEF in women. With passive longitudinal follow up, changes in EF over time differed in patients with preserved and reduced EF. In those with HFpEF, EF declined with greater reductions noted in older patients and those with significant CAD. In patients with HFrEF, EF increased with greater improvements in women, younger patients, those without CAD, and those treated with evidence-based medications. At one and five years after diagnosis, significant proportions of patients with HFpEF had a decline in EF to <50% and a similar proportion of patients with HFrEF experienced an increase in EF to ≥50%. Greater decline or less improvement in EF was associated with worse prognosis in HFpEF and HFrEF patients, respectively. While prospective longitudinal studies with serial measurement of EF are needed to confirm these observations, these data provide insight into the natural history of HF in the community. The finding that EF declines over time in HFpEF patients is particularly interesting and consistent with other studies suggesting that contractile dysfunction may contribute to the pathophysiology of HFpEF.
HFpEF vs. HFrEF
The clinical syndrome of HF occurs in patients with preserved EF in approximately 50% of cases1, 2. The prevalence of HFpEF is increasing,2 either due to a change in the clinical recognition of the syndrome or evolving risk factors and population demographics. The bimodal distribution of EF among HF patients noted here and in previous studies18, 19, suggests unique pathophysiology in the two forms of HF. Further, there are significant differences in the two populations with HFpEF patients tending to be older, more frequently female, and less likely to have CAD. Differential response to therapies has been demonstrated with clinical trials demonstrating that ACE-I or ARBs do not impact outcomes in HFpEF as they do in HFrEF3, 20 although the response to beta-adrenergic receptor or aldosterone receptor antagonists in HFpEF have not been rigorously characterized. Herein, we found a differential response to ACE/ARBs and beta blockers in patients with HFpEF vs. HFrEF. While HFrEF patients treated with these medications had a greater improvement in EF compared to those who were untreated, we found that treatment with ACE/ARBs and beta blockers had no impact on the change in EF over time in patients with HFpEF. As we characterized patients by medication treatment at initial diagnosis and did not account for longitudinal treatment changes, we cannot fully evaluate the impact that medical therapy has had on the changes in EF observed. Furthermore, a greater proportion of patients with HFrEF vs. HFpEF were treated with these medications, which may have influenced observed results.
Change in EF Over Time in HFpEF
On average, EF declined over time in patients with HFpEF with greater decreases noted in those who were older and had CAD. Interestingly, a large proportion (39%) of patients who were categorized as HFpEF at diagnosis, had an EF decline during follow-up that placed them in the range of HFrEF (EF<50%). HFpEF is commonly thought to represent maladaptive age- and hypertension-related remodeling which results in diastolic dysfunction, elevation of filling pressures and limitations in resting or exercise cardiac output.18 However, recent studies have reported that subtle impairments in myocardial contractility and systolic reserve also exist in patients with HFpEF.21, 22 Some human and animal studies suggest the potential for a transition from pressure overload induced concentric hypertrophy to systolic dysfunction23–25. While interim MI has been invoked to explain the progression from HFpEF to HFrEF26, excluding patients with clinically-apparent interim MI yielded similar results, suggesting other mechanisms. Neurohormonal activation has not been extensively characterized in HFpEF, but the limited studies available suggest that activation of the adrenergic and renin-angiotensin-aldosterone (RAAS) systems occur in HFpEF27–29 and may contribute to progressive remodeling and contractile dysfunction. However, the lack of response to ACE/ARBs and beta blockers observed would underscore that the mechanisms of progressive systolic dysfunction in HFpEF remain unclear.
While progressive contractile dysfunction may represent a fundamental component of the pathogenesis of HFpEF, altered loading conditions, tachycardia, sepsis, development of valvular disease, infiltrative processes or cardio-toxin ingestion may have contributed to systolic dysfunction in some patients. However, as patients with HFrEF were also elderly and burdened with comorbidities, the divergent trends in changes in EF in the two forms of HF may suggest unique mechanisms but underscore the need for further studies investigating the pathogenesis of these changes.
Change in EF Over Time in HFrEF
In contrast to HFpEF patients, on average, EF improved in patients with HFrEF although patients with CAD had no significant change in EF over time. Spontaneous improvement in EF in patients with profound systolic dysfunction has been reported30. In trial populations with HFrEF, adding beta blockers and RAAS antagonists resulted in between 4–8% improvements in EF within the first year after treatment5, 6. Some30–32, but not all33, studies have suggested greater improvements in EF in non-ischemic dilated cardiomyopathy, presumably reflecting a greater extent of viable, non-fibrotic myocardium and a greater degree of adrenergic activation34 compared to those with an ischemic etiology. Herein, women, particularly younger women, had a greater improvement in EF compared with men. As women and the elderly have historically been severely underrepresented in HF clinical trials35, few data exist to inform us on differential changes in EF by age or sex in HFrEF. While women with HF have been demonstrated in some settings to have lower risk for cardiovascular hospitalization and death, the mechanisms for these differential outcomes are unexplained. Sex-related differences in cardiac remodeling and the protective effects of estrogen on apoptosis may be among the explanations for differential improvement in EF and clinical outcomes36.
Change in EF and Prognosis
In patients with HFrEF, lower EF may be associated with worse prognosis.37 However, in most community cohorts, overall survival is similar in patients with HFpEF and HFrEF1, 38 despite the normal EF in HFpEF patients. Little is known about how changes in EF over time correspond to prognosis, particularly in HFpEF patients.
Our findings indicate that a decline in EF is associated with an increase in mortality in HFpEF, a finding consistent with the observation that subtle impairment in resting myocardial contractility (assessed with stress corrected midwall fiber shortening) was associated with increased mortality in a separate HFpEF cohort21. Given the reliance on clinically-obtained echocardiograms, confirmation in prospective longitudinal studies is needed.
While the current study design precludes the ability to relate changes in EF to therapy, we speculate that much of the improvement or lack of progressive impairment in EF over time in patients with HFrEF may reflect appropriate HF therapy. In HFrEF, short-term improvements in EF in response to therapy have been associated with improved survival31, 32. However, as clinical trial patients are frequently highly selected35 and therefore differ from patients in the community, it is difficult to extrapolate results from clinical trials to community patients. Thus, the current observations provide unique insight into the natural history of HFrEF in the community, albeit during a period where therapies and clinical practice were evolving.
Limitations and Strengths
All echocardiograms were obtained at the discretion of the patient’s providers rather than at pre-specified intervals. This is a source of potential bias with EF assessment being influenced by clinical status, age, provider and therapeutic era. Patients excluded from the study due to a lack of an echocardiogram at HF diagnosis were older and more often female, a population who would be more likely to have HFpEF. We must consider whether our findings regarding the change in EF over time represent regression to the mean, as EF improved for those with HFrEF and declined for those with HFpEF. However, divergent patterns of change according to age, sex, CAD and medication use make regression to the mean a less plausible explanation. Furthermore, the average EF in the general adult (>45 years) population is 64%39, which is already higher than the observed EF at diagnosis in HFpEF (59%), such that further declines over time would be moving further from the mean. The prevalence of significant CAD or frequency of interim MI may be underestimated by the diagnostic criteria utilized. These data do not provide information on the mechanism(s) responsible for the decline in EF in patients with HFpEF, which are likely diverse and potentially different from the mechanisms for systolic dysfunction in HFrEF. While it would be interesting to understand whether differences in cardiovascular, rather than all-cause, mortality exist according to change in EF over time, we do not have access to data on cause of death. Olmsted County remains a largely Caucasian community; findings may differ in populations of varying racial and ethnic composition. Several strengths should also be acknowledged. This is a large community population of patients who have been followed longitudinally from the time of incident, validated, HF diagnosis, often until death. Further, all echocardiograms have been performed at a single echocardiography laboratory which has followed strict standards of practice such that EF assessment likely has high internal validity.
Conclusions
EF changes differentially for HF patients with preserved and reduced EF at diagnosis. The observation that EF declines over time in HFpEF may suggest that progressive contractile dysfunction or unique remodeling contribute to the pathophysiology of HFpEF and thus, may represent therapeutic targets. However, prospective longitudinal studies are needed to confirm these observations and if confirmed, establish the mechanism and clinical relevance of decline in EF over time in HFpEF patients.
Supplementary Material
COMMENTARY.
Heart failure (HF) can occur in patients with preserved (HFpEF) and reduced (HFrEF) ejection fraction (EF). These may represent distinct diseases, as they occur in different patient populations, and respond differently to therapies. Among 1233 community HF patients followed longitudinally, we found that changes in EF over time also differed in patients with HFpEF vs. HFrEF. In those with HFpEF, EF declined with greater reductions noted in older patients and those with significant coronary artery disease. In patients with HFrEF, EF increased with greater improvements in women, younger patients, those without coronary artery disease, and those treated with evidence-based medications. Greater decline or less improvement in EF was associated with worse prognosis in HFpEF and HFrEF patients, respectively. These findings suggest that progressive contractile dysfunction may contribute to the pathophysiology of HFpEF. The clinical implications of these findings are two-fold. First, when evaluating patients with HFpEF, it is important to recognize that progressive decline in EF can occur, and may be associated with worse prognosis. Second, HFpEF and HFrEF, while sharing similar clinical manifestations, represent distinct pathophysiological entities and require differential approaches to care.
Acknowledgments
Sources of Funding
This study was supported by grants from the NIH (RO1HL72435), and was made possible by the Rochester Epidemiology Project (R01-AR30582). Dr Redfield is supported by HL76611 and HL110262.
Footnotes
Disclosures
None.
References
- 1.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. New Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 4.Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, Weingarten SR. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med. 2004;141:693–704. doi: 10.7326/0003-4819-141-9-200411020-00011. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Antonopoulos GV, Berlin JA, Chittams J, Konstam MA, Udelson JE. Comparative effects of carvedilol and metoprolol on left ventricular ejection fraction in heart failure: results of a meta-analysis. Am Heart J. 2001;141:899–907. doi: 10.1067/mhj.2001.115584. [DOI] [PubMed] [Google Scholar]
- 6.van Campen LC, Visser FC, Visser CA. Ejection fraction improvement by beta-blocker treatment in patients with heart failure: an analysis of studies published in the literature. J Cardiovasc Pharmacol. 1998;32 (Suppl 1):S31–35. doi: 10.1097/00005344-199800003-00006. [DOI] [PubMed] [Google Scholar]
- 7.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 8.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. New Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 10.Amico AF, Lichtenberg GS, Reisner SA, Stone CK, Schwartz RG, Meltzer RS. Superiority of visual versus computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am Heart J. 1989;118:1259–1265. doi: 10.1016/0002-8703(89)90018-5. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Garber A, Goldberg R, Havas S, Holman R, Lamendola C, Howard WJ, Savage P, Sowers J, Vega GL. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group IV: lifestyle and medical management of risk factors. Circulation. 2002;105:e153–158. doi: 10.1161/01.cir.0000014022.85836.96. [DOI] [PubMed] [Google Scholar]
- 15.Roger VL, Killian J, Henkel M, Weston SA, Goraya TY, Yawn BP, Kottke TE, Frye RL, Jacobsen SJ. Coronary disease surveillance in Olmsted County objectives and methodology. J Clin Epidemiol. 2002;55:593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2013. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaasch WH, Delorey DE, Kueffer FJ, Zile MR. Distribution of left ventricular ejection fraction in patients with ischemic and hypertensive heart disease and chronic heart failure. Am J Cardiol. 2009;104:1413–1415. doi: 10.1016/j.amjcard.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 21.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia EH, Perna ER, Farias EF, Obregon RO, Macin SM, Parras JI, Aguero MA, Moratorio DA, Pitzus AE, Tassano EA, Rodriguez L. Reduced systolic performance by tissue Doppler in patients with preserved and abnormal ejection fraction: new insights in chronic heart failure. Int J Cardiol. 2006;108:181–188. doi: 10.1016/j.ijcard.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Boluyt MO, Bing OH, Lakatta EG. The ageing spontaneously hypertensive rat as a model of the transition from stable compensated hypertrophy to heart failure. Eur Heart J. 1995;16(Suppl N):19–30. doi: 10.1093/eurheartj/16.suppl_n.19. [DOI] [PubMed] [Google Scholar]
- 24.Desai RV, Ahmed MI, Mujib M, Aban IB, Zile MR, Ahmed A. Natural history of concentric left ventricular geometry in community-dwelling older adults without heart failure during seven years of follow-up. Am J Cardiol. 2011;107:321–324. doi: 10.1016/j.amjcard.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 26.El-Gharbawy AH, Nadig VS, Kotchen JM, Grim CE, Sagar KB, Kaldunski M, Hamet P, Pausova Z, Gaudet D, Gossard F, Kotchen TA. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension. 2001;37:845–850. doi: 10.1161/01.hyp.37.3.845. [DOI] [PubMed] [Google Scholar]
- 27.Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Stork S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 28.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 29.Bishu KG, Borlaug BA, Chen HH, LeWinter MM, Deswal A, Semigran MJ, Lewis GD, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Humoral Activation in Decompensated Heart Failure with Preserved or Reduced Ejection Fraction. Circulation. 2010;122:A12658. doi: 10.1016/j.ahj.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis GS, Johnson TH, Ziesche S, Berg M, Boosalis P, Cohn JN. Marked spontaneous improvement in ejection fraction in patients with congestive heart failure. Am J Med. 1990;89:303–307. doi: 10.1016/0002-9343(90)90342-b. [DOI] [PubMed] [Google Scholar]
- 31.de Groote P, Delour P, Mouquet F, Lamblin N, Dagorn J, Hennebert O, Le Tourneau T, Foucher-Hossein C, Verkindere C, Bauters C. The effects of beta-blockers in patients with stable chronic heart failure. Predictors of left ventricular ejection fraction improvement and impact on prognosis. Am Heart J. 2007;154:589–595. doi: 10.1016/j.ahj.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Metra M, Nodari S, Parrinello G, Giubbini R, Manca C, Dei Cas L. Marked improvement in left ventricular ejection fraction during long-term beta-blockade in patients with chronic heart failure: clinical correlates and prognostic significance. Am Heart J. 2003;145:292–299. doi: 10.1067/mhj.2003.105. [DOI] [PubMed] [Google Scholar]
- 33.Maurer MS, Sackner-Bernstein JD, El-Khoury Rumbarger L, Yushak M, King DL, Burkhoff D. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail. 2009;2:189–196. doi: 10.1161/CIRCHEARTFAILURE.108.806240. [DOI] [PubMed] [Google Scholar]
- 34.Bristow MR, Anderson FL, Port JD, Skerl L, Hershberger RE, Larrabee P, O’Connell JB, Renlund DG, Volkman K, Murray J. Differences in beta-adrenergic neuroeffector mechanisms in ischemic versus idiopathic dilated cardiomyopathy. Circulation. 1991;84:1024–1039. doi: 10.1161/01.cir.84.3.1024. [DOI] [PubMed] [Google Scholar]
- 35.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 36.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–1065. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 37.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. New Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 39.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


