Abstract
The macrophage (MΦ) has been the focus of causality, research, and therapy of Gaucher disease, but recent evidence casts doubt its solitary role in the disease pathogenesis. The excess of glucosylceramide (GC) in such cells accounts for some of the disease manifestations. Evidence of increased expression of C-C and C-X-C chemokines (i.e., CCL2,CXCL1, CXCL8) in Gaucher disease could be critical for monocytes (MOs) transformation to inflammatory subsets of (MΦs) and dendritic cells (DCs) as well as neutrophil (PMNs) recruitment to visceral organs. These immune responses could be essential for activation of T- and B-cell subsets, and the induction of numerous cytokines and chemokines that participate in the initiation and propagation of the molecular pathogenesis of Gaucher disease. The association of Gaucher disease with a variety of cellular and humoral immune responses is reviewed here to provide a potential foundation for expanding the complex pathophysiology of Gaucher disease.
Keywords: Monocyte (MO), Macrophage (MΦ), Neutrophil (PMN), Dendritic cell (DC), T cell, B cell, Cytokine, Chemokine, Antibody
I. INTRODUCTION
Gaucher disease results from mutations in the glucosidase, beta, acid1(GBA1) that cause functional disruption of the encoded lysosomal enzyme, acid β-glucosidase (β-D-glucosyl-N-acylsphingosine glucohydrolase (EC 4.2.1.25; GCase). The consequent excess accumulation of glucosylceramide (GC) and glucosylsphingosine (GS) in lysosomes is central to the disease pathogenesis, with classical involvement of macrophage (MΦ) lineage cells of visceral organs, bone, and brain of humans and other vertebrates with Gaucher disease.1–8 The clinical classification, epidemiology, and other aspects of Gaucher disease are available in detailed reviews.6,8 All variants of Gaucher disease have shown involvement of monocytes (MOs),9 macrophages (MΦs),10 dendritic cells (DCs)11, T cells,9,11 and B cells. Also, B-cell or plasma cell malignancies have increased frequencies in affected individuals as do hypergammaglobulinemia (IgA, IgM, and IgG) and plasmacytosis.10,12 In addition, many cytokines and chemokines are increased (Table 1). Here, the spectrum of immunological cell types and molecules involved in Gaucher disease is presented as a basis for understanding the complex evolution of the disease phenotypes (Figure 1).
TABLE 1.
C-C and C-X-C chemokines in Gaucher disease
| Cytokines/chemokines | Full Name | References |
|---|---|---|
| IFN-γ | Interferon gamma | (26, 257) |
| TNFα | Tumor necrosis factor-alpha | (26, 254, 258) |
| IL-1α | Interleukin-1 alpha | (254) |
| IL-1β | Interleukin-1 Beta | (254, 259, 260) |
| IL-1Ra | IL-1 receptor antagonist | (26, 259, 261) |
| sIL-2R | IL-1a soluble IL-2 receptor | (259) |
| IL-4 | Interleukin-4 | (26) |
| IL-6 | Interleukin-6 | (26, 254, 257, 259, 262) |
| IL-10 | Interleukin-10 | (167, 257, 262) |
| IL-18 | Interleukin-18 | (263) |
| TGF-β1 | Transforming growth factor-beta1 | (263) |
| HGF | Hepatocyte growth factor | (167) |
| M-CSF | Macrophage colony-stimulating factor | (46) |
| G-CSF | Granulocyte colony stimulating factor | (257) |
| MCP-1/CCL2 | Monocyte chemotactic protein-1 or Chemokine, (C-C motif) ligand 2 | (26, 257, 261) |
| MIP-1α/CCL3 | Macrophage-inflammatory protein-1alfa or Chemokine (C-C motif) ligand 3 | (26, 261, 264) |
| MIP-1β/CCL4 | Macrophage-inflammatory protein-1beta or Chemokine (C-C motif) ligand 4 | (261, 264) |
| CCL6 | Chemokine (C-C motif) ligand 6 | (26) |
| CCL9 | Chemokine (C-C motif) ligand 9 | (26) |
| CCL17 | Chemokine (C-C motif) ligand 17 | (26) |
| PARC/CCL18 | Pulmonary and Activation Regulated Chemokine or Chemokine (C-C motif) ligand 18 | (167, 261, 265) |
| CCL22 | Chemokine (C-C motif) ligand 22 | (26) |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 | (26) |
| IL-8/CXCL8 | Interleukin-8 or Chemokine (C-X-C motif) ligand 8 | (46, 257, 261) |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 | (26) |
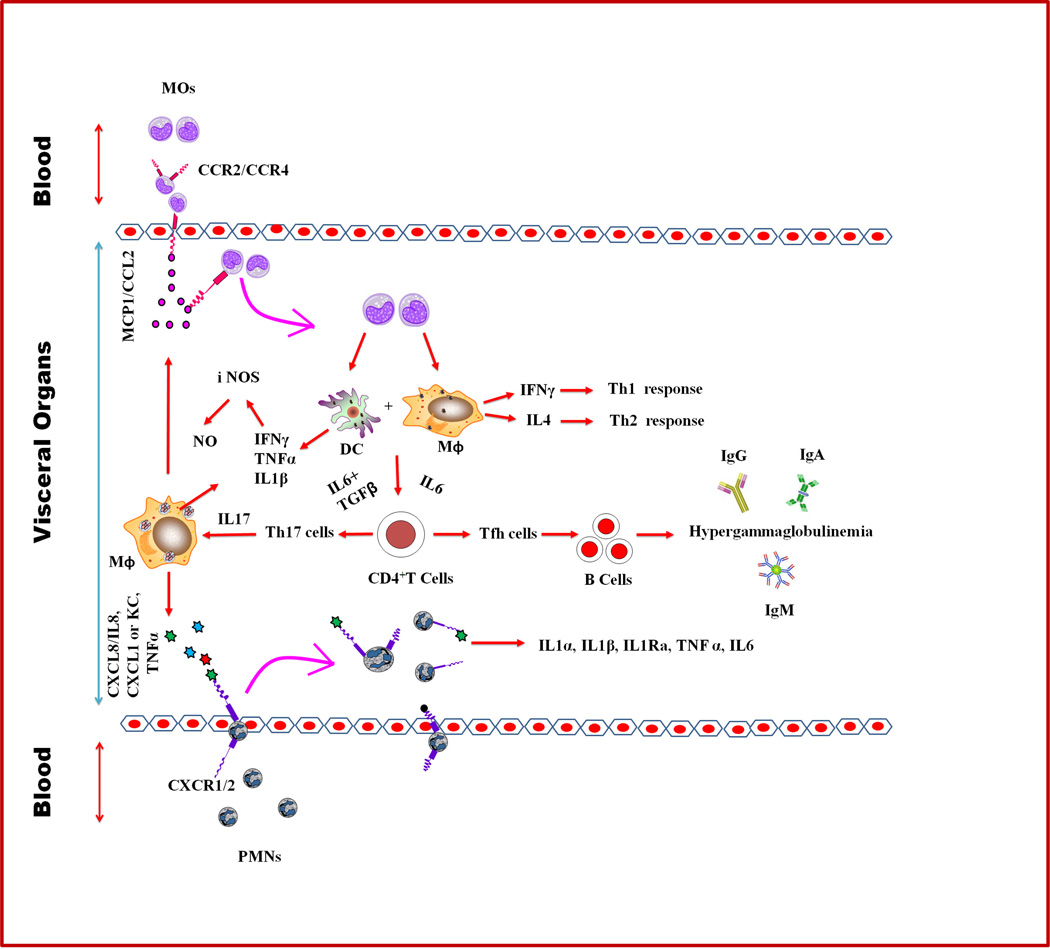
FIGURE 1. A schematic for a model of inflammatory propagation of Gaucher.
MΦ activation due to excess of glucosylceramide (GC) could trigger the release of C-C chemokines, e.g., monocyte chemoat-tractant protein-1(MCP1) or CC chemokine ligand-2 (CCL2),which cause the recruitment of blood MOs into the different visceral organs. These cells then mature into MΦs and DCs subsets. Because of the GCase defects in these cells, excess of GC accumulates and in turn activates the release of interferon-γ (IFN-γ), interleukin-4 (IL-4), IL-6, and transforming growth factor-β (TGF-β). The cytokines IFN-γ and Il-4 cause the development of T helper-1 (Th1) and Th2 cell-mediated responses, whereas IL-6 facilitates the development of follicular T cells (Tfh). These responses lead to the formation and activation of the germinal center that triggers B-cell differentiation and immunoglobulin (IgG, IgA, and IgM) production, and hypergammaglobulinemia. IL-6 together with TGF-β impact Th17 cell development, which induces the production of IL-17 and subsequently the production of CXCL8/IL8 to recruit blood PMNs into Gaucher disease visceral organs. In addition to CXCL8/IL8, GC-engorged MΦs also secrete KC/CXCL1, IL-1β, IFN-γ, and TNF-α, which are critical for the recruitment of PMNs and release of their activation products (e.g., TNF-α, IL-6, IL-1α, IL-1β, and IL-1Ra) into the visceral organs. Also, TNF-α together with IFN-γ and IL-1β induce iNOS followed by the production of NO to trigger immunological inflammation in Gaucher disease.
II. MONOCYTES (MOS)
MOs are blood mononuclear cells with bean-shaped nuclei that are present in the blood, bone marrow, and spleen; they do not proliferate in a steady state.13,14 Upon activation, they migrate from blood into tissues, elaborate inflammatory cytokines, and differentiate into inflammatory DCs or MΦs.13,15 MOs are defined by the expression of CD11b, CD11c, and CD14 in humans and by CD11b and F4/80 in mice. They lack markers of B-cells, T-cells, NK cells, and DCs.16,17
Based on differential expression of Gr1/Ly6C, MOs are classified into Gr1−/Ly-6Clow and Gr1+/Ly-6Chigh subsets. Gr1−/Ly-6ClowMOs represent a functionally distinct subset, which is characterized by CX3CR1high LFA-1high CCR2-L, and causes angiogenesis.17–19 The Gr1+/Ly-6Chigh MOs are characterized by CX3CR1low CCR2+ and are considered as inflammatory. They differentiate into MΦ and DC subsets that are critical for immune responses.15,20–22
Circulatory MOs in patients with Gaucher disease showed significant suppression of superoxide generation upon stimulation with phorbol 12-myristate 13-acetate (PMA), opsonized zymosan, and formyl-methionyl-leucylphenylalanine (FMLP) as well as diminished potential for staphylococcal killing, and phagocytosis.23 In contrast, up-regulation of the major histocompatibility complex-II (MHCII) and CD1d molecules in Gaucher disease MOs cause increased activation of CD4+T cells.9,24 GCase treatment improved some of these MOs functional activities and reduced the numbers of peripheral blood CD4+ T-cells in patients with Gaucher disease24,25 and verified a contribution to its pathophysiology.23,25 Significant increases in gene and protein expression of MOs chemo-attracting protein-1(MCP-1) or CC-chemokine ligand 2 (CCL2) were found in the lung tissue of Gba1 point-mutated mice26 and other models as well as patients with Gaucher disease (Table 1). These could be critical for tissue recruitment of inflammatory MOs and their differentiation into DCs and classically activated MΦs.21,22,27–29 However, combining FACS staining with antibodies to Gr1, Ly-6C, CX3CR1, and CCR2 could reveal, with further investigation, clear subset identification and specific functions in Gaucher disease. Additionally, IFNγ, IL-4, and MCSF (Table 1), which are critical for differentiation of MOs to MΦ and DC subsets,17,30–32 had increased expression and could facilitate the MOs transformation to inflammatory subsets of MΦs and DCs in Gaucher disease.
III. MACROPHAGES (MΦS)
Elie Metchnikoff classified phagocytes into MΦs (large eaters) and microphages (a smaller type of phagocytic cell), and the polymorphonuclear leukocyte now known as granulocytes. He argued that both types of phagocytes played an important role in host resistance against infections.33
Metchnikoff also recognized the close relationship between mononuclear phagocytic cells in the spleen, lymph nodes, bone marrow, and connective tissues, leading him to introduce the term MΦ system.34 Karl Albert Ludwig Aschoff, a German physician and pathologist, developed this concept further and grouped several cell types into the reticuloendothelial system and, subsequently, the reticulo-histiocyte system. This system encompassed the reticular cells or fixed MΦs of the spleen and lymph nodes, endothelial cells of the lymph and blood sinuses, MOs, and histiocytes, (a term used for “tissue wandering” as opposed to “fixed” MΦs). In addition to these tissues, MΦs also populate the brain as infiltrating microglial cells. MΦ functions shared by most tissues include high phagocytic function and degradative potential, allowing them to clear foreign and damaged cells.27 MΦs also participate in the induction of innate immunity in response to tissue infection, which plays a critical role in the killing of micro-organisms and processing their pathogenic factors.27 MΦs also load extracellular antigens in MHC class II compartments, but primarily interact with effector CD4+ T-cells and are less efficient than DCs at priming naïve T-cells.
MΦs have been classified into two main groups, M1 and M2. M1 MΦs are characterized by increased expression of interleukins IL-12 and IL-23, with decreased expression of IL-10, reactive oxygen species (ROS), nitrogen species, and other Th1-type inflammatory cytokines. M1 MΦs are critical for the clearance of microbial infections and necrotic cell death remnants.13,35 M2 MΦs have increased expression of IL-1ra, decoy IL-1 type II receptor, IL-4, IL-10, IL-13, scavenger receptors, and mannose and galactose-type receptors, immune complexes, gluco-corticoid hormones, arginase I, resistin-like molecule alpha (RELMα), and Ym1/2, a chitinase. M2 MΦs have decreased expression of IL-1β, caspase1, IL-12, and IL-23. M2 MΦs are involved in T-helper 2 (Th2) response immunoregulatory functions, encapsulation and containment of parasites, tissue repair, remodeling, and tumor progression.36–39 Inflammatory reactions involving M2 MΦ, e.g., a response to parasitic or helminth invasion, “type 2” inflammation, results in the accumulation of large numbers of these cells in the affected tissues.13,39–43
The delineation of tissue-specific MΦ cell markers make this classification inadequate for understanding the functional differences among and between MΦs from various organs or regions. Several of these MΦ populations in different organs are detailed in Table 2. Clearly, the multitude of differences among MΦs in tissues highlights the need to examine their differential functions that lead their specific involvement in Gaucher disease.
TABLE 2.
Specific Phenotypes of Macrophages (MΦs) in Different Tissues.
| Organ | Location and types | Markers |
|---|---|---|
| Liver (266–268) | Sinusoids Kupffer cells | F4/80highCD11blowCD169+ CD68+Mac-2+ |
| Spleen (269–273) | Red pulp MΦs | F4/80high CD11blow CD169low MHCIIlowD163+CD68+CD115+CD172a+ |
| Marginal zone MΦs | F4/80−SIGN-R1+MARCO+ | |
| Marginal zone Metalophilic MΦs, | F4/80−CD169+ | |
| Tingible body MΦs | F4/80−CD11b−CD68+MFG-E8+ | |
| Lung (267, 274–278) | Alveolar MΦs | F4/80+CD11blowCD169+CD11chighCD68+SiglecF+ MARCO+Mac-2+ |
| Alveolar interstitialMΦs | CD11c−F480+CD68+MHCII+ | |
| Lymph Node (279, 280) | Subcapsular sinus MΦs | F4/80lowCD11b+CD169+CD11low |
| Medullary MΦs | F4/80high CD11b+CD169+CD11clow | |
| Boundary between the sinus and the T cell zone | CD11chighCD169+MΦ | |
| B cell follicle MΦs | MHCII+F4/80+CD169+CD11chighCD8+ | |
| Germinal centertingible body MΦs | F4/80−CD11b−CD68+MFG-E8+ | |
| Gut (281, 282) | Lamina propria MΦs, | MHCII+F4/80+CD11b+CD11c+CD103−CD115+CX3CR1+CD172a+ |
| Muscular layer and serosa MΦs | MHCIIhighF4/80+CD11b+CD169+CD11clowCX3CR1+CD103−CD115+ | |
| Bone (283–285) | Bone marrow MΦs | CD169+F4/80+CD11blowCD169+CD11clowCD68+CX3CR1−CD115+ |
| Pro-osteoclasts | CD11b−F4/80 | |
| Pre-osteoclasts | CD11b−F4/80lowlow | |
| Mature osteoclasts | CD11b+F4/80medium | |
| Thymus (286) | Germinal center subcapsular MΦs, | MHCII+F4/80+Mac-2−FcγRII+FcγRIII+ |
| Germinal center cortex MΦs | MHCII−F4/80+Mac-2+FcγRII+FcγRIII+ | |
| Germinal center cortico-medullaryMΦs, | MHCII+F4/80+Mac-2+ FcγRIIhighFcγRIIIhigh | |
| Germinal center medullar MΦs | MHCIIhighF4/80+Mac-2+ FcγRII+FcγRIII+ | |
| Skin (287, 288) | Dermal MΦs | F4/80+CD11b+CD11clowmMGL+CD206+MHCIIlowCD169+ |
| Peritoneal lavage (289) | Peritoneal MΦs | CD11bhigh F4/80high CD11c− |
| Brain (290) | Microglial cells | CD11b+CD14+CSFR1+CX3CR1+CD172+CD200+CD45+ |
MΦs express a broad range of pathogen-recognition receptors induced within the microbial and cytokine milieu, which drives them to specialized and polarized functions.36,44,45 Interferon-gamma (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor (TNF-α), and microbial products (lipopolysaccharide) elicit a M1 MΦ activation to trigger extensive pro-inflammatory responses required to kill intracellular pathogens.
In Gaucher disease, MΦs have been functionally and numerically assigned as the dominant disease effectors. These cells, which become engorged with un-degraded GC, are present in nearly all visceral tissues,26,46 and are termed Gaucher cells. They contain twisted and flat layers of tubular-like structures of GC that are a hallmark of Gaucher disease. These tubular structures are bilayered membrane-like inclusions containing phospholipids and large amounts of GC.8,47 By immunohistochemistry, splenic Gaucher cells are positive for CD163, CD14, chitotriosidase, CD68, and HLA II, but negative for CD11b. Such cells resemble those in red pulp and in the tingible body48 (Table 2). These findings indicate that the splenic MΦs in Gaucher disease have M2 characteristics. Also, similar MΦs were positive for Mac3, CD68, and F4/80 in several tissues of mice with Gba1 mutations;7,26,49 these findings indicate their activated state in Gaucher disease.
The high degree of CD68+ and F4/80+positive Gaucher cells in the lung tissue of Gba1 point-mutated mice suggest that these cells are alveolar macrophages, but a more refined characterization that includes CD11b, CD169, and MHCII would facilitate subset assignment to the alveolar interstitium or airspaces (Table 2). Global gene expression profiling in the MΦs enriched lung tissue of such mice showed alterations in ~0.9–3% of all genes. The genes associated with MΦs activation and immune response were significantly enriched, e.g., INF-γ network (CCL2, CCL3, CCL9, NOS2, TNF, and IL-6) and IL-4 networks (CD163 and MMP12).26 These findings implicate M1 and M2 MΦs, but in addition, storage cells were present that had characteristic markers for both M1 and M2 MΦs. Such findings suggest a disruption in the differentiation and subset formation of MΦs in Gaucher disease that may be tissue specific. The complete characterization of the tissue specificity of the M1/M2 subsets of MΦs will require the use of purified cells and in vitro analyses to dissect the tissue-specific effects of Gaucher disease.
IV. NEUTROPHILS (PMNS)
PMNs or polymorphonuclear neutrophils are the most abundant white blood cells normally found in the blood, bone marrow, and areas of acute inflammation. PMNs are Gr1high and CD11b+ cells,50 which influence adaptive immune responses through pathogen shuttling to draining lymph nodes,51,52 antigen presentation,53 and modulation of Th1/Th2 responses.54 In addition to their primary role as professional phagocytes, PMNs express a variety of cytokines and chemokines in response to physiological stimuli,55 and they play an important role in inflammatory and immune reactions.56–64 PMNs are classically first-responders to inflammation. They are recruited to local regions from the blood and are mobilized from bone marrow.65–67 Circulating PMNs are signaled to migrate into the interstitial spaces by chemo-attractants, including leukotriene B4 (LTB4), CXC chemokines, CXCL1 or KC, CXCL2 or MIP-2, IL-8 or CXCL8, C5a, IL-1, CXCL12 or stromal cell derived factor-1(SDF-1).68–71
Some studies have found increased plasma levels of C-X-C chemokines (CXCL1/KC and CXCL8/IL8), IL1α, TNFα, and MIP1α (Table 1) in Gaucher disease, which are important for activation and recruitment of PMNs.72–75 However, these have not been used to evaluate the chemotactic profiles of PMNs in Gaucher disease. Such studies would be important because decreased ability of PMNs migration and increased susceptibility to infection has been suggested in patients with Gaucher disease. One study reported that ~33% of affected patients had a significant decrease in ex vivo PMNs directed chemotaxis toward zymosan-activated serum or N-formyl-methionyl-leucyl-phenylalanine and that ~50% of patients showed defects in PMNs random migration.76 Most of the patients with impaired PMNs chemotaxis had more severe disease manifestations. Also, 33% of patients (3/9) with impaired chemotaxis suffered from recurrent pyogenic infections, whereas this type of infection was not found in 20 patients with normal PMNs function.76
Mean chemotaxis rates of granulocytes (which form part of the PMNs family together with basophils and eosinophils)77,78 were not decreased in patients with Gaucher disease, although they had normal functional assays, including superoxide generation, staphylococcal killing, and phagocytosis.23,25 These chemotaxis experiments used traditional Boyden chamber ex vivo methods79 in which cells are placed and migrate through porous membrane (3 μM) to the chamber containing a chemotactic agent. An increase in the cell number is considered as chemotactic influence. This abnormal migration of PMNs in selected patients23,25,76 and could be independent of other disease manifestations. However, the relevance of these findings to clinical infection susceptibility and/or clearance in affected patients is not clearly apparent from clinical observations. Several severe infections following orthopedic procedures and recurrent pyogenic infections have been reported in severely affected patients, but such infections do not appear to be a major disease complication in the majority of patients.
V. DENDRITIC CELLS (DCS)
Steinman and Cohn identified a population of hematopoietic cells in mouse spleen that excelled at antigen presentation and T-cell stimulation; these were named DCs because of their unique morphology. 80,81 Similar to MΦs and PMNs, DCs are antigen presenting cells (APCs) that are present in lymphoid and non-lymphoid organs.82 DCs derive from bone marrow and migrate as precursors through the circulation to become resident in tissues, such as Langerhans cells of the epidermis. After pathogen invasion, DCs get recruited to sites of inflammation.83 DCs capture the antigen,8–87 migrate to the draining lymph nodes, present extracellular antigens, and initiate tissue-specific T-cell immunity.88–94 During their migration from peripheral tissues to lymphoid organs, DCs undergo maturation with altered phenotypes and functions.90,95,96 The two DC subsets in mice are termed myeloid (mDCs) and plasmacytoid DCs (pDCs).97 mDCs are CD11c+ and precursors of Langerhans cells, and dermal interstitial DCs, which express myeloid markers, intercept invading pathogens in the periphery, and then migrate to the secondary lymphoid tissue where they present pathogen-derived peptides to antigen-specific T cells.98 In comparison, pDCs are CD11c−, lack myeloid markers, and migrate directly from blood to the secondary lymphoid tissue, where they differentiate into cells that were originally termed plasmacytoid T cells because of their extensive endoplasmic reticuli.99,100 The mDCs secrete interleukin 12 (IL-12), which drives type 1 helper T-cell immune responses associated with cellular immunity.101 The pDCs secrete lesser amounts of IL-12 but are potent producers of interferon-α (IFN-α) and may play an important role in controlling viral infections.101,102 mDCs are subdivided into CD4+ mDCs, CD8α+ mDCs, and CD8α−CD4−mDCs.103 The CD4+ mDCs do not produce cytokines, but they effectively present antigens to CD4 T-cells.104 CD8α+ mDCs perform cross-presentation of foreign antigens to CD8 T-cells and are a major producer of IL-12.104–107 CD8α−CD4−cDCs produce IFN-γ.104 mDCs and pDCs can be generated from their progenitor cells in BM cells in vitro by granulocyte macrophage colony stimulating factor (GM-CSF) and Fms-like tyrosine kinase 3 ligand (FLT3L) stimulation.108,109
In untreated Gaucher disease type 1, mDCs and pDCs as well as MOs-derived DCs were decreased in the peripheral blood, but no change from healthy controls was found in the expression of the DC-associated surface molecules (i.e., CD80, CD83, CD40, HLA-DR, and CD54). Lipopolysaccharide and TNF-α stimulated immature MOs-derived DCs from patients and healthy controls had similar degrees of up-regulation of CD83, CD80, CD54 and HLA-DR, indicating an efficient maturation of MOs-derived DCs. The endocytic and allostimulatory capacities of the immature and mature MOs-derived DCs were similar to those obtained with healthy controls.
In comparison, mice with conditionally deleted Gba1 in hematopoietic and mesenchymal cell lineages showed an increase in the inflammatory subset of mDCs [CD11b+CD11c+B220+CD4+MHCII (IA/IE)+cells] in thymus cells.11 This could be due to infiltration of MOs due to the CCL2/MCP1 chemokine26,110,111 overexpression. Mature DCs of lymphoid organs are poor at antigen capture and processing, but they are markedly efficient in priming naïve T-cells84,87,112,113 and inducing high expression of MHC class II and B7 molecules.114–116 Thus, studies are needed to evaluate the relationships between MO and DC subsets and their positivity for specific activation molecules (e.g., CD40, CD80, CD86, PDL1, and PDL2) in Gaucher disease.
DCs are activated by damage-associated molecular patterns through FcγRI, II, III, and IV,117,118 CD88,119,120 and several TLRs.121,122 However, expression levels of these molecules have not been evaluated with DCs from visceral tissues of human and murine Gaucher disease.
VI. B LYMPHOCYTES
B cells are critical for humoral immune responses, and they have APC-like functions to generate T-cell-mediated immune responses.123 Activated B cells express high levels of MHC class II and T-cell-activation-associated molecules that facilitate their effective antigen presentation to T cells.124,125 Moreover, DCs and MΦs promote antigen specific Th1 cell differentiation as well as B-cell antigen presentation that usually induces T-cell anergy and influences naïve T-cell differentiation to the anti-inflammatory Th2 phenotype.126–129 Thus, B cells have dual roles in modulating T-cell immunity and potentially contributing to the maintenance of immunological homeostasis.
B lymphocytes are classified into two subtypes: B-1 and B-2. B1 cells are present in the pleural cavities, peritoneal cavities, spleen, and Peyer’s patches of the intestine, whereas B2 cells are present in spleen and lymph nodes.130–132 B1 cells develop from self-replenishing peritoneal precursor cells and secrete natural IgM antibodies,133 whereas B2 cells (conventional B cells) develop from bone-marrow precursors,134,135 and are distributed in lymphoid organs, where they mature and lead to immunoglobulin isotype switching and differentiation into memory B cells and plasma cells.
B1 cells are characterized by increased IgM and decreased IgD, B220, and CD11b surface expression, as compared to B2 cells.130,136–138 Based on the differential expression of CD5, B-1 cells are further divided into IgMhigh IgDlowCD23−CD11b+CD43+CD5+B1a cells and IgMhighIgDlow,CD11b+CD5−B1b cells.130,137,139 B1 cells often have specificities for self-antigens, such as phosphatidylcholine,140 single-stranded DNA,141 ribonucleoprotein,142 cell-surface Thy-1 antigen,143 and rheumatoid factor.144,145 The absolute numbers of B1 cells are increased in rheumatoid arthritis,145 Sjogren’s syndrome,146 chronic inflamed gingival tissue, 147 and systemic lupus erythematosis.148
B2 B cells express intermediate levels of IgM and IgD, as well as increased and absent cell-surface B220 and CD5, respectively. B2 cells have a major role in the pathogenesis of many inflammatory and autoimmune diseases such ase rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, atherosclerosis, thyroiditis, and autoimmune diabetes.
B1 cells can develop into B-cell chronic lymphocytic leukemia149–151 and Gaucher disease has been associated with B-cell and plasma cell malignancies. 10,152–158 In addition, Gaucher disease type 1 patients develop immunoglobulin abnormalities, IgG- and IgM-specific hypergammaglobulinemia, and plasmacytosis.153,154,159–167
Enzyme therapy or splenectomy decreases the IgG and IgM levels in affected patients.162,164,165,168 However, the effects of these interventions on the reduction of specific subsets of B cells (IgMhigh IgDlowCD23−CD11b+CD43+CD5+B1a,IgMhighIgDlo w,CD11b+CD5−B1b, and B220high CD19high B2 cells) need investigation. Also, the antigens for several autoantibodies in sera of patients with Gaucher disease have been characterized as pyruvate dehydrogenease, DNA, sulfatide, and rheumatoid factor.155,169 The subsets of B cells that secrete these autoantibodies may have different effector functions and impacts on the disease pathogenesis in individual patients. The exact nature, source, and mechanism(s) of production of these autoantibodies, and their interaction with different FcγRs on MΦs, PMNs, and DCs could be important to the pathophysiology of Gaucher disease.
VII. T LYMPHOCYTES
T cells are classified into two major groups: CD4+ T-helper cells and CD8+ T-cytotoxic cells. CD4+T-helper cells are further subdivided into Th1, Th2, Th17, CD4+ CD25+ regulatory T-cells (Treg), and follicular helper T-cell (Tfh) subsets based on their cytokine production and activation by lineage-specific transcription factors: T-bet for Th1, GATA-3 for Th2, RORγt for Th17, Foxp3 for Treg, and Bcl-6 for Tfh.170–187 Th1 and Th2 cells produce the signature cytokines IFN-γ, IL-4, and IL-13, respectively, and are important for the elimination of microbial pathogens and intracellular microbes.176 Th17 cells selectively produce IL-17 cytokines.180 CD4+ CD25+ Tregs are critical for preservation of immune tolerance,177–179 where TGF-β plays a critical role.188 Tfh cells help B cells mount antibody responses to T-cell–dependent antigens, to the development of germinal centers, and to immunoglobulin class switching.182–184 CD8+ T-cells are critical for killing virally infected cells predominantly through the release of lytic proteins, mainly perforin and granzymes, which are secreted via exocytosis of preformed granules following recognition of infected targets.189–191
T-cell lymphomas occur in Gaucher patients, for example, in a 6-year-old female with concomitant neurofibromatosis type 1192 and in a 16-year-old male. The latter individual had increased expression of CD45, CD45R0, CD3, and CD15193 indicating a disruption of the T- and B-cell networks. T-cell deficiency and poor T-cell response to lectins were found in spleen tissue and peripheral blood of patients with Gaucher disease.194,195 A deficiency of CD4+ and CD8+ T-cell subsets was found in peripheral blood of 21 non-splenectomized and 10 splenectomized affected patients.196 In contrast, the thymus of mice with a conditionally deleted Gba1 gene (hematopoetic and mesenchymal cell lineage deletions) showed increased percentages of the CD4+T-cell subsets.11 Beyond the associations of increased production of IFNγ, IL-4, IL-6, TGF-β (Table1), the alterations in CD4+ T-cell subsets (Th1, Th2, Th17, Treg) and their transcription factors (T-bet, GATA -3, RORγt, Foxp3, and Bcl-6), which are critical for production of these cytokines,170–187 have not been examined in Gaucher disease.
Expression of IL-6 (Table 1) is critical for Tfh cell responses197,198 and is central to the development of fully matured germinal center B cells and the production of high-affinity antibodies.197 The association of increased IL-6 and T-and B-cell lymphomas and hypergammaglobulinemia in patients with Gaucher disease26,164,192,193 suggests the need to examine Tfh cells, including their antigen specificity for CXCR5, PD-1, CD200, inducible T-cell costimulator (ICOS), as well as the potential absence of the signaling lymphocytic activation marker (SLAM) in CD4+ T cells.197 Enhanced crosstalk between Tfh cells and B1-/B2-cell subsets could provide a mechanism for the hypergammaglobulinemia in Gaucher disease.182–184
VIII. NATURAL KILLER T (NK-T) CELLS AND THE CD1 SYSTEM
In contrast to the peptide antigen associated MHC-I/II processing through CD4+ and CD8+T cells,199–201 NK-T cells are subsets of regulatory T cells that co-express T-cell (CD3, α/βTCRs) and NKT (NK1.1)-cell surface receptors202 these T cells which recognize various lipid and glycolipid antigens through the CD1 antigen-presenting system on APCs.203–209 Five CD1 isoforms are present in humans and are classified into two groups based on sequence similarity; CD1a, -b, and -c constitute group I, and CD1d forms group II.210 CD1e represents an intermediate between the two CD1 groups and acts as a chaperone to facilitate lipid transfer onto CD1b and CD1d.211 Most of the mycobacterially derived lipids (e.g., mycolic acid, glucose-monomycolate, phosphatidylinositol mannoside,212 lipoarabinomannan, mannosyl-β-1-phosphoisoprenoid, and mannosyl-β-1-mycoketide213) bind to CD1 group 1. Mice express only two homologues of CD1d: CD1d1 and CD1d2.203 CD1d presents endogenous and exogenous lipid antigens to NKT cells in humans and mice.207,214 The most well-known subset of CD1d-restricted NKT cells uses an invariant TCRα chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans), that is, invariant NKT (iNKT) cells.215 iNKT cells rapidly secrete IFN-γ, IL-4, IL-17, and other cytokines upon TCR stimulation).215–217 Activated iNKT cells in turn activate DCs, MΦs, and NK cells, and thereby impact subsequent B- and T-cell responses.218 These interactions highlight the critical role played by iNKT cells in bridging innate and adaptive immune responses in diseases such as cancer,219 bacterial, viral, parasitic, and fungal infections, 220–222 and autoimmune diseases.223
Up-regulation of CD1d and MHC-class II was found in MOs from patients with Gaucher disease and in ex vivo MOs models induced with GCase inhibitors. Based on such observations, CD1d up-regulation was postulated to be secondary to alterations in intracellular trafficking as a consequence of excess lipid accumulation. The increased expression of MHC-class II was attributed to the patient’s inflammatory status.24 This finding was supported by the decreased chitotriosidase activity and surface presence of MHC-class II in MOs of patients with Gaucher disease who had received enzyme therapy, suggesting up-regulation of MHC-class II expression by ex vivo MOs of Gaucher patients.9,24
Sphingolipid activator proteins (SAPs) are ~10-kDa glycoproteins that have differential affinity for various glycosphingolipids. They have been postulated to facilitate membrane extraction and/or membrane structure of specific glycosphingolipids to enhance their degradation.224 In addition, of the four SAPs (A, B, C, D) that derive from a common precursor, prosaposin, SAP B has a significant ability to transfer lipids to CD1d molecules.225 Also, prosaposin-deficient mice fail to develop or stimulate invariant NKT cells,226,227 suggesting that SAPs are critical for the development of NK-T cells. The excess SAPs present in Gaucher disease have not yet been implicated in its disease-related immune system defects. Curiously, decreased numbers of NK cells are found in Gaucher disease.194
IX. CYTOKINES
Each of the CD4+T-effector lymphocytes are characterized by their production of specific cytokines that drive their effector functions.228 In addition to their signature effector cytokines (e.g., IFN-γ (TH1), IL-4 (TH2),229,230 IL-17 (TH17),172,231 IL-35,and TGF-β (Treg), IL-6 and IL-21 (Tfh)198,232), all helper T-cell subsets can produce IL-10, a cytokine with broad immunoregulatory properties.233 Th1 cells produce IFN-γ, IL-2, and TNF-α to clear intracellular pathogens and evoke cell-mediated immunity, whereas Th2 cells produce IL-4, IL-5 and IL-13 to clear extracellular organisms and evoke strong allergic responses.229,234–237 In contrast to Th1 and Th2 cell differentiation, which depends on their respective effector cytokines (IFN-γ and IL-4), Th17 cell differentiation does not require IL-17, but has a critical need for TGF-β and IL-6.238,239 IL-17 cytokines are highly concentrated in collagen-induced arthritis, multiple sclerosis, and rheumatoid arthritis.240 T (reg) cells produce IL-10, IL-35, and TGF-β to cause immune tolerance and inhibit IFN-γ synthesis241 as well as blocking T-helper cell differentiation of naïve T-cells into effector T cells.242 Tfh cells facilitate B-cell differentiation and induce IL-21 production, which is critical for immunoglobulin switching.243 Several reports show changes in gene expression, and/or serum and tissue levels of several cytokines and chemokines in human and murine Gaucher disease (Table 1). Age-dependent progressive accumulation of GC and enhanced MΦ activation and immune response genes (i.e., INFγ, TNF, IL-1ra, IL-4, IL-6, CCL2, CCL3, CCL6, CCL9, CXCL1, CXCL12, CCL17, and CCL22) have been observed in lungs and livers of viable Gba1 point-mutated mice.26 These studies suggest that GC accumulation in MΦs triggers events that contribute to the increased production of MOs and PMNs chemo-attracting proteins (e.g., MCP1/CCL2, CXCL8/IL8, KC/CXCL1) as well as IL-1β, IFNγ, and TNFα in Gaucher disease (Table 1), which could cause the recruitment of PMNs and transformation of MOs into the effector MΦs and DCs to initiate inflammatory reactions in visceral tissues of Gaucher disease.
X. NITRIC OXIDE (NO) AND REACTIVE OXYGEN SPECIES (ROS)
NO production from many cell types is an important effector for a variety of cytokines and chemokines.244 NO is produced from L-arginine by the actions of NO synthases (NOS), a family of enzymes encoded by at least three distinct genes: NOS1, NOS2, and NOS3.245,246 NOS1 is found mainly in neuronal247 and skeletal muscle cells.248 NOS3 is found mainly in endothelial cells.249 NOS2, which is also called inducible NOS (iNOS), is expressed by variety of immunological cells upon activation by IFN-γ, TNF-α, and specific chemokines.250–253 The increased levels of these cytokines and chemokines in Gaucher disease (Table 1) and Gba1 mutant mice may underlay an increased expression of the NOS2 gene,26 and NO and ROS proteins in the brain of Gba1 knockout mice.254 Such a mechanism could be highly relevant to the modulation of inflammatory and perfusion alterations observed in Gaucher disease. This concept is supported by the association of NO and the generation of ROS, including superoxide anions, hydroxyl radicals, lipid hydroperoxides, and hydrogen peroxide, which promote endothelial cell dysfunction directly or indirectly by promoting formation of lipid inflammatory mediators.255 The enhancement of inflammatory and oxidative responses by long chain fatty acids256 might implicate GC in the induction of IFN-γ, TNF-α, and IL-1β leading to enhanced iNOS expression. Clearly, such a mechanism requires direct experimental validation.
XI. PATHOGENESIS AND PROPAGATION OF GAUCHER DISEASE—A MODEL
The delineation of the numerous inflammatory and APC cell types involved in Gaucher disease pathophysiologic propagation implies a more complex disease model than that focused primarily on the MΦs. A possible pathway for this expanded propagation pathway can be proposed. The initial MΦs activation resulting from excess GC triggers a vicious cycle for the release of MOs and PMNs, attracting cytokines as well as C-C and C-X-C chemokines (Table 1). For example, MCP-1 recruits circulating MOs, whereas CXCL8/IL8, CXCL1/KC, and TNF-α are chemoattractants for PMNs migration into the different visceral organs. Once MOs enter visceral organs, they mature into a variety of tissue-specific MΦs and DCs subsets (Table 2). These cells with GCase defects lead to increasing numbers of GC-containing cells with the resultant release of additional IFN-γ, IL-4, IL-6, and TGF-β cytokines, as well as the promotion of continued development of Th1- and Th2-cell-mediated inflammation. IL-6 induces T-follicular cells (Tfh), leading to activation of B cells in germinal centers and hypergammaglobulinemia. Moreover, IL-6 and TGF-β induce Th17 cell production with subsequent IL-17 production, leading to myeloid cell release of C-X-C chemokines i.e., CXCL8/IL8, with recruitment of circulating PMNs. IL-1β, IFN-γ, and TNF-α, which are also secreted by Gaucher cell MΦs lead to the induction of iNOS/NO2 expression and the release of NO. Together, TNF-α and KC/CXCL1 leads to additional PMNs recruitment into the visceral organs (Figure 1). The detailed mechanisms of such a pathophysiology remain to be delineated. The diversity of these cell subsets in various tissues implies a similar diversity of their functions in different tissues. The translation of such diversity into the individual and tissue-specific reaction of or to GCase defects remains to be elucidated. Clearly, the mechanistic basis for the initiation and propagation of the immunological cell involvement in Gaucher disease should provide approaches to alternative therapies for this and other lipid storage diseases.
ACKNOWLEDGMENT
This work was supported by grant from the NIH (Grant No. DK 36749) to GAG.
ABBREVIATIONS
- APC
antigen presenting cells
- CCL
CC chemokine ligand
- CXCL
CXC chemokine ligand
- DCs
dendritic cells
- FLT3L
Fms-like tyrosine kinase 3 ligand
- FMLP
formyl – methionyl–leucyl–phenylalanine
- GC
glucosylceramide
- GCSF
granulocyte colony stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HGF
hepatocyte growth factor
- IFN-γ
interferon-gamma
- IL
interleukin
- LTB4
leukotriene B4
- MCP-1
monocyte chemoattracting protein - 1
- MCSF
macrophage colony stimulating factor
- mDC
myeloid dendritic cell
- MHC
major histocompatibility complex
- MΦs
macrophages
- MOs
monocytes
- pDC
plasmacytoid dendritic cell
- PMA
phorbol 12-myristate 13-acetate
- PMNs
neutrophils
- SDF-1
stromal cell derived factor-1
- TGF-β
transforming growth factor-beta
- TNF-α
tumor necrosis factor-alpha
REFERENCES
- 1.Jonsson LM, Murray GJ, Sorrell SH, Strijland A, Aerts JF, Ginns EI, Barranger JA, Tager JM, Schram AW. Biosynthesis and maturation of glucocerebrosidase in Gaucher fibroblasts. Eur J Biochem. 1987;164(1):171–179. doi: 10.1111/j.1432-1033.1987.tb11008.x. [DOI] [PubMed] [Google Scholar]
- 2.Liou B, Kazimierczuk A, Zhang M, Scott CR, Hegde RS, Grabowski GA. Analyses of variant acid beta-glucosidases: effects of Gaucher disease mutations. J Biol Chem. 2006;281(7):4242–4253. doi: 10.1074/jbc.M511110200. [DOI] [PubMed] [Google Scholar]
- 3.Ohashi T, Hong CM, Weiler S, Tomich JM, Aerts JM, Tager JM, Barranger JA. Characterization of human glucocerebrosidase from different mutant alleles. J Biol Chem. 1991;266(6):3661–3667. [PubMed] [Google Scholar]
- 4.Sawkar AR, Schmitz M, Zimmer KP, Reczek D, Edmunds T, Balch WE, Kelly JW. Chemical chaperones and permissive temperatures alter localization of Gaucher disease associated glucocerebrosidase variants. ACS Chem Biol. 2006;1(4):235–251. doi: 10.1021/cb600187q. [DOI] [PubMed] [Google Scholar]
- 5.Zimmer KP, le Coutre P, Aerts HM, Harzer K, Fukuda M, O’Brien JS, Naim HY. Intracellular transport of acid beta-glucosidase and lysosome-associated membrane proteins is affected in Gaucher’s disease (G202R mutation) J Pathol. 1999;188(4):407–414. doi: 10.1002/(SICI)1096-9896(199908)188:4<407::AID-PATH377>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 7.Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol. 2003;163(5):2093–2101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski GA, Petsko GA, Kolodny EH. Gaucher Disease. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, editors. The online metabolic and molecular bases of inherited disease. 146 ed. New York: Mc Graw Hill; 2010. pp. 1–18. [Google Scholar]
- 9.Balreira A, Cavallari M, Sa Miranda MC, Arosa FA. Uncoupling between CD1d upregulation induced by retinoic acid and conduritol-B-epoxide and iNKT cell responsiveness. Immunobiology. 2010;215(6):505–513. doi: 10.1016/j.imbio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Camou F, Viallard JF. Extended remission of B-cell lymphoma with monoclonal gammopathy in a patient with type 1 Gaucher disease treated with enzyme replacement therapy. Blood Cells Mol Dis. 2011 doi: 10.1016/j.bcmd.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Mistry PK, Liu J, Yang M, Nottoli T, McGrath J, Jain D, Zhang K, Keutzer J, Chuang WL, Mehal WZ, Zhao H, Lin A, Mane S, Liu X, Peng YZ, Li JH, Agrawal M, Zhu LL, Blair HC, Robinson LJ, Iqbal J, Sun L, Zaidi M. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc Natl Acad Sci U S A. 2010;107(45):19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brisca G, Di Rocco M, Picco P, Damasio MB, Martini A. Coxarthritis as the presenting symptom of Gaucher disease type 1. Arthritis. 2011;2011:361279. doi: 10.1155/2011/361279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 14.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller WA. New mechanisms and pathways for monocyte recruitment. J Exp Med. 2001;194(9):F47–F51. doi: 10.1084/jem.194.9.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 18.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 19.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narni-Mancinelli E, Campisi L, Bassand D, Cazareth J, Gounon P, Glaichenhaus N, Lauvau G. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204(9):2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29(2):306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201(11):1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liel Y, Rudich A, Nagauker-Shriker O, Yermiyahu T, Levy R. Monocyte dysfunction in patients with Gaucher disease: evidence for interference of glucocerebroside with superoxide generation. Blood. 1994;83(9):2646–2653. [PubMed] [Google Scholar]
- 24.Balreira A, Lacerda L, Miranda CS, Arosa FA. Evidence for a link between sphingolipid metabolism and expression of CD1d and MHC-class II: monocytes from Gaucher disease patients as a model. Br J Haematol. 2005;129(5):667–676. doi: 10.1111/j.1365-2141.2005.05503.x. [DOI] [PubMed] [Google Scholar]
- 25.Marodi L, Kaposzta R, Toth J, Laszlo A. Impaired microbicidal capacity of mononuclear phagocytes from patients with type I Gaucher disease: partial correction by enzyme replacement therapy. Blood. 1995;86(12):4645–4649. [PubMed] [Google Scholar]
- 26.Xu YH, Jia L, Quinn B, Zamzow M, Stringer K, Aronow B, Sun Y, Zhang W, Setchell KD, Grabowski GA. Global gene expression profile progression in Gaucher disease mouse models. BMC Genomics. 2011;12:20. doi: 10.1186/1471-2164-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 32.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86(5):398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann SH. Immunology’s foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008;9(7):705–712. doi: 10.1038/ni0708-705. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S. Elie Metchnikoff: father of natural immunity. Eur J Immunol. 2008;38(12):3257–3264. doi: 10.1002/eji.200838855. [DOI] [PubMed] [Google Scholar]
- 35.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201(9):1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 39.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7(12):975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12(8):955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siracusa MC, Reece JJ, Urban JF, Jr, Scott AL. Dynamics of lung macrophage activation in response to helminth infection. J Leukoc Biol. 2008;84(6):1422–1433. doi: 10.1189/jlb.0308199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 45.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111(7):927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 46.Hollak CE, Evers L, Aerts JM, van Oers MH. Elevated levels of M-CSF, sCD14 and IL8 in type 1 Gaucher disease. Blood Cells Mol Dis. 1997;23(2):201–212. doi: 10.1006/bcmd.1997.0137. [DOI] [PubMed] [Google Scholar]
- 47.Glew RH, Lee RE. Composition of the membranous deposits occurring in Gaucher’s disease. Arch Biochem Biophys. 1973;156(2):626–639. doi: 10.1016/0003-9861(73)90314-7. [DOI] [PubMed] [Google Scholar]
- 48.Boven LA, van Meurs M, Boot RG, Mehta A, Boon L, Aerts JM, Laman JD. Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am J Clin Pathol. 2004;122(3):359–369. doi: 10.1309/BG5V-A8JR-DQH1-M7HN. [DOI] [PubMed] [Google Scholar]
- 49.Xu YH, Reboulet R, Quinn B, Huelsken J, Witte D, Grabowski GA. Dependence of reversibility and progression of mouse neuronopathic Gaucher disease on acid beta-glucosidase residual activity levels. Mol Genet Metab. 2008;94(2):190–203. doi: 10.1016/j.ymgme.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasenberg M, Kohler A, Bonifatius S, Borucki K, Riek-Burchardt M, Achilles J, Mann L, Baumgart K, Schraven B, Gunzer M. Rapid immunomagnetic negative enrichment of neutrophil granulocytes from murine bone marrow for functional studies in vitro and in vivo. PLoS One. 2011;6(2):e17314. doi: 10.1371/journal.pone.0017314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106(5):1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 52.Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, Moron VG, Pistoresi-Palencia MC. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood. 2006;108(9):3094–3102. doi: 10.1182/blood-2006-04-016659. [DOI] [PubMed] [Google Scholar]
- 53.Beauvillain C, Delneste Y, Scotet M, Peres A, Gascan H, Guermonprez P, Barnaba V, Jeannin P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110(8):2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 54.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, Louis JA. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165(5):2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 55.Cassatella MA, Locati M, Mantovani A. Never underestimate the power of a neutrophil. Immunity. 2009;31(5):698–700. doi: 10.1016/j.immuni.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994;10(2):148–153. doi: 10.1165/ajrcmb.10.2.8110470. [DOI] [PubMed] [Google Scholar]
- 57.Ivey CL, Williams FM, Collins PD, Jose PJ, Williams TJ. Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role for C5a and interleukin-8. J Clin Invest. 1995;95(6):2720–2728. doi: 10.1172/JCI117974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denkers EY, Del Rio L, Bennouna S. Neutrophil production of IL-12 and other cytokines during microbial infection. Chem Immunol Allergy. 2003;83:95–114. doi: 10.1159/000071557. [DOI] [PubMed] [Google Scholar]
- 59.Bennouna S, Denkers EY. Microbial antigen triggers rapid mobilization of TNF-alpha to the surface of mouse neutrophils transforming them into inducers of high-level dendritic cell TNF-alpha production. J Immunol. 2005;174(8):4845–4851. doi: 10.4049/jimmunol.174.8.4845. [DOI] [PubMed] [Google Scholar]
- 60.von Stebut E, Metz M, Milon G, Knop J, Maurer M. Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1alpha/beta released from neutrophils recruited by mast cell-derived TNFalpha. Blood. 2003;101(1):210–215. doi: 10.1182/blood-2002-03-0921. [DOI] [PubMed] [Google Scholar]
- 61.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 62.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat Med. 2005;11(6):666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFNgamma- mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120(1):331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuboi N, Asano K, Lauterbach M, Mayadas TN. Human neutrophil Fcgamma receptors initiate and play specialized nonredundant roles in anti-body- mediated inflammatory diseases. Immunity. 2008;28(6):833–846. doi: 10.1016/j.immuni.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobs L, Nawrot TS, de Geus B, Meeusen R, Degraeuwe B, Bernard A, Sughis M, Nemery B, Panis LI. Subclinical responses in healthy cyclists briefly exposed to traffic-related air pollution: an intervention study. Environ Health. 2010;9:64. doi: 10.1186/1476-069X-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 67.De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10(15):4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 68.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33(2):266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14(1):3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125(3):281–288. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delano MJ, Kelly-Scumpia KM, Thayer TC, Winfield RD, Scumpia PO, Cuenca AG, Harrington PB, O’Malley KA, Warner E, Gabrilovich S, Mathews CE, Laface D, Heyworth PG, Ramphal R, Strieter RM, Moldawer LL, Efron PA. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol. 2011;187(2):911–918. doi: 10.4049/jimmunol.1100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smart SJ, Casale TB. TNF-alpha-induced transendothelial neutrophil migration is IL-8 dependent. Am J Physiol. 1994;266(3 Pt 1):L238–L245. doi: 10.1152/ajplung.1994.266.3.L238. [DOI] [PubMed] [Google Scholar]
- 73.Bittleman DB, Casale TB. Interleukin-8 mediates interleukin-1 alpha-induced neutrophil trans-cellular migration. Am J Respir Cell Mol Biol. 1995;13(3):323–329. doi: 10.1165/ajrcmb.13.3.7654388. [DOI] [PubMed] [Google Scholar]
- 74.Kinoshita M, Miyazaki H, Ono S, Inatsu A, Nakashima H, Tsujimoto H, Shinomiya N, Saitoh D, Seki S. Enhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureus. Infect Immun. 2011;79(7):2670–2680. doi: 10.1128/IAI.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson EA, Dao TL, Guignet MA, Geddes CE, Koemeter-Cox AI, Kan RK. Increased expression of the chemokines CXCL1 and MIP-1alpha by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J Neuroinflammation. 2011;8:41. doi: 10.1186/1742-2094-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aker M, Zimran A, Abrahamov A, Horowitz M, Matzner Y. Abnormal neutrophil chemotaxis in Gaucher disease. Br J Haematol. 1993;83(2):187–191. doi: 10.1111/j.1365-2141.1993.tb08270.x. [DOI] [PubMed] [Google Scholar]
- 77.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80(5):617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 78.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 79.Zigmond SH, Hirsch JG. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 83.Kaplan G, Walsh G, Guido LS, Meyn P, Burkhardt RA, Abalos RM, Barker J, Frindt PA, Fajardo TT, Celona R, Cohn ZA. Novel responses of human skin to intradermal recombinant granulocyte/macrophage-colony-stimulating factor: Langerhans cell recruitment, keratinocyte growth, and enhanced wound healing. J Exp Med. 1992;175(6):1717–1728. doi: 10.1084/jem.175.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inaba K, Schuler G, Witmer MD, Valinksy J, Atassi B, Steinman RM. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Streilein JW, Grammer SF. In vitro evidence that Langerhans cells can adopt two functionally distinct forms capable of antigen presentation to T lymphocytes. J Immunol. 1989;143(12):3925–3933. [PubMed] [Google Scholar]
- 86.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone AM, Fathman CG, Inaba K, Steinman RM. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989;169(3):1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pure E, Inaba K, Crowley MT, Tardelli L, Witmer- Pack MD, Ruberti G, Fathman G, Steinman RM. Antigen processing by epidermal Langerhans cells correlates with the level of biosynthesis of major histocompatibility complex class II molecules and expression of invariant chain. J Exp Med. 1990;172(5):1459–1469. doi: 10.1084/jem.172.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Austyn JM, Kupiec-Weglinski JW, Hankins DF, Morris PJ. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988;167(2):646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kupiec-Weglinski JW, Austyn JM, Morris PJ. Migration patterns of dendritic cells in the mouse. Traffic from the blood, and T cell-dependent and -independent entry to lymphoid tissues. J Exp Med. 1988;167(2):632–645. doi: 10.1084/jem.167.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172(5):1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fossum S. Lymph-borne dendritic leucocytes do not recirculate, but enter the lymph node paracortex to become interdigitating cells. Scand J Immunol. 1988;27(1):97–105. doi: 10.1111/j.1365-3083.1988.tb02326.x. [DOI] [PubMed] [Google Scholar]
- 92.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166(6):1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kripke ML, Munn CG, Jeevan A, Tang JM, Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145(9):2833–2838. [PubMed] [Google Scholar]
- 94.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 95.Streilein JW, Grammer SF, Yoshikawa T, Demidem A, Vermeer M. Functional dichotomy between Langerhans cells that present antigen to naive and to memory/effector T lymphocytes. Immunol Rev. 1990;117:159–183. doi: 10.1111/j.1600-065x.1990.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 96.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med. 1996;184(4):1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10(12):1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 98.Ito T, Inaba M, Inaba K, Toki J, Sogo S, Iguchi T, Adachi Y, Yamaguchi K, Amakawa R, Valladeau J, Saeland S, Fukuhara S, Ikehara S. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999;163(3):1409–1419. [PubMed] [Google Scholar]
- 99.Facchetti F, De Wolf-Peeters C, van den Oord JJ, De vos R, Desmet VJ. Plasmacytoid T cells: a cell population normally present in the reactive lymph node. An immunohistochemical and electronmicroscopic study. Hum Pathol. 1988;19(9):1085–1092. doi: 10.1016/s0046-8177(88)80091-1. [DOI] [PubMed] [Google Scholar]
- 100.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185(6):1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283(5405):1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 102.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5(8):919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 103.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 104.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166(9):5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 105.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192(12):1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 107.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186(11):1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174(11):6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 109.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996;60(3):365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 112.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9(1):10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 114.Romani N, Lenz A, Glassel H, Stossel H, Stanzl U, Majdic O, Fritsch P, Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989;93(5):600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- 115.Larsen CP, Ritchie SC, Pearson TC, Linsley PS, Lowry RP. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, Muramatsu S, Hodes RJ, Steinman RM. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180(5):1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de la Salle H, Galon J, Bausinger H, Spehner D, Bohbot A, Cohen J, Cazenave JP, Fridman WH, Sautes C, Hanau D. Soluble CD16/Fc gamma RIII induces maturation of dendritic cells and production of several cytokines including IL-12. Adv Exp Med Biol. 1997;417:345–352. doi: 10.1007/978-1-4757-9966-8_56. [DOI] [PubMed] [Google Scholar]
- 118.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 119.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116(3):783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weaver DJ, Jr, Reis ES, Pandey MK, Kohl G, Harris N, Gerard C, Kohl J. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010;40(3):710–721. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kool M, Geurtsvankessel C, Muskens F, Madeira FB, van Nimwegen M, Kuipers H, Thielemans K, Hoogsteden HC, Hammad H, Lambrecht BN. Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen-presenting potential of plasmacytoid DCs. J Leukoc Biol. 2011;90(6):1177–1190. doi: 10.1189/jlb.0610342. [DOI] [PubMed] [Google Scholar]
- 122.Cruz LJ, Rueda F, Cordobilla B, Simon L, Hosta L, Albericio F, Domingo JC. Targeting nanosystems to human DCs via Fc receptor as an effective strategy to deliver antigen for immunotherapy. Mol Pharm. 2011;8(1):104–116. doi: 10.1021/mp100178k. [DOI] [PubMed] [Google Scholar]
- 123.Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140(11):3773–3778. [PubMed] [Google Scholar]
- 124.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76(2):275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 125.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155(8):3734–3741. [PubMed] [Google Scholar]
- 126.Rossi-Bergmann B, Muller I, Godinho EB. TH1 and TH2 T-cell subsets are differentially activated by macrophages and B cells in murine leishmaniasis. Infect Immun. 1993;61(5):2266–2269. doi: 10.1128/iai.61.5.2266-2269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hollsberg P, Batra V, Dressel A, Hafler DA. Induction of anergy in CD8 T cells by B cell presentation of antigen. J Immunol. 1996;157(12):5269–5276. [PubMed] [Google Scholar]
- 128.Adorini L, Guery JC, Ria F, Galbiati F. B cells present antigen to CD4+ T cells, but fail to produce IL-12. Selective APC for Th2 cell development? Ann N Y Acad Sci. 1997;815:401–411. doi: 10.1111/j.1749-6632.1997.tb52091.x. [DOI] [PubMed] [Google Scholar]
- 129.Croft M, Joseph SB, Miner KT. Partial activation of naive CD4 T cells and tolerance induction in response to peptide presented by resting B cells. J Immunol. 1997;159(7):3257–3265. [PubMed] [Google Scholar]
- 130.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 131.Kroese FG, Ammerlaan WA, Deenen GJ. Location and function of B-cell lineages. Ann N Y Acad Sci. 1992;651:44–58. doi: 10.1111/j.1749-6632.1992.tb24592.x. [DOI] [PubMed] [Google Scholar]
- 132.Marcos MA, Huetz F, Pereira P, Andreu JL, Martinez AC, Coutinho A. Further evidence for coelomic-associated B lymphocytes. Eur J Immunol. 1989;19(11):2031–2035. doi: 10.1002/eji.1830191110. [DOI] [PubMed] [Google Scholar]
- 133.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2011 doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The ‘Ly-1 B’ cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hayakawa K, Hardy RR. Development and function of B-1 cells. Curr Opin Immunol. 2000;12(3):346–353. doi: 10.1016/s0952-7915(00)00098-4. [DOI] [PubMed] [Google Scholar]
- 137.Tung JW, Parks DR, Moore WA, Herzenberg LA. Identification of B-cell subsets: an exposition of 11-color (Hi-D) FACS methods. Methods Mol Biol. 2004;271:37–58. doi: 10.1385/1-59259-796-3:037. [DOI] [PubMed] [Google Scholar]
- 138.Hardy RR, Carmack CE, Li YS, Hayakawa K. Distinctive developmental origins and specificities of murine CD5+ B cells. Immunol Rev. 1994;137:91–118. doi: 10.1111/j.1600-065x.1994.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 139.Youinou P, Renaudineau Y. CD5 expression in B cells from patients with systemic lupus erythematosus. Crit Rev Immunol. 2011;31(1):31–42. doi: 10.1615/critrevimmunol.v31.i1.30. [DOI] [PubMed] [Google Scholar]
- 140.Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988;168(2):687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 142.Qian Y, Santiago C, Borrero M, Tedder TF, Clarke SH. Lupus-specific antiribonucleoprotein B cell tolerance in nonautoimmune mice is maintained by differentiation to B-1 and governed by B cell receptor signaling thresholds. J Immunol. 2001;166(4):2412–2419. doi: 10.4049/jimmunol.166.4.2412. [DOI] [PubMed] [Google Scholar]
- 143.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285(5424):113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 144.Nakamura M, Burastero SE, Notkins AL, Casal P. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1)+ B cells are polyreactive. J Immunol. 1988;140(12):4180–4186. [PubMed] [Google Scholar]
- 145.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 146.Dauphinee M, Tovar Z, Talal N. B cells expressing CD5 are increased in Sjogren’s syndrome. Arthritis Rheum. 1988;31(5):642–647. doi: 10.1002/art.1780310509. [DOI] [PubMed] [Google Scholar]
- 147.Aramaki M, Nagasawa T, Koseki T, Ishikawa I. Presence of activated B-1 cells in chronic inflamed gingival tissue. J Clin Immunol. 1998;18(6):421–429. doi: 10.1023/a:1023234823783. [DOI] [PubMed] [Google Scholar]
- 148.Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011 doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kipps TJ. The CD5 B cell. Adv Immunol. 1989;47:117–185. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- 150.Casey SC, Nelson EL, Turco GM, Janes MR, Fruman DA, Blumberg B. B-1 cell lymphoma in mice lacking the steroid and xenobiotic receptor, SXR. Mol Endocrinol. 2011;25(6):933–943. doi: 10.1210/me.2010-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 152.Burstein Y, Rechavi G, Rausen AR, Frisch B, Spirer Z. Association of Gaucher’s disease and lymphoid malignancy in 2 children. Scand J Haematol. 1985;35(4):445–447. doi: 10.1111/j.1600-0609.1985.tb02269.x. [DOI] [PubMed] [Google Scholar]
- 153.Marti GE, Ryan ET, Papadopoulos NM, Filling- Katz M, Barton N, Fleischer TA, Rick M, Gralnick HR. Polyclonal B-cell lymphocytosis and hypergammaglobulinemia in patients with Gaucher disease. Am J Hematol. 1988;29(4):189–194. doi: 10.1002/ajh.2830290403. [DOI] [PubMed] [Google Scholar]
- 154.Shoenfeld Y, Gallant LA, Shaklai M, Livni E, Djaldetti M, Pinkhas J. Gaucher’s disease: a disease with chronic stimulation of the immune system. Arch Pathol Lab Med. 1982;106(8):388–391. [PubMed] [Google Scholar]
- 155.McAlarney T, Pastores GM, Hays AP, Latov N. Anti-sulfatide antibody and neuropathy in a patient with Gaucher’s disease. Neurology. 1995;45(8):1622–1623. doi: 10.1212/wnl.45.8.1622. [DOI] [PubMed] [Google Scholar]
- 156.Rosenbloom BE, Weinreb NJ, Zimran A, Kacena KA, Charrow J, Ward E. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood. 2005;105(12):4569–4572. doi: 10.1182/blood-2004-12-4672. [DOI] [PubMed] [Google Scholar]
- 157.de Fost M, Vom Dahl S, Weverling GJ, Brill N, Brett S, Haussinger D, Hollak CE. Increased incidence of cancer in adult Gaucher disease in Western Europe. Blood Cells Mol Dis. 2006;36(1):53–58. doi: 10.1016/j.bcmd.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 158.Hawkesford MP, Bowey AJ, Rao J, Meara NJ. Synchronous presentation of Gaucher disease and solitary plasmacytoma with progression to multiple myeloma. Scott Med J. 2011;56(4):236. doi: 10.1258/smj.2011.011178. [DOI] [PubMed] [Google Scholar]
- 159.Kaloterakis A, Filiotou A, Koskinas J, Raptis I, Zouboulis C, Michelakakis H, Hadziyannis S. Systemic AL amyloidosis in Gaucher disease. A case report and review of the literature. J Intern Med. 1999;246(6):587–590. doi: 10.1046/j.1365-2796.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- 160.Marie JP, Tulliez M, Tricottet-Paczinski V, Reynes M, Diebold J. Gaucher’s disease with monoclonal gammopathy. Significance of splenic plasmacytosis. Scand J Haematol. 1982;28(1):54–58. doi: 10.1111/j.1600-0609.1982.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 161.Airo R, Gabusi G, Guindani M. Gaucher’s disease associated with monoclonal gammapathy of undetermined significance: a case report. Haematologica. 1993;78(2):129–131. [PubMed] [Google Scholar]
- 162.Arikan-Ayyildiz Z, Yuce A, Uslu-Kizilkan N, Demir H, Gurakan F. Immunoglobulin abnormalities and effects of enzyme replacement therapy in children with Gaucher disease. Pediatr Blood Cancer. 2011;56(4):664–666. doi: 10.1002/pbc.22863. [DOI] [PubMed] [Google Scholar]
- 163.Arikan-Ayyildiz Z, Yuce A, Uslu-Kizilkan N, Demir H, Gurakan F. Immunoglobulin abnormalities and effects of enzyme replacement therapy in children with Gaucher disease. Pediatr Blood Cancer. 2010 doi: 10.1002/pbc.22863. [DOI] [PubMed] [Google Scholar]
- 164.Wine E, Yaniv I, Cohen IJ. Hyperimmunoglobulinemia in pediatric-onset type 1 Gaucher disease and effects of enzyme replacement therapy. J Pediatr Hematol Oncol. 2007;29(7):451–457. doi: 10.1097/MPH.0b013e31806451d3. [DOI] [PubMed] [Google Scholar]
- 165.Brautbar A, Elstein D, Pines G, Abrahamov A, Zimran A. Effect of enzyme replacement therapy on gammopathies in Gaucher disease. Blood Cells Mol Dis. 2004;32(1):214–217. doi: 10.1016/j.bcmd.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 166.Pratt PW, Kochwa S, Estren S. Immunoglobulin abnormalities in Gaucher’s disease. Report of 16 cases. Blood. 1968;31(5):633–640. [PubMed] [Google Scholar]
- 167.de Fost M, Out TA, de Wilde FA, Tjin EP, Pals ST, van Oers MH, Boot RG, Aerts JF, Maas M, Vom Dahl S, Hollak CE. Immunoglobulin and free light chain abnormalities in Gaucher disease type I: data from an adult cohort of 63 patients and review of the literature. Ann Hematol. 2008;87(6):439–449. doi: 10.1007/s00277-008-0441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fujita T, Matai K, Kohno S, Itsubo K. Impact of splenectomy on circulating immunoglobulin levels and the development of postoperative infection following total gastrectomy for gastric cancer. Br J Surg. 1996;83(12):1776–1778. doi: 10.1002/bjs.1800831236. [DOI] [PubMed] [Google Scholar]
- 169.Shoenfeld Y, Beresovski A, Zharhary D, Tomer Y, Swissa M, Sela E, Zimran A, Zevin S, Gilburd B, Blank M. Natural autoantibodies in sera of patients with Gaucher’s disease. J Clin Immunol. 1995;15(6):363–372. doi: 10.1007/BF01541326. [DOI] [PubMed] [Google Scholar]
- 170.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 171.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 173.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 174.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 175.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 176.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 177.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2(6):389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 178.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 179.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30(5):616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19(6):353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 181.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 182.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 183.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 185.Awasthi A, Kuchroo VK. Immunology. The yin and yang of follicular helper T cells. Science. 2009;325(5943):953–955. doi: 10.1126/science.1178752. [DOI] [PubMed] [Google Scholar]
- 186.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21(3):274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 189.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 190.Shiver JW, Su L, Henkart PA. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992;71(2):315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- 191.Hersperger AR, Migueles SA, Betts MR, Connors M. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr Opin HIV AIDS. 2011;6(3):169–173. doi: 10.1097/COH.0b013e3283454c39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cajaiba MM, Reyes-Mugica M. Gaucher or pseudo-Gaucher? The challenge of several diseases colliding in a pediatric patient. Hum Pathol. 2009;40(4):594–598. doi: 10.1016/j.humpath.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 193.Sanchez R, Etzell J, Kim G, Packman S, Fairley C, Goldsby R. Pediatric malignancies. Case 2. Peripheral T-cell lymphoma in an adolescent with unsuspected Gaucher disease. J Clin Oncol. 2005;23(21):4792–4793. doi: 10.1200/JCO.2005.05.141. [DOI] [PubMed] [Google Scholar]
- 194.Burstein Y, Zakuth V, Rechavi G, Spirer Z. Abnormalities of cellular immunity and natural killer cells in Gaucher’s disease. J Clin Lab Immunol. 1987;23(3):149–151. [PubMed] [Google Scholar]
- 195.Burns GF, Cawley JC, Flemans RJ, Higgy KE, Worman CP, Barker CR, Roberts BE, Hayhoe FG. Surface marker and other characteristics of Gaucher’s cells. J Clin Pathol. 1977;30(10):981–988. doi: 10.1136/jcp.30.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lacerda L, Arosa FA, Lacerda R, Cabeda J, Porto G, Amaral O, Fortuna A, Pinto R, Oliveira P, McLaren CE, Sa Miranda C, de Sousa M. T cell numbers relate to bone involvement in Gaucher disease. Blood Cells Mol Dis. 1999;25(2):130–138. doi: 10.1006/bcmd.1999.0237. [DOI] [PubMed] [Google Scholar]
- 197.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 198.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334(6057):825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pamer E, Cresswell P. Mechanisms of MHC class I--restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 200.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 201.Yin L, Huseby E, Scott-Browne J, Rubtsova K, Pinilla C, Crawford F, Marrack P, Dai S, Kappler JW. A single T cell receptor bound to major histocompatibility complex class I and class II glycoproteins reveals switchable TCR conformers. Immunity. 2011;35(1):23–33. doi: 10.1016/j.immuni.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250(4981):679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- 203.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 204.Vincent MS, Xiong X, Grant EP, Peng W, Brenner MB. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J Immunol. 2005;175(10):6344–6351. doi: 10.4049/jimmunol.175.10.6344. [DOI] [PubMed] [Google Scholar]
- 205.Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, Wilson IA, Porcelli SA, Brenner MB. Molecular recognition of lipid antigens by T cell receptors. J Exp Med. 1999;189(1):195–205. doi: 10.1084/jem.189.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29(5):1667–1675. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 207.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 208.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 209.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 210.Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7(12):929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- 211.de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Maitre B, Mourey L, Salamero J, Cazenave JP, Hanau D, Mori L, Puzo G, De Libero G. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310(5752):1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 212.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. Am J Pathol. 2004;165(6):1853–1863. doi: 10.1016/S0002-9440(10)63238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vogt AB, Kropshofer H, Moldenhauer G, Hammerling GJ. Kinetic analysis of peptide loading onto HLA-DR molecules mediated by HLA-DM. Proc Natl Acad Sci U S A. 1996;93(18):9724–9729. doi: 10.1073/pnas.93.18.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 215.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 216.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178(5):2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 218.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372(6507):691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 219.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 220.Nieuwenhuis EE, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, Corazza N, Colgan SP, Onderdonk AB, Blumberg RS. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8(6):588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 221.Hansen DS, Siomos MA, Buckingham L, Scalzo AA, Schofield L. Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity. 2003;18(3):391–402. doi: 10.1016/s1074-7613(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 222.Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, Nakayama T, Taniguchi M, Saito A. Monocyte chemoattractant protein-1-dependent increase of V alpha 14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J Immunol. 2001;167(11):6525–6532. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- 223.Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7(9):1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 224.Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc Natl Acad Sci U S A. 1996;93(22):12654. doi: 10.1073/pnas.93.22.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yuan W, Qi X, Tsang P, Kang SJ, Illarionov PA, Besra GS, Gumperz J, Cresswell P. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci U S A. 2007;104(13):5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, Savage P, Bendelac A, Teyton L. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303(5657):523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5(2):175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 228.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 230.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 231.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 232.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jankovic D, Kugler DG, Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol. 2010;3(3):239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8(3):275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 235.Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 236.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 237.Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- 238.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 240.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sojka DK, Fowell DJ. Regulatory T cells inhibit acute IFN-gamma synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10. Proc Natl Acad Sci U S A. 2011;108(45):18336–18341. doi: 10.1073/pnas.1110566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 Axis Converts Human TH1 Cells into Regulatory T Cells. Sci Transl Med. 2011;3(111):111ra20. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179(12):8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 244.Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 245.Michel T, Feron O. Nitric oxide synthases: which, where, how, why? J Clin Invest. 1997;100(9):2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269(19):13725–13728. [PubMed] [Google Scholar]
- 247.Oztuzcu S, Igci YZ, Arslan A, Sivasli E, Ozkara E, Igci M, Demiryurek S, Cengiz B, Gogebakan B, Namiduru M, Coskun MY, Cakmak EA. mRNA expressions of inducible nitric oxide synthase, endothelial nitric oxide synthase, and neuronal nitric oxide synthase genes in meningitis patients. Genet Test Mol Biomarkers. 2011;15(3):147–152. doi: 10.1089/gtmb.2010.0142. [DOI] [PubMed] [Google Scholar]
- 248.Suzuki N, Mizuno H, Warita H, Takeda S, Itoyama Y, Aoki M. Neuronal NOS is dislocated during muscle atrophy in amyotrophic lateral sclerosis. J Neurol Sci. 2010;294(1–2):95–101. doi: 10.1016/j.jns.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 249.Binti Md Isa K, Kawasaki N, Ueyama K, Sumii T, Kudo S. Effects of cold exposure and shear stress on endothelial nitric oxide synthase activation. Biochem Biophys Res Commun. 2011;412(2):318–322. doi: 10.1016/j.bbrc.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 250.Portillo JA, Feliciano LM, Okenka G, Heinzel F, Cecilia Subauste M, Subauste CS. CD40 and Tnf-Alpha Cooperate to Upregulate Nos2 Expression in Macrophages. Immunology. 2012;135(2):140–150. doi: 10.1111/j.1365-2567.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Do H, Kang NS, Pyo S, Billiar TR, Sohn EH. Differential regulation by fucoidan of IFN-gamma-induced NO production in glial cells and macrophages. J Cell Biochem. 2010;111(5):1337–1345. doi: 10.1002/jcb.22860. [DOI] [PubMed] [Google Scholar]
- 252.Gutierrez FR, Mineo TW, Pavanelli WR, Guedes PM, Silva JS. The effects of nitric oxide on the immune system during Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 2009;104 Suppl 1:236–245. doi: 10.1590/s0074-02762009000900030. [DOI] [PubMed] [Google Scholar]
- 253.Chatterjee M, Saluja R, Tewari S, Barthwal MK, Goel SK, Dikshit M. Augmented nitric oxide generation in neutrophils: oxidative and proinflammatory implications in hypertension. Free Radic Res. 2009;43(12):1195–1204. doi: 10.3109/10715760903247256. [DOI] [PubMed] [Google Scholar]
- 254.Hong YB, Kim EY, Jung SC. Upregulation of proinflammatory cytokines in the fetal brain of the Gaucher mouse. J Korean Med Sci. 2006;21(4):733–738. doi: 10.3346/jkms.2006.21.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Grisham MB, Granger DN, Lefer DJ. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med. 1998;25(4–5):404–433. doi: 10.1016/s0891-5849(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 256.Yanagisawa N, Shimada K, Miyazaki T, Kume A, Kitamura Y, Sumiyoshi K, Kiyanagi T, Iesaki T, Inoue N, Daida H. Enhanced production of nitric oxide, reactive oxygen species, and pro-inflammatory cytokines in very long chain saturated fatty acidaccumulated macrophages. Lipids Health Dis. 2008;7:48. doi: 10.1186/1476-511X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wakusawa K, Haginoya K, Ishitobi M, Hino-Fukuyo N, Togashi N, Sato I, Ohura T, Yokoyama H, Kikuchi M, Iinuma K, Tsuchiya S. The cytokine and chemokine profiles in rhabdomyolysis in a patient with Gaucher disease type II. Neuropediatrics. 2010;41(1):39–42. doi: 10.1055/s-0030-1253425. [DOI] [PubMed] [Google Scholar]
- 258.Michelakakis H, Spanou C, Kondyli A, Dimitriou E, Van Weely S, Hollak CE, Van Oers MH, Aerts JM. Plasma tumor necrosis factor-a (TNF-a) levels in Gaucher disease. Biochim Biophys Acta. 1996;1317(3):219–222. doi: 10.1016/s0925-4439(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 259.Barak V, Acker M, Nisman B, Kalickman I, Abrahamov A, Zimran A, Yatziv S. Cytokines in Gaucher’s disease. Eur Cytokine Netw. 1999;10(2):205–210. [PubMed] [Google Scholar]
- 260.Lichtenstein M, Zimran A, Horowitz M. Cytokine mRNA in Gaucher disease. Blood Cells Mol Dis. 1997;23(3):395–401. doi: 10.1006/bcmd.1997.0156. [DOI] [PubMed] [Google Scholar]
- 261.Pavlova EV, Deegan PB, Tindall J, McFarlane I, Mehta A, Hughes D, Wraith JE, Cox TM. Potential biomarkers of osteonecrosis in Gaucher disease. Blood Cells Mol Dis. 2011;46(1):27–33. doi: 10.1016/j.bcmd.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 262.Allen MJ, Myer BJ, Khokher AM, Rushton N, Cox TM. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: increased release of interleukin-6 and interleukin-10. QJM. 1997;90(1):19–25. doi: 10.1093/qjmed/90.1.19. [DOI] [PubMed] [Google Scholar]
- 263.Yoshino M, Watanabe Y, Tokunaga Y, Harada E, Fujii C, Numata S, Harada M, Tajima A, Ida H. Roles of specific cytokines in bone remodeling and hematopoiesis in Gaucher disease. Pediatr Int. 2007;49(6):959–965. doi: 10.1111/j.1442-200X.2007.02502.x. [DOI] [PubMed] [Google Scholar]
- 264.van Breemen MJ, de Fost M, Voerman JS, Laman JD, Boot RG, Maas M, Hollak CE, Aerts JM, Rezaee F. Increased plasma macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels in type 1 Gaucher disease. Biochim Biophys Acta. 2007;1772(7):788–796. doi: 10.1016/j.bbadis.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 265.Boot RG, Verhoek M, de Fost M, Hollak CE, Maas M, Bleijlevens B, van Breemen MJ, van Meurs M, Boven LA, Laman JD, Moran MT, Cox TM, Aerts JM. Marked elevation of the chemokine CCL18/PARC in Gaucher disease: a novel surrogate marker for assessing therapeutic intervention. Blood. 2004;103(1):33–39. doi: 10.1182/blood-2003-05-1612. [DOI] [PubMed] [Google Scholar]
- 266.Crocker PR, Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Flotte TJ, Springer TA, Thorbecke GJ. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983;111(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- 268.Kinoshita M, Uchida T, Sato A, Nakashima M, Nakashima H, Shono S, Habu Y, Miyazaki H, Hiroi S, Seki S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol. 2010;53(5):903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 269.Lloyd CM, Phillips AR, Cooper GJ, Dunbar PR. Three-colour fluorescence immunohistochemistry reveals the diversity of cells staining for macrophage markers in murine spleen and liver. J Immunol Methods. 2008;334(1–2):70–81. doi: 10.1016/j.jim.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 270.Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, Frazier WA, Murphy TL, Murphy KM. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457(7227):318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117(8):2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.You Y, Myers RC, Freeberg L, Foote J, Kearney JF, Justement LB, Carter RH. Marginal zone B cells regulate antigen capture by marginal zone macrophages. J Immunol. 2011;186(4):2172–2181. doi: 10.4049/jimmunol.1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304(5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 274.Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27(16):5699–5710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ducreux J, Crocker PR, Vanbever R. Analysis of sialoadhesin expression on mouse alveolar macrophages. Immunol Lett. 2009;124(2):77–80. doi: 10.1016/j.imlet.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 276.Palecanda A, Paulauskis J, Al-Mutairi E, Imrich A, Qin G, Suzuki H, Kodama T, Tryggvason K, Koziel H, Kobzik L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med. 1999;189(9):1497–1506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10(5):488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 278.Lagranderie M, Nahori MA, Balazuc AM, Kiefer- Biasizzo H, Lapa e Silva JR, Milon G, Marchal G, Vargaftig BB. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology. 2003;108(3):352–364. doi: 10.1046/j.1365-2567.2003.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10(7):786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34(1):85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 281.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Flores-Langarica A, Meza-Perez S, Calderon- Amador J, Estrada-Garcia T, Macpherson G, Lebecque S, Saeland S, Steinman RM, Flores-Romo L. Network of dendritic cells within the muscular layer of the mouse intestine. Proc Natl Acad Sci U S A. 2005;102(52):19039–19044. doi: 10.1073/pnas.0504253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Crocker PR, Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med. 1986;164(6):1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15(8):1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- 286.Soga H, Nakamura M, Yagi H, Kayaba S, Ishii T, Gotoh T, Itoh T. Heterogeneity of mouse thymic macrophages: I. Immunohistochemical analysis. Arch Histol Cytol. 1997;60(1):53–63. doi: 10.1679/aohc.60.53. [DOI] [PubMed] [Google Scholar]
- 287.Dupasquier M, Stoitzner P, van Oudenaren A, Romani N, Leenen PJ. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol. 2004;123(5):876–879. doi: 10.1111/j.0022-202X.2004.23427.x. [DOI] [PubMed] [Google Scholar]
- 288.Dupasquier M, Stoitzner P, Wan H, Cerqueira D, van Oudenaren A, Voerman JS, Denda-Nagai K, Irimura T, Raes G, Romani N, Leenen PJ. The dermal microenvironment induces the expression of the alternative activation marker CD301/mMGL in mononuclear phagocytes, independent of IL-4/IL-13 signaling. J Leukoc Biol. 2006;80(4):838–849. doi: 10.1189/jlb.1005564. [DOI] [PubMed] [Google Scholar]
- 289.Kojima N, Kato C, Igarashi M, Ishii M. Development of peritoneal macrophage along a dendritic cell lineage in response to uptake of oligomannose-coated liposomes. Cell Immunol. 2011;271(2):335–341. doi: 10.1016/j.cellimm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 290.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]