Abstract
Intracellular membrane networks including the endoplasmic reticulum (ER) and the Golgi apparatus experience dramatic reorganization upon entry into mitosis. However, the mechanisms driving these rearrangements and their importance for cell division are poorly understood. The GTPase Sar1 is a component of the secretory pathway and a key activator of anterograde transport of cargo from the ER to the Golgi. Here we show that Sar1 mutant proteins added to metaphase-arrested Xenopus laevis egg extracts cause dramatic effects on membrane organization. Live analysis of membrane structures in egg extract cytoplasm revealed a distinct network of sheets and tubules reflective of the organization of the ER in other systems. Addition of a constitutively active Sar1 GTPase mutant (H79G) increased membrane tubulation, while a dominant negative version Sar1 (T39N) impaired tubule organization. Although microtubule pelleting assays revealed that Sar1 associates with microtubules in the egg extract, and addition of Sar1 (H79G) mutant slightly destabilized spindle poles, bipolar spindle assembly was largely unaffected. Thus, spindles are stable to dramatic changes in mitotic membrane organization at metaphase, suggesting that mitotic membrane is not an upstream regulator of the mitotic spindle apparatus in Xenopus egg extracts.
Introduction
Cell division sees dramatic changes in cellular organization, including dynamic transformation of the cytoskeleton and condensation of chromosomes. However, less understood are changes in membrane organelles such as the endoplasmic reticulum (ER), a dynamic, continuous membrane network composed of sheet-like cisternae and interconnected tubules contiguous with the nuclear envelope. Entry into mitosis is accompanied by a breakdown of the nuclear envelope and dramatic reorganization of ER structure, characterized by a reduction in membrane sheets and an increase in tubular structures (Puhka et al. 2007). Studies of these dramatic changes of membrane have largely focused on the partitioning and inheritance of these organelles at the end of mitosis (Barr 2002; Shorter and Warren 2002). However, little is known about the cell cycle regulation of changes in ER dynamics and the relationship between these changes and other mitotic events.
Daughter cells acquire a full complement of chromosomes during mitosis due to the activity of a bipolar microtubule-based apparatus known as the mitotic spindle. Spindle assembly and organization relies on the coordinated activity of microtubule associated proteins (MAPs), microtubule motors, and hundreds of other regulatory proteins (Gadde and Heald 2004). Recently, several studies have indicated the existence of a membranous spindle matrix, raising the possibility that intracellular membrane organelles influence microtubule organization and spindle assembly (Zheng and Tsai 2006). The endocytic adapter protein epsin and lamin B intermediate filaments are both associated with membranes and their inhibition leads to defects in spindle assembly (Liu and Zheng 2009; Tsai et al. 2006). In addition, disruption of the Golgi-associated protein GRASP65 causes defects in spindle dynamics (Sutterlin et al. 2005). It is well documented that the ER network associates closely with the mitotic apparatus, however it is unknown if ER membranes are involved in assembly and structural organization of the spindle (Chaldakov and Vankov 1985; Terasaki 2000; Waterman-Storer et al. 1993).
To examine the importance of mitotic membrane organization for spindle assembly, we utilized the small GTPase Sar1, a component of the ER involved in vesicle biogenesis and export of cargo from the ER to the Golgi in the secretory pathway (Lee et al. 2004). Sar1 activity is known to promote membrane curvature and tubulation during vesicle formation (Bielli et al. 2005; Lee et al. 2005; Long et al. 2010). Addition of Sar1 mutants, either dominant negative or constitutively active versions, to metaphase-arrested Xenopus egg extracts caused dramatic effects in membrane organization. However, Sar1 mutants did not impair bipolar spindle assembly and organization, indicating that, despite the close association of membrane with the spindle, its morphology does not play an important role in mitotic microtubule dynamics and organization at metaphase.
Results and Discussion
Sar1 alters membrane organization
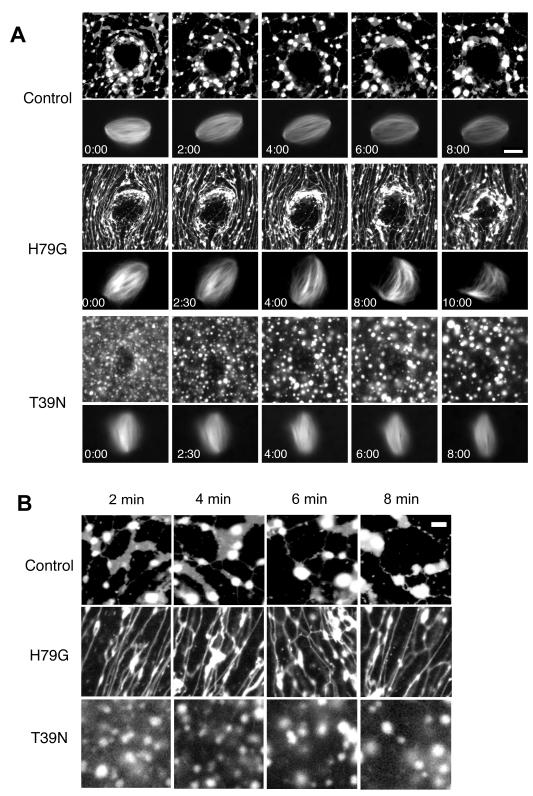
ER and Golgi membrane structures display a dramatic rearrangement upon entry into mitosis, but the mechanisms underlying this transition and the consequences for spindle assembly are poorly understood. As a tool to alter mitotic membrane morphology, we utilized mutants of the small GTPase Sar1. Sar1 has been previously shown to cause curvature and tubulation of membrane structures in other systems (Aridor et al. 2001; Lee et al. 2005). Mutation of amino acid 79 from histidine to glycine (H79G) inhibits its GTP hydrolysis and maintains Sar1 in an activated state, while mutation of amino acid threonine 39 to asparagine (T39N) blocks its nucleotide exchange and therefore has a dominant negative effect, restricting Sar1 to a GDP inactive state (Altan-Bonnet et al. 2004; Stroud et al. 2003). Recombinant Sar1 wild-type and mutant proteins purified from bacteria were added to Xenopus laevis egg extracts, which can reconstitute cell cycle progression in vitro. Calcium addition to cytostatic factor (CSF) metaphase-arrested egg extracts containing sperm nuclei induces transit through interphase allowing nuclear assembly, DNA and centrosome replication. Spindle assembly around sperm chromosomes is then induced by addition of fresh CSF extract, which returns the reaction to a metaphase arrest (Desai et al. 1999). Extract reactions contained Alexa 488-labeled tubulin that incorporated into microtubules and a rhodamine-labeled lipophilic membrane dye (CM-DiI), which has been shown to label the ER network in previous studies in both mammalian cells and Xenopus laevis egg extracts (Anderson and Hetzer 2007; Liu and Zheng 2009). Time-lapse fluorescence microscopy of control reactions showed flattened sheets connected by tubules surrounding the mitotic spindle during its assembly, similar to ER organization described in other systems (Anderson and Hetzer 2008; Shibata et al. 2006; Voeltz et al. 2002) (Figure 1AB, top panels, Supplemental Video 1). During metaphase arrest, sheet structures appeared to diminish while an increase in length and number of ER tubules was observed (Figure 1B, top panel). To test the effects of Sar1, recombinant mutant proteins were added at metaphase. Purified GST was added to extracts at metaphase, and served as a control. The constitutively active Sar1 (H79G) mutant caused dramatic changes in mitotic membrane organization characterized by abundant and abnormally long membrane tubules compared to the control (Figure 1AB, middle panels, Figure 2B, Supplemental Video 2). In contrast, addition of the dominant negative Sar1 (T39N) mutant caused a loss of both sheet and tubule structures and an increase in membrane punctate structures (Figure 1AB, bottom panels). Interestingly, changes in membrane organization did not appear to disrupt the spindle.
Figure 1. Sar1 mutants alter membrane organization in metaphase Xenopus egg extracts.
(A) Metaphase-arrested spindles were generated in Xenopus egg extracts cycled through interphase in the presence of a rhodamine-labeled lipophilic dye CM-DiI to label membranes and Alexa 488-labeled tubulin to label microtubules. Reactions were treated with 5 μM GST (control), 5 μM constitutively active Sar1 (H79G) mutant, or 5 μM dominant-negative Sar1 (T39N) mutant, and images captured at 30 second intervals by time-lapse fluorescence microscopy. Scale Bar, 10 μm. (B) Enlarged images of mitotic membrane organization. Higher magnification images were created from 1A to demonstrate the interconnected sheet tubule network seen in mitotic Xenopus egg extracts (top row). Time-lapse images show that there appears to be an increase of tubule length over time. Scale Bar, 5 μm
Figure 2. Constitutively active Sar1 mutant decreases membrane sheet width and increases tubule length.
(A) Measurement of membrane sheet width. 20 different widths of sheets at their widest diameter were taken per experiment and averaged. Addition of dominant negative Sar1 T39N caused a decrease in sheet width. (B) Measurement of membrane tubule length. 20 different measurements of tubule length were taken per experiment and averaged. Addition of the constitutively active Sar1 H79G caused a dramatic increase in tubule length. (C) Ratio of average width / length upon addition of Sar1 proteins. Addition of Sar1 H79G caused a decrease in width / length ratio of membrane compared to Sar1 T39N which showed a slight increase in the width / length ratio. Measurements were taken from 3 separate extract experiments per condition. Error bars represent standard deviation.
Changes in membrane morphology were quantified by measuring average tubule lengths and sheet widths in the different reactions (Figure 2). Addition of the dominant negative Sar1 (T39N) mutant resulted in a decrease in the average sheet width compared to the GST control and Sar1 (H79G). In contrast, addition of the constitutively active Sar1 (H79G) caused a considerable increase in tubule length (Figure 2B), overall resulting in a dramatic decrease in the width / length ratio upon H79G addition, reflecting the significant increase in membrane tubulation (Figure 2C). Thus, under the conditions used, Sar1 can dramatically modify membrane organization and ER tubule formation, but neither GTPase obviously disrupts the spindle.
Altering membrane morphology does not affect spindle stability
Although we observed no gross spindle defects upon alteration of membrane structure at metaphase, we wondered whether spindle size or stability were affected, since membrane-associated factors such as the Golgi-associated protein GRASP65 the endocytotic adapter protein Epsin and nuclear intermediate filament lamin-B have been shown to regulate spindle dynamics (Liu and Zheng 2009; Sutterlin et al. 2005), We examined more closely the effects of altering membrane organization on spindle size and stability. Spindle length and area were quantified in reactions to which wildtype Sar1, Sar1H79G, Sar1T39N and GST were added at metaphase and fixed 10 minutes later (Figure 3). Spindle length was not affected, while Sar1 (H79G) caused a relative decrease in spindle area and Sar1 (T39N) caused an increase. However, both averages were within the standard error of control measurements and p-values show that these were not statistically significant changes. In addition, immunofluorescence analysis of fixed samples revealed that the spindle pole factor TPX2 (Gruss and Vernos 2004) localized normally in the presence of Sar1 mutants (data not shown). In summary, Sar1-mediated alteration of membrane organization does not affect spindle stability in Xenopus egg extracts.
Figure 3. Sar1 mutants do not affect bipolar spindle size and organization.
Length (pole to pole) and area of mitotic spindles were measured on preformed metaphase spindle reactions treated with either 5 μM GST (control), wildtype Sar1, constitutively active Sar1 (H79G) mutant, and dominant-negative Sar1 (T39N). N = ~80 spindles for each condition. Bars represent standard error.
Sar1 binds microtubules in egg extracts but does not cause significant defects in spindle assembly
Since ER membrane associates closely with the mitotic spindle and Sar1 binding to microtubules has been demonstrated in a biochemical screen in budding yeast (Barnes et al. 1992), we tested whether addition of wild type Sar1 or mutants affected microtubule organization during spindle assembly. Recombinant proteins were added to cycled reactions along with CSF extract to induce spindle assembly, and reactions were fixed at metaphase and immunostained for the spindle pole marker NuMA. Phenotypes were categorized as: normal bipolar spindle, multipolar spindle, splitting pole spindle, and aberrant spindle (Figure 4A). NuMA localization appeared unaffected in all phenotypic categories except aberrant spindle. Quantification of the different phenotypes revealed that addition of wildtype Sar1 or the constitutively active Sar1 (H79G) caused a small increase in pole organization defects characterized by outward bending of polar microtubules and a split pole (Figure 4B). However, examination of standard error and p-values suggest that this increase is not significant. Furthermore, it is important to note that a large percentage of the spindles formed normally in the presence of Sar1 mutants.
Figure 4. Sar1 mutants do not disrupt assembly of the mitotic spindle.
(A) Mitotic spindle assembly reactions were performed in Xenopus egg extract in the presence of buffer control, 5 μM Sar1 wt, 5 μM constitutively active (H79G) mutant, or 5 μM dominant-negative Sar1 (T39N) mutant, and allowed to progress to metaphase in the presence of X-rhodamine-labeled tubulin (red) to label microtubules. Samples were fixed and immunostained for NuMA (green), a spindle pole marker. Hoeschst dye was used for visualization of DNA (blue). Defects were classified as bipolar, muiltipolar, splitting pole, aberrant spindle. (B) Counts of spindle defects were performed on fixed samples described above. Buffer control N=459 spindles, Sar1wt N=468 spindles, Sar1H79G N=1037, Sar1T39N N=697 spindles. Bars represent standard error.
During interphase, activated Sar1 associates with the ER at the sites of cargo export, but its localization and function during mitosis remain unclear. There has been considerable difficulty visualizing Sar1 by immunofluorescence analysis and previous studies relied on cells specialized for high levels of protein secretion (Kuge et al. 1994). Likewise, we were unable to detect endogenous Sar1 using immunofluorescence analysis in Xenopus laevis egg extracts, most likely due to very low protein levels. In order to examine Sar1 localization in spindle assembly reactions in egg extracts, immunofluorescence analysis was performed after addition of recombinant Sar1 (Figure 5). Control reactions lacking exogenous Sar1 were devoid of Sar1 staining (Figure 5, top row). Sar1 wildtype displayed a polar localization with slight spreading of Sar1 along the spindle. Interestingly, the Sar1 constitutively active mutant, H79G, localized to the poles and more intensely along spindle microtubules, while the dominant negative Sar1 mutant, T39N, showed slightly reduced polar staining and little staining along spindle microtubules. This polar localization of Sar1 is consistent with several published studies demonstrating polar localization of ER membrane at mitosis (Audhya et al. 2007; Bobinnec et al. 2003; Frescas et al. 2006).
Figure 5. Sar1 localizes at the poles in the mitotic spindle.
Spindle assembly reactions in Xenopus egg extracts were performed in the presence of 5 μM GST (control), Sar1 wildtype (wt), constitutively active Sar1 (H79G) mutant, and dominant-negative Sar1 (T39N) mutant. Rhodamine-labeled tubulin (red) was added to the reactions to visualize the mitotic spindle and DNA (blue) was visualized using Hoeschst dye. Reactions were fixed and immunostained for Sar1 (green) localization on the mitotic spindle. Sar1 wt and constitutively active Sar1 (H79G) mutant, and to a lesser extent, dominant-negative Sar1 (T39N) localized to spindle poles. Control reactions do not show Sar1 localization, as we were unable to detect endogenous Sar1 in spindle assembly reactions. Scale Bar, 5 μM
To further examine the association between Sar1 mutants and mitotic microtubules, we employed a biochemical mirotubule pelleting assay to test whether Sar1 binds to spindle microtubules in metaphase-arrested egg extracts. CSF spindle assembly reactions in Xenopus laevis egg extracts were supplemented with Sar1wt, constitutively active Sar1 mutant protein (H79G), or Sar1 dominant-negative mutant protein (T39N). A control reaction containing the constitutive active Sar1 (H79G) mutant plus the microtubule depolymerizing agent nocodazole was also included. These reactions were allowed to incubate for 30 minutes (the time necessary for bipolar spindles to form in CSF extracts) then subjected to centrifugation and pelleted microtubules were probed for the presence of Sar1 by SDS-PAGE and immunoblot analysis. Sar1wt, H79G mutant and T39N mutant all co-pelleted with microtubules binding, but Sar1 H79G did not pellet by itself when microtubule polymerization was inhibited with nocodazole (Figure 6). Taken together, these data suggest that Sar1 specifically associates with microtubule structures, but is not involved in mitotic spindle assembly.
Figure 6. Sar1 co-pellets with microtubules.

CSF spindle assembly reactions in Xenopus egg extracts were incubated with 5 μM Sar1wildtype (wt), constitutive active Sar1 (H79G) mutant, and dominant-negative Sar1 (T39N) mutant. Control reaction for the 5 μM Sar1 (H79G) mutant contained 20 μM nocodazole, a microtubule depolymerizing agent. Reactions were incubated for 30 min then pelleted through a 40% glycerol cushion and the pellet was probed with α-Sar1 antibody by SDS-PAGE and immunoblot.
Sar1 addition does not affect the mitotic localization of the lamin B network
Mitotic spindle assembly is a dynamic process requiring a rapid reorganization of the microtubule network to form the bipolar array. The existence of a scaffold or matrix has been postulated to support the assembly and dynamics of the spindle and recently several studies have identified components that may play a role in this process (Scholey et al. 2001; Zheng and Tsai 2006). In particular, the intermediate filament protein lamin B, a component of the nuclear lamina has been proposed to be a structural component of the mitotic spindle matrix (Tsai et al. 2006). To investigate if altering the membrane organization at mitosis affects the lamin B network at the spindle, wildtype Sar1 or mutant versions were added to spindle assembly reactions, which were fixed and immunostained for lamin B3 at metaphase (Figure 7A). Lamin B3 localization in the presence of the wildtype Sar1 and the constitutively active Sar1 (H79G) mutant, appeared to be slightly reduced at spindle poles (Figure 7A) but could be due to the decreased density of microtubules. This result was quantified by measurement of lamin B3 and tubulin fluorescence intensity (Figure 7B), which showed that lamin B3 and tubulin intensity are well correlated. From this, we can conclude that Sar1 is not affecting lamin B3 localization and suggest that specific organization of membrane structures is not required for the lamin network to form on the spindle.
Figure 7. The intermediate filament, Lamin B3 localizes properly in the presence of Sar1 mutants.
(A) Mitotic spindle assembly reactions were performed in the presence of 5 μM GST (control), 5 μM Sar1 wildtype (wt), 5 μM constitutively active Sar1 (H79G) mutant, or 5 μM dominant-negative Sar1 (T39N) mutant, with each being added individually prior to entry into mitosis and mitotic spindle formation. Rhodamine-labeled tubulin (red) was added to the reactions to visualize the mitotic spindle and DNA (blue) was visualized using Hoeschst dye. Reactions were fixed and immunostained for lamin B3 (green) localization on the mitotic spindle. Lamin B3 localization is unaffected with addition of Sar1 wt and Sar1 mutant proteins. Scale Bar, 10μm. (B) Measurement of fluorescence intensity of lamin B3 in the presence of Sar1 mutants. Measurement of fluorescence intensity in control reactions show that lamin B3 levels correlates with tubulin intensity. This correlation does not change in the presence of Sar1 wt and Sar1 mutants. Yellow line represents measurement of intensity.
Entry into mitosis sees major changes in cellular architecture including a dramatic rearrangement of the ER network, however many questions remain in understanding this reorganization and its role in other mitotic events. In this study, we examined the role of membrane organization on mitotic events by modulating of the activity of the small GTPase Sar1. Our experiments show that mitotic membranes are a combination of sheets and tubules, and that Sar1GTP drives tubulation of the membrane network, which is not surprising given that Sar1 has been shown to promote membrane curvature in other in vitro systems (Bielli et al. 2005; Lee et al. 2005). Whether Sar1 is normally required for proper mitotic ER morphology is unclear. A previous study of Golgi disassembly and partitioning during mitosis suggested that Sar1 is inactivated at the start of mitosis as export from the ER to the Golgi is inhibited, allowing for the redistribution of Golgi enzymes to the ER (Altan-Bonnet et al. 2006). However, our experiments show that dominant negative Sar1 (T39N), which prevents GTP exchange and inactivates endogenous Sar1, strongly inhibited tubule formation in the metaphase egg extract. Immunodepletion of Sar1 would help to address its requirement, but is not yet possible with the reagents currently available. Nevertheless, Sar1 mutants provide a valuable tool to study the consequences of membrane alteration for mitosis and organelle inheritance.
Our study indicates that alteration of the ER membrane network does not play a vital role in mitotic spindle assembly and organization in Xenopus egg extracts. This finding is intriguing in light of other studies that showing that Golgi and endocytic membrane associated proteins are involved in spindle organization (Liu and Zheng 2009; Sutterlin et al. 2005; Tsai et al. 2006). Perhaps these membrane-associated spindle factors exist in a separate intracellular membrane population or their function does not require a particular membrane topology. This study establishes an assay to examine spindle assembly and membrane morphology in the same reaction. In the future, addition or depletion of other factors, and/or the use of more specific membrane labels, will better define the behavior of distinct membrane populations and the requirements for membrane-associated factors for spindle assembly and other mitotic events.
Methods and Materials
Expression and purification of recombinant proteins
Plasmids encoding N-terminal, GST-tagged human SAR1A wild type, H79G and T39N mutants were gifts from R. Schekman (Fromme et al. 2007). All proteins were expressed in E. coli according to standard protocols. Proteins were purified by glutathione sepharose 4B chromatography, and dialyzed into phosphate-buffered saline containing 150 mM KCl, 1 mM GTP and 3% glycerol. All proteins were diluted in XB (10 mM Hepes pH 7.7, 1 mM MgCl2, 0.1 mM CaCl2, 100 mM KCl, 100 μM GTP and 50 mM Sucrose) to a concentration of 100 μM before addition to egg extracts.
Preparation of Xenopus egg extracts and in vitro assays
Xenopus laevis egg extracts were prepared, and spindle assembly reactions with replicated sperm chromosomes preformed as previously described (Hannak and Heald 2006). For membrane visualization in spindle assembly reactions, the lipophilic general membrane dye CM-DiI (Invitrogen) was added to cytostatic factor-arrested (CSF) extracts at a concentration of 1 μM along with sperm nuclei. For visualization of the mitotic spindle, X-rhodamine- or alexa 488-labeled porcine brain tubulin was added to extracts at a final concentration of 50 μg/ml (Maresca and Heald 2006). Slides used for immunofluorescence analysis were precleaned in 1M sodium hydroxide (NaOH) for 2+ hours, rinsed in water (3X), and dried overnight prior to usage. Microtubule pelleting assays were preformed as previously described (Budde et al. 2006), by diluting spindles with 30% glycerol in BRB80 (80mM K-Pipes, pH 6.8, 1mM MgCl2, and 1mM EGTA) and centrifuging through a cushion of 40% glycerol / BRB80. Pellets were analyzed by immunoblotting with a monoclonal α-tubulin antibody (1:5000; E7 Developmental Studies Hybridoma Bank) and a polyclonal α-Sar1 antibody (gift from R. Schekman) using standard techniques. Control reactions contained 20 μM Nocodazole (Sigma-Aldrich).
Immunofluorescence and time-lapse microscopy
Spindle reactions were spun onto coverslips and fixed as previously described (Maresca and Heald 2006). Rabbit polyclonal α-Sar1 antibody (R. Schekman), Strategic Diagnostics Inc. (SDI) antibodies against TPX2, and NuMA were diluted 1:1000, and rabbit α-Lamin-B3 was used at 1:500. Images were collected with a fluorescence microscope (model BX51; Olympus) with a dry 40X NA 0.75 objective, a cooled CCD camera (model Orcall; Hamamatsu), and MetaMorph software (Molecular Devices). Live movies of spindle reactions were obtained at 30s intervals in the presence of an oxygen scavenging mix (Hartman et al. 1998). Spindle length and area were quantified using MATLAB. Images were processed using Adobe Photoshop.
Supplementary Material
Acknowledgements
We thank E. Kieserman and D. Halpin for discussions and technical assistance, R. Loughlin for analytical analysis and Matlab scripts used in this paper, E. Hannak for technical assistance with Arf1 constructs. We would also like to thank J. Fromme and R. Schekman for the generous gift of antibodies and constructs of Sar1, N. Cheng and R. Khodary for assistance with protein purification assays. B. Riggs is supported by a NRSA postdoctoral fellowship F32 CA126249-01. R. Heald is supported by the National Institutes of Health grant R01 GM057839.
References
- Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J. Molecular basis for Golgi maintenance and biogenesis. Curr Opin Cell Biol. 2004;16(4):364–72. doi: 10.1016/j.ceb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Altan-Bonnet N, Sougrat R, Liu W, Snapp EL, Ward T, Lippincott-Schwartz J. Golgi inheritance in mammalian cells is mediated through endoplasmic reticulum export activities. Mol Biol Cell. 2006;17(2):990–1005. doi: 10.1091/mbc.E05-02-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol. 2007;9(10):1160–6. doi: 10.1038/ncb1636. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Hetzer MW. Shaping the endoplasmic reticulum into the nuclear envelope. J Cell Sci. 2008;121(Pt 2):137–42. doi: 10.1242/jcs.005777. [DOI] [PubMed] [Google Scholar]
- Aridor M, Fish KN, Bannykh S, Weissman J, Roberts TH, Lippincott-Schwartz J, Balch WE. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152(1):213–29. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. J Cell Biol. 2007;178(1):43–56. doi: 10.1083/jcb.200701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G, Louie KA, Botstein D. Yeast proteins associated with microtubules in vitro and in vivo. Mol Biol Cell. 1992;3(1):29–47. doi: 10.1091/mbc.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA. Inheritance of the endoplasmic reticulum and Golgi apparatus. Curr Opin Cell Biol. 2002;14(4):496–9. doi: 10.1016/s0955-0674(02)00345-9. [DOI] [PubMed] [Google Scholar]
- Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171(6):919–24. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinnec Y, Marcaillou C, Morin X, Debec A. Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil Cytoskeleton. 2003;54(3):217–25. doi: 10.1002/cm.10094. [DOI] [PubMed] [Google Scholar]
- Budde PP, Desai A, Heald R. Analysis of microtubule polymerization in vitro and during the cell cycle in Xenopus egg extracts. Methods. 2006;38(1):29–34. doi: 10.1016/j.ymeth.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Chaldakov GN, Vankov VN. Association of smooth membranes with spindle microtubules in the arterial smooth muscle cell. Acta Biol Hung. 1985;36(2):179–83. [PubMed] [Google Scholar]
- Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- Frescas D, Mavrakis M, Lorenz H, Delotto R, Lippincott-Schwartz J. The secretory membrane system in the Drosophila syncytial blastoderm embryo exists as functionally compartmentalized units around individual nuclei. J Cell Biol. 2006;173(2):219–30. doi: 10.1083/jcb.200601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, Cosson P, Schekman R, Orci L. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13(5):623–34. doi: 10.1016/j.devcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde S, Heald R. Mechanisms and molecules of the mitotic spindle. Curr Biol. 2004;14(18):R797–805. doi: 10.1016/j.cub.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gruss OJ, Vernos I. The mechanism of spindle assembly: functions of Ran and its target TPX2. J Cell Biol. 2004;166(7):949–55. doi: 10.1083/jcb.200312112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak E, Heald R. Investigating mitotic spindle assembly and function in vitro using Xenopus laevis egg extracts. Nat Protoc. 2006;1(5):2305–14. doi: 10.1038/nprot.2006.396. [DOI] [PubMed] [Google Scholar]
- Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93(2):277–87. doi: 10.1016/s0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125(1):51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122(4):605–17. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zheng Y. A requirement for epsin in mitotic membrane and spindle organization. J Cell Biol. 2009;186(4):473–80. doi: 10.1083/jcb.200902071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KR, Yamamoto Y, Baker AL, Watkins SC, Coyne CB, Conway JF, Aridor M. Sar1 assembly regulates membrane constriction and ER export. J Cell Biol. 2010;190(1):115–28. doi: 10.1083/jcb.201004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Heald R. Methods for studying spindle assembly and chromosome condensation in Xenopus egg extracts. Methods Mol Biol. 2006;322:459–74. doi: 10.1007/978-1-59745-000-3_33. [DOI] [PubMed] [Google Scholar]
- Puhka M, Vihinen H, Joensuu M, Jokitalo E. Endoplasmic reticulum remains continuous and undergoes sheet-to-tubule transformation during cell division in mammalian cells. J Cell Biol. 2007;179(5):895–909. doi: 10.1083/jcb.200705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey JM, Rogers GC, Sharp DJ. Mitosis, microtubules, and the matrix. J Cell Biol. 2001;154(2):261–6. doi: 10.1083/jcb.200101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126(3):435–9. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Shorter J, Warren G. Golgi architecture and inheritance. Annu Rev Cell Dev Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- Stroud WJ, Jiang S, Jack G, Storrie B. Persistence of Golgi matrix distribution exhibits the same dependence on Sar1p activity as a Golgi glycosyltransferase. Traffic. 2003;4(9):631–41. doi: 10.1034/j.1600-0854.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- Sutterlin C, Polishchuk R, Pecot M, Malhotra V. The Golgi-associated protein GRASP65 regulates spindle dynamics and is essential for cell division. Mol Biol Cell. 2005;16(7):3211–22. doi: 10.1091/mbc.E04-12-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M. Dynamics of the endoplasmic reticulum and golgi apparatus during early sea urchin development. Mol Biol Cell. 2000;11(3):897–914. doi: 10.1091/mbc.11.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311(5769):1887–93. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3(10):944–50. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Sanger JW, Sanger JM. Dynamics of organelles in the mitotic spindles of living cells: membrane and microtubule interactions. Cell Motil Cytoskeleton. 1993;26(1):19–39. doi: 10.1002/cm.970260104. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Tsai MY. The mitotic spindle matrix: a fibro-membranous lamin connection. Cell Cycle. 2006;5(20):2345–7. doi: 10.4161/cc.5.20.3365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.