Abstract
Nitrite, long considered a biologically inert metabolite of nitric oxide (NO) oxidation, is now accepted as a physiological storage pool of NO that can be reduced to bioactive NO in hypoxic conditions to mediate a spectrum of physiological responses in blood and tissue. This graphical review will provide a broad overview of the role of nitrite in physiology, focusing on its formation and reduction to NO as well as its regulation of the mitochondrion—an emerging subcellular target for its biological actions in tissues.
Keywords: Nitrite, Nitrate, Mitochondria, Hypoxia
Highlights
► Nitrite is a stable physiological reservoir of nitric oxide. ► Nitrite reductase enzymes reduce nitrite to NO to mediate physiological responses. ► The mitochondrion is an important physiological target for nitrite.
Introduction
While nitrite (NO2−) was for decades considered to be physiologically inert, it is now accepted that NO2− represents a stable reservoir that can be reduced to bioactive NO and other reactive nitrogen species during hypoxia to mediate physiological signaling [1]. Concentrations of the anion are maintained at low micromolar levels in tissues (1–20 μM) and nanomolar levels in blood (100–200 nM) [2,3]. The majority of NO2− is derived from the oxidation of NO Synthase (NOS)-generated NO. While this one electron auto-oxidation of NO proceeds relatively slowly (k=2×106 M−2 s−1) compared to the two electron oxidation of NO to nitrate (NO3−) by heme proteins in the blood and tissue (k=8×107 M−1 s−1), NO2− formation can be catalyzed by the multicopper oxidase ceruloplasmin in the plasma or cytochrome c oxidase (ccox) in tissues [4–6]. A smaller proportion (∼30%) of NO2− is derived from dietary sources. Nitrite itself is present in cured meats, however green leafy vegetable are a principal source of NO3−, which is reduced to NO2− in the body by commensal bacteria in the oral cavity and the gastrointestinal tract and to a lesser extent by mammalian xanthine oxidoreductase (XOR) in the liver [7] (Fig. 1).
Fig. 1.
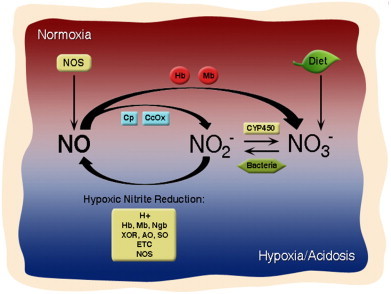
The nitrite–NO cycle. In normoxia, NOS is functional and generates NO, which is oxidized by Mb and Hb to nitrate and by cytochrome c oxidase (ccox) and ceruloplasmin (Cp) to nitrite. Nitrite is also derived from the diet as well as the normoxic oxidation of nitrite by cytochrome P450 enzymes. In hypoxia, nitrate is reduced to nitrite by anaerobic commensal bacteria and nitrite is reduced to bioactive NO by a number of mammalian nitrite reductase enzymes including Hb, Mb, neuroglobin (Ngb), xanthine oxidoreductase (XOR), aldehyde oxidase (AO), sulfite oxidase (SO), components of the mitochondrial electron transport chain (ETC) and NOS.
Once formed, NO2− is reduced to bioactive NO through acidification or via reaction with a number of proteins possessing NO2− reductase activity, including heme globins [8–10], molybdenum-containing enzymes [11,12], NOS [13], and components of the mitochondrial electron transport chain (ETC) [14–16]. While the reaction mechanism by which each of these systems reduce NO2− has been elucidated to differing degrees, it is clear that NO2− reduction by all mammalian reductases is optimized in conditions of hypoxia and acidosis (Fig. 2). Thus, NO2− reduction represents a physiological mechanism by which NO production is sustained in hypoxic conditions, during which catalytic NO generation by NOS, which relies on oxygen as a substrate, is compromised (Fig. 1).
Fig. 2.
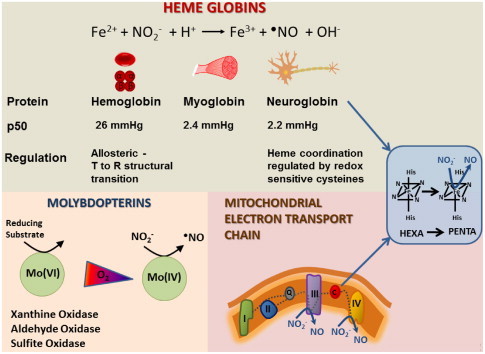
Major classes of mammalian nitrite reductases. Heme globins (hemoglobin, myoglobin, neuroglobin) reduce nitrite through the reaction of nitrite with deoxyheme (ferrous) in the presence of a proton, to generate NO and yielding oxidized heme. Molybdenum containing enzymes (xanthine oxidoreductase, aldehyde oxidase, sulfite oxidase) reduce nitrite at their molybdenum site in hypoxic conditions when reduction of the molybdenum co-factor is favored. Within the mitochondrial ETC, complexes III and IV reduce nitrite in hypoxia. Cytochrome c, like neuroglobin exists as a hexacoordinate heme protein. Cytochrome c and neuroglobin efficiently reduce nitrite when the bond between the iron and the distal histidine is broken such that the heme is penta-coordinate.
Perhaps the most well-characterized mammalian NO2− reductases are the heme globins, which catalyze the following reaction:
| deoxy(FeII)+NO2−+H+→(FeIII)+•NO+OH− |
For hemoglobin (Hb), the rate of this reaction is regulated by the allosteric structural transition of the protein from its R (relaxed) to T (tense) state, such that the maximal rate of Hb-catalyzed NO2− reduction occurs around the p50 of the protein (26 mmHg) [17]. This reaction has been implicated in the mechanism underlying hypoxic vasodilation. In tissues, the monomeric heme globins, myoglobin (Mb) and neuroglobin (Ngb), catalyze NO2− reduction by the same reaction but at lower oxygen tensions (p50 Mb=2.4 mmHg; p50 Ngb=2.2 mmHg). Mb-dependent NO2− reduction has been implicated in the protective effects of NO2− after ischemia/reperfusion (I/R) in the heart as well as in vasodilation [18,19]. Neuroglobin, present in the brain and retina contains a hexa-coordinated group, which can be converted to a penta-coordinate heme capable of reducing NO2− at a greater reaction rate than Mb and Hb. This transition of the heme coordination is regulated by the oxidation of two surface cysteine residues on the protein [10]. Molybdenum containing enzymes, of which XOR is best characterized, have been implicated in the mechanism underlying nitrite-dependent protection after I/R as well as protective vascular remodeling after vascular injury [12,20–22]. While the exact reaction scheme underlying XOR-mediated NO2− reduction is unclear, it is known that this reaction occurs at the molybdenum cofactor of XOR and aldehyde oxidase [11,12]. Nitrite reduction by the mitochondrial ETC has been shown to occur in near anoxic conditions, predominantly at pH less than 7 and with relatively high (millimolar) concentrations of NO2− [15]. Within the ETC, complexes III and IV predominate, while the hexacoordinate protein cytochrome c can reduce NO2− to NO when it is converted to its pentacoordinate form, similarly to Ngb [14] (Fig. 2). Nitrite reduction by these enzymes with differing oxygen affinities, tissue distribution and rates of reduction, ensures NO generation and nitrosative modification of target proteins over a wide range of physiological hypoxia in the cell [1]. This leads to downstream signaling to induce a wide spectrum of biological responses including hypoxic vasodilation [8], stimulation of angiogenesis [23], modulation of glucose metabolism [24], augmentation of exercise efficiency [20], regulation of mitochondrial function [9,25,26] and tolerance to I/R [22,27–29] (Fig. 3).
Fig. 3.
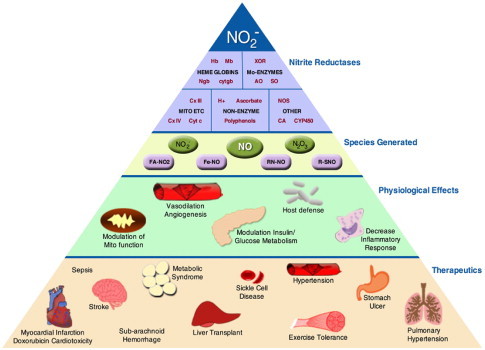
The nitrite pyramid. Nitrite is reduced by a number of nitrite reductase enzymes in hypoxia including heme globins, molybdenum containing enzymes, components of the mitochondrial ETC, other enzymes (NOS, cytochrome P450—CYP450, and carbonic anhydrase—CA) as well as non-enzymatic reactions (acidification, reaction with polyphenols and ascorbate). Reduction of nitrite generates NO as well as nitrosating (N2O3) and nitrating (•NO2) species, which can modify protein and lipids to form nitrated fatty acids (FA-NO2), iron nitrosyl (Fe-NO), nitrosamines (RN-NO) and S-nitrosothiols (RSNO). These species mediate signaling leading to downstream physiological effects including modulation of mitochondrial function, vasodilation, stimulation of angiogenesis, modulation of glucose metabolism, decrease inflammation, and modulate host defenses. These species also mediate therapeutic benefits in a number of pathologies in virtually all organ systems.
It is now well-established that NO2− mediates a number of beneficial tissue responses. While the downstream molecular signaling underlying these effects remains unclear, the mitochondrion has emerged as a major sub-cellular target of NO2−. Accumulating evidence demonstrates that NO2− differentially regulates mitochondrial function through the modulation of specific proteins within the organelle in both physiology and pathology (Fig. 4). The inhibition of mitochondrial complexes I and IV have been implicated in NO2−-mediated cytoprotection after I/R [18,26]. The mitochondrion plays a central role in the progression of I/R injury. During ischemia, ATP production is limited, contributing to the depletion of high energy phosphate stores. Upon reperfusion, overwhelming influx of oxygen into the respiratory chain results in excessive reactive oxygen species generation at complexes I and III and oxidation of critical proteins leading to opening of the mitochondrial permeability transition pore (PTP) as well as release of cytochrome c to initiate apoptosis [30,31]. Inhibitors of complex I have been demonstrated to attenuate I/R injury by limiting electron flow through the ETC at reperfusion, thereby limiting ROS generation [32]. It has now been demonstrated in a number of animal models of I/R that NO2− inhibits complex I activity specifically after ischemia [26,33,34]. This inhibition is attributed to the NO2−-dependent S-nitrosation of complex I and results in an attenuation of mitochondrial ROS generation, as well as inhibition of PTP opening and cytochrome c release after I/R [26].
Fig. 4.
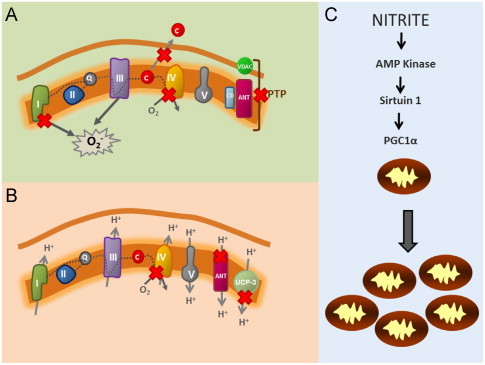
Nitrite-dependent modulation of mitochondrial function. Red “X” denotes points of modulation by nitrite. (A) During ischemia/reperfusion, nitrite inhibits complex I by S-nitrosation of the complex, leading to decreased mitochondrial reactive oxygen species generation. This decreases cytochrome c release and inhibits opening of the permeability transition pore (PTP), which consists of the Adenine nucleotide translocase (ANT), Cyclophylan D (CD) and the Voltage Gated Anion Channel (VDAC). Nitrite also inhibits complex IV during ischemia to potentially mediate cardiac hibernation. (B) Slight inhibition of complex IV by nitrite can decrease oxygen consumption, while not impacting ATP generation during exercise. Nitrite also decreases expression of the ANT (adenine nucleotide translocase) and Uncoupling protein 3 (UCP-3), both of which dissipate the proton gradient by allowing the re-entry of protons into the mitochondrial matrix. In aggregate, these effects would decreases respiratory rate as well as proton leak, as observed in human subjects with increased exercise efficiency after oral nitrate ingestion. (C) Chronic nitrite treatment activates AMP kinase and sirtuin-1 which de-acetylates PPARγ co-activator-1α (PGC1α) to increase mitochondrial number in hypoxia.
The reversible inhibition of cytochrome c oxidase (ccox; complex IV) has also been implicated in NO2−-mediated protection after I/R [18]. Ccox, the terminal complex of the etc to which oxygen binds at the copperB/hemea3 binuclear center and is reduced to water, is the primary target of NO within the mitochondrion. Binding of NO to the binuclear center excludes oxygen binding and inhibits respiration [35]. This NO-dependent inhibition of mitochondrial oxygen consumption is greater as oxygen tension is decreased and fully reversible [35]. We have demonstrated that Mb-dependent reduction of NO2− to NO results in the inhibition of ccox in the heart [9,18]. This inhibition of mitochondrial respiration potentially underlies the downregulation of metabolism, a protective phenomenon termed “short-term hibernation” that is responsible for conserving oxygen as well as high energy phosphates during prolonged ischemic episodes [18]. Once reperfusion commences, this inhibition is removed and metabolic function returns (Fig. 4A).
Nitrite dependent inhibition of ccox also potentially regulates responses to physiological hypoxia, such as that present in the muscle during exercise. Larsen and colleagues recently demonstrated that ingestion of NO3− decreased whole body oxygen consumption during exercise without changing maximal attainable work rate in human subjects [20]. This increase in exercise efficiency, which was associated with augmented plasma NO2− levels, has now been corroborated by a number of studies in various exercise models. While the underlying mechanism of this beneficial effect is not completely elucidated, a decrease in the rate of oxygen consumption due to proton leak and state 4 respiration in the skeletal muscle of subjects receiving NO3− was reported [25]. Further, the authors reported a NO3−-induced decrease in the expression of uncoupling protein 3 (UCP-3) and the adenine nucleotide translocase (ANT), two proteins which facilitate proton leak [25]. Notably, numerous studies of respiratory control suggest that oxygen consumption by ccox can be inhibited to a certain degree without significantly affecting ATP production by the ETC [36,37]. Hence, it is possible that NO2−-mediated inhibition of ccox could decrease oxygen consumption without negatively impacting ATP generation, contributing to the augmentation of the ratio of ATP generated per mole of oxygen consumed that was observed in subjects after NO3− ingestion.
In addition to modulating specific proteins within the mitochondrion, NO2− has also recently been shown to stimulate hypoxic mitochondrial biogenesis [38]. Treatment of cells with physiological levels of NO2− during chronic hypoxia induced a significant increase in mitochondrial number per cell. This effect is mediated through the classical mitochondrial biogenesis pathway involving the nitrite-dependent activation of AMP Kinase, Sirtuin-1, PPARγ-coactivator-1α and upregulation of mitochondrial transcription factors. This effect, observed both in vitro as well as in a rat model of restenosis, is associated with NO2−-dependent protective vascular remodeling [38].
While the field of nitrite biology has advanced rapidly in the last decade, several challenges remain. The mechanisms underlying the regulation of individual nitrite reductases as well as the assessment of crosstalk between mammalian nitrite reductases are currently being elucidated. Ongoing study in a number of labs is identifying downstream targets through which nitrite mediates its effects. Future study will further delineate the role of nitrite reduction versus NOS-dependent NO generation in physiological NO signaling.
Acknowledgments
This work was supported by the NIH 1R01HL096973, the AHA 09SDG2150066, the Institution of Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 2.Bryan N.S., Rassaf T., Maloney R.E., Rodriguez C.M., Saijo F., Rodriguez J.R. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejam A., Hunter C.J., Pelletier M.M., Hsu L.L., Machado R.F., Shiva S. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunori M., Giuffre A., Forte E., Mastronicola D., Barone M.C., Sarti P. Control of cytochrome c oxidase activity by nitric oxide. Biochimica et Biophysica Acta. 2004;1655:365–371. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Doyle M.P., Hoekstra J.W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. Journal of Inorganic Biochemistry. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 6.Shiva S., Wang X., Ringwood L.A., Xu X., Yuditskaya S., Annavajjhala V. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nature Chemical Biology. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 7.Jansson E.A., Huang L., Malkey R., Govoni M., Nihlen C., Olsson A. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nature Chemical Biology. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 8.Cosby K., Partovi K.S., Crawford J.H., Patel R.P., Reiter C.D., Martyr S. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 9.Shiva S., Huang Z., Grubina R., Sun J., Ringwood L.A., MacArthur P.H. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 10.Tiso M., Tejero J., Basu S., Azarov I., Wang X., Simplaceanu V. Human neuroglobin functions as a redox-regulated nitrite reductase. The Journal of Biological Chemistry. 2011;286:18277–18289. doi: 10.1074/jbc.M110.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Kundu T.K., Zweier J.L. Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. The Journal of Biological Chemistry. 2009;284:33850–33858. doi: 10.1074/jbc.M109.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H., Samouilov A., Liu X., Zweier J.L. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry. 2003;42:1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 13.Vanin A.F., Bevers L.M., Slama-Schwok A., van Faassen E.E. Nitric oxide synthase reduces nitrite to NO under anoxia. Cellular and Molecular Life Sciences: CMLS. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu S., Azarova N.A., Font M.D., King S.B., Hogg N., Gladwin M.T. Nitrite reductase activity of cytochrome c. The Journal of Biological Chemistry. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castello P.R., David P.S., McClure T., Crook Z., Poyton R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Nohl H., Staniek K., Sobhian B., Bahrami S., Redl H., Kozlov A.V. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochimica Polonica. 2000;47:913–921. [PubMed] [Google Scholar]
- 17.Huang Z., Shiva S., Kim-Shapiro D.B., Patel R.P., Ringwood L.A., Irby C.E., Huang K.T. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. The Journal of Clinical Investigation. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendgen-Cotta U.B., Merx M.W., Shiva S., Schmitz J., Becher S., Klare J.P. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Totzeck M., Hendgen-Cotta U.B., Luedike P., Berenbrink M., Klare J.P., Steinhoff H.J. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen F.J., Weitzberg E., Lundberg J.O., Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiologica (Oxford) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 21.Alef M.J., Vallabhaneni R., Carchman E., Morris S.M., Jr., Shiva S., Wang Y. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. The Journal of Clinical Investigation. 2011;121:1646–1656. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webb A., Bond R., McLean P., Uppal R., Benjamin N., Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar D., Branch B.G., Pattillo C.B., Hood J., Thoma S., Simpson S. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlstrom M., Larsen F.J., Nystrom T., Hezel M., Borniquel S., Weitzberg E., Lundberg J.O. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen F.J., Schiffer T.A., Borniquel S., Sahlin K., Ekblom B., Lundberg J.O., Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabolism. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Shiva S., Sack M.N., Greer J.J., Duranski M., Ringwood L.A., Burwell L. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. The Journal of Experimental Medicine. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duranski M.R., Greer J.J., Dejam A., Jaganmohan S., Hogg N., Langston W. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. The Journal of Clinical Investigation. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung K.H., Chu K., Ko S.Y., Lee S.T., Sinn D.I., Park D.K. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke: A Journal of Cerebral Circulation. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 29.Dezfulian C., Raat N., Shiva S., Gladwin M.T. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovascular Research. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burwell L.S., Brookes P.S. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxidants & Redox Signaling. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 31.Murphy E., Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiological Reviews. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burwell L.S., Nadtochiy S.M., Brookes P.S. Cardioprotection by metabolic shut-down and gradual wake-up. Journal of Molecular and Cellular Cardiology. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dezfulian C., Shiva S., Alekseyenko A., Pendyal A., Beiser D.G., Munasinghe J.P. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raat N.J., Noguchi A.C., Liu V.B., Raghavachari N., Liu D., Xu X. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radical Biology & Medicine. 2009;47:510–517. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleeter M.W., Cooper J.M., Darley-Usmar V.M., Moncada S., Schapira A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Letters. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 36.Brand M.D., Murphy M.P. Control of electron flux through the respiratory chain in mitochondria and cells. Biological Reviews of the Cambridge Philosophical Society. 1987;62:141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 37.Gnaiger E., Lassnig B., Kuznetsov A., Rieger G., Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. Journal of Experimental Biology. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- 38.Mo L., Wang Y., Geary L., Corey C., Alef M.J., Beer-Stolz D. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radical Biology & Medicine. 2012;53:1440–1450. doi: 10.1016/j.freeradbiomed.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]


