Abstract
Herpes simplex virus type-1 (HSV-1) is among the most common human pathogens worldwide. Its entry into host cells is an intricate process that relies heavily on the ability of the viral glycoproteins to bind host cellular proteins and to efficiently mediate fusion of the virus envelope with the cell membrane. Acquisition of HSV-1 results in a lifelong latent infection. Due to the cycles of reactivation from a latent state, much emphasis has been placed on the management of infection through the use of DNA synthesis inhibitors. However, new methods are needed to provide more effective treatment at earlier phases of the viral infection and to prevent the development of drug resistance by the virus. This review outlines the infection process and the common therapeutics currently used against the fundamental stages of HSV-1 replication and fusion. The remainder of this article will focus on a new approach for HSV-1 infection control and management, the concept of glycoprotein-receptor targeting.
Keywords: Herpes Simplex Virus, Antivirals, Pathogenesis
INTRODUCTION
HSV-1 infections are endemic throughout the world. As a ubiquitous human pathogen, HSV-1 ranks as one of the most common viruses acquired by humans [1–3]. In the United States alone, 57% of adults are seropositive for HSV-1 [4]. Worldwide, 50% to 90% of individuals are seropositive for this neurotropic virus [5, 6]. Once HSV-1 is acquired, whether through sexual or nonsexual contact, the virus successfully avoids clearance by the immune system by entering into a state of latency, resulting in a lifelong infection [7–14]. As a result of the latent stage, the viral infection is considered incurable, leaving infected persons to live as lifelong reservoirs for the virus as complete clearance of the virus is seldom achieved [15–18]. The fact that viral reactivation may occur by many environmental and non-environmental stimuli such as ultraviolet light, immunosuppressant state, menstruation, emotional stress, and skin trauma only serves to highlight the problems associated with latency. Common clinical manifestations include blisters at the initial site of infection, but blindness, encephalitis, and neonatal infections can also occur [19–21].
As knowledge of HSV-1 infection grows, innovative antiviral therapeutic design and potential new targets for viral management are being identified. These new therapeutic approaches are challenging to develop as they have to fit within the parameters of high bioavailability, low cost, low toxicity, and easy synthesis. It is also important for new drugs to be based on non-traditional targets, as this will allow prescription of multi-drug therapy with relatively lower risk of drug resistance development. But regardless of the challenge inherent in developing new antivirals against HSV-1, novel therapies are needed. The currently available antivirals against HSV-1 infections are not always effective in given conditions and viral resistance may develop. In addition, the current antivirals as a whole also do little to preclude the establishment of viral latency and the resulting consequences following viral reactivation [19–22]. This article presents an overview of the current therapeutic approaches against HSV-1 with specific focus on currently available viral DNA synthesis inhibitors and the blockers of viral fusion activity. We will then present a promising alternative to current therapeutics (i.e. glycoprotein-receptor targeting) as a new proposed means to clinically inhibit HSV-1 pathogenesis.
CURRENT THERAPIES
HSV-1 DNA REPLICATION INHIBITORS
The fact that most current therapies target HSV-1 replication is not surprising given that the initiation of replication is a highly ordered process and any disturbance to this process adversely affects viral propagation. Once HSV-1 enters the host cell, the viral capsid is transported to nuclear pores in a process mediated by the dynein/dynactin motor protein complex [23] where it delivers double stranded linear DNA into the nucleus [22]. Within 30 minutes following virus entry [24], the viral DNA is then converted to a circular form prior to the initiation of replication [25]. Shortly after cell-entry, HSV-1 initiates a series of viral gene expression that is regulated by VP16, which is a late viral protein that is a component of the virion particles [26]. VP16, along with the cellular proteins Oct-1 and HCF, activate RNA polymerase II-dependent transcription [27]. All the following viral transcripts are synthesized via this cellular RNA polymerase II [26]. ICP0, ICP4, ICP22, and ICP27, all of which are IE proteins, further transduce gene expression [26]. ICP4 is a protein essential to the virus and is necessary for the efficient expression of viral DE and other late proteins [28]. ICP22 is not considered to be an essential viral protein, but regulates RNA polymerase II activity via regulation of DE and viral late gene expression [29]. ICP27, in addition to also regulating viral DE and late gene expression, is involved in inhibiting the host cell gene expression following viral cell entry [30, 31]. ICP0 is also considered a nonessential viral protein, but it is important in both lytic and latent infection as it activates viral gene expression during the former and is necessary for efficient reactivation during the latter [32]. Additionally important to viral gene expression is Vhs (virion host shut-off), a tegument protein that is able to regulate the cascade of viral gene expression by influencing the stability of viral transcripts [33]. However, this protein is also important in compromising the stability of cellular transcripts, a factor that favors expression of viral transcripts instead [26].
Following this initiation of the cascade of viral gene expression and proper localization to the nucleus [26], lytic replication is initiated by recruitment of seven essential viral proteins that include an origin binding protein, UL9; a helicase-primase heterotrimer containing UL5, UL8, and UL 52; a single-stranded–DNA-binding protein, ICP8 (or UL29); a viral DNA polymerase, UL30, and a polymerase accessory protein, UL42 [34, 35]. The formation of a scaffold consisting of UL5, UL8, UL52, and UL9 promotes the recruitment of IPC8. UL9 and ICP8 are necessary to distort the immediate origin in an ATP-independent manner [36] and then in an ATP-dependent mechanism, are able to facilitate unwinding of the origin [37, 38]. Additionally, together, these five proteins – UL5, UL8, UL52, UL9, and ICP8 - create a platform for UL42 to recruit viral DNA polymerase (UL30), the enzyme necessary to initiate the synthesis of a new DNA strand [39]. The important role in viral DNA synthesis of HSV-1 DNA polymerase has made the enzyme an ideal target for the development of antiviral drugs. Its close interactions with a polymerase accessory protein, UL42, helps establish the rate of DNA synthesis during an infection [40, 41]. An additional role of UL30 includes an exonuclease activity that is required for the removal of mismatched or unpaired deoxynucleotides [42]. The sensitivity of the polymerase for proper base pairing has provided validation for the use of nucleoside and pyrophosphate analogs as anti-HSV-1 agents. Incorporation of either into a growing DNA chain halts viral DNA synthesis and leads to the termination of chain extension.
Current therapies belonging to this class of nucleoside, nucleotide, and pyrophosphate analogs are relatively abundant within the realm of HSV-1 therapeutics. Acyclovir (ACV) is one of the oldest antiviral drugs used for HSV-1 treatment [43]. ACV functions as a substrate for viral DNA polymerase, competing with deoxyguanosine tripohosphate for incorporation into the elongation chain. It is a nucleoside analog with potent inhibitory effects and relatively low toxicity. ACV is selectively phosphorylated in virally infected cells by viral thymidine kinase, a process required for the activation of ACV. This phosphorylation step is the first of three steps that generates a non-functional GTP analog [44]. The two later phosphorylation steps are performed by cellular kinases, which generate a GTP analog that strongly inhibits viral DNA synthesis by competing with endogenous GTP [45]. The incorporation of ACV-triphosphate into the growing viral DNA chain forces the termination of HSV-1 DNA synthesis, effectively preventing viral replication. Since the development of ACV in the early 1980s, it has been administered intravenously, orally, and as a topical ointment.
Although ACV initially displayed potent inhibitory activity against HSV-1, the bioavailability of ACV at recommended dosages was limited. As a result of the limited bioavailability, plasma levels adequate to inhibit the less sensitive herpesvirus strains were more difficult to achieve [46]. Due to this low bioavailability, efforts were placed on the development of a prodrug that would retain the safety and efficacy profiles of ACV while greatly improving the oral bioavailability [47], a process which lead to the discovery of valaciclovir. As a prodrug of ACV, oral valaciclovir enhances the bioavailability of ACV to levels three-to-five fold higher than that obtained with oral ACV [48–50]. Upon oral administration, valaciclovir is rapidly converted to ACV by compounds within the small intestine, promoting high bioavailability [51]. The usage of valaciclovir as a suppressive therapy is advantageous to patients as daily dosage requirements is significantly decreased in comparison to those needed with its parent compound [52]. Valaciclovir has very few adverse effects and is generally recommended for long-term usage. Commons side effects include anemia, neutropenia, and thrombocytopenia, thus requiring careful monitoring of a patient’s blood count.
Another drug developed during this period of antiviral development was famciclovir. Famciclovir, a synthetic acyclic guanine derivative, is a prodrug which, after oral administration, is rapidly metabolized to the highly bioavailable antiviral compound penciclovir via a two-step process of enzymatic hydrolysis and oxidation [53, 54]. The first step of activation results from the removal of an ester group in the intestines. The second step of activation occurs in the liver, in which penciclovir undergoes deacetylation and oxidation [55]. Upon entry into virally infected cells, penciclovir is phosphorylated by viral thymidine kinase. Additionally this drug displays higher affinity for HSV-1 thymidine kinase than does ACV and promotes a higher production of penciclovir triphosphate within infected cells when compared to the production of ACV triphosphate. The active form of penciclovir is much more stable than that of ACV and, thus, it resides within infected cells for a longer period of time [56, 57]. The function of penciclovir as a short chain terminator allows limited DNA chain elongation [58]. As a result of the high bioavailability, famciclovir can be administered in low doses for long periods of time[58]
Ganciclovir, a homolog of ACV, is the only guanine analog that potently inhibits herpes viruses. Through the use of the cellular enzymes deoxyguanosine kinase, guanylated kinase and phosphoglycerate kinase, ganciclovir is metabolized to a triphosphate form that allows for potent inhibition of the viral DNA polymerase [59]. Of the three enzymes mentioned above, deoxyguanosine kinase is only induced in a herpes infected cell, a finding that aids in the specificity of this drug. Ganciclovir is initially phosphorylated to ganciclovir monophosphate by the viral protein kinase and then to ganciclovir diphosphate and finally to ganciclovir triphosphate by these cellular kinases [60, 61]. The mechanism of action of ganciclovir is similar to that of its homolog ACV. Through competitive inhibition of endogenous deoxyguanosine triphosphate, ganciclovir triphosphate incorporates into the growing DNA chain, initiating chain termination by inhibiting herpes virus DNA polymerase and arresting HSV replication [62, 63]. The ability of ganciclovir to be phosphorylated by cytomegalovirus (CMV), HSV, and Epstein-Barr virus (EBV)-infected cells has made ganciclovir an excellent broad-spectrum anti-viral [62].
Foscarnet, a pyrophosphate analog, is not added to the growing DNA chain, but rather acts by inhibiting the action of the viral polymerase [64]. However, despite a relatively common use of foscarnet as an alternative drug during resistance to ACV and ganciclovir, it is nephrotoxic and cannot be taken for long periods of time. In addition, foscarnet has also become limited in its usage due to development of viral resistance against its mechanism of action [65, 66].
Cidofovir is a monophosphate nucleotide analogue of deoxycytidine (dCTP) and the first nucleotide analog approved for clinical usage. Like ganciclovir, cidofovir is a broad-spectrum therapeutic that can be utilized to target numerous herperviruses. However unlike ganciclovir, cidofovir is an acyclic cytidine phosphonate analog, making it mechanistically different from ganciclovir [67]. Upon entry into host cells, cidofovir is phosphorylated by pyrimidine nucleoside monophosphate kinase to cidofovir monophosphate. Cidofovir monophosphate is then phosphorylated by nucleoside diphosphate kinase, pyruvate kinase, or creatine kinase, respectively, to form the active metabolite, cidofovir diphosphate [68, 69]. Cidofovir is resistant to phosphostase and, unlike ACV and ganciclovir, it is not dependent on phosphorylation by viral kinases for activation, allowing phosphorylation steps to occur in infected and uninfected cells [68]. Studies by Mendel et al. revealed that the antiviral activity of cidofovir was maintained against HSV with mutant thymidine kinase [70]. The structural similarity between cidofovir diphosphate and cytosine nucleotide allows cidofovir to act as a competitive inhibitor and alternative substrate for viral DNA polymerase. Once incorporated into the DNA strand, cidofovir decreases viral DNA synthesis and promotes chain termination upon the incorporation of two consecutive molecules cidofovir [67]. Cidofovir is very advantageous as it has a long half-life. Additionally, patients who have developed drug resistance against ACV, ganciclovir and/or foscarnet, remain sensitive to cidofovir treatment [71].
Since the introduction of ACV in the 1980’s, ACV and its subsequent derivatives mentioned above have become the first line of drugs for prophylaxis and treatment of HSV infections [58]. While these drugs have been successful in the prevention of primary infection such as in cases of neonates [72–74] and are able to reduce the duration of infection outbreak, the therapeutics mentioned above do not abolish viral latency within the trigeminal ganglion.
HSV-1 FUSION INIHIBITORS
In addition to regulating viral replication, current therapies have also targeted the exterior structure of HSV-1, specifically the envelope which contains the glycoproteins, to block fusion of the virus with the cell membrane. Currently, docosanol is the only marketed HSV-1 drug that inhibits this viral fusion to the host cell [75, 76]. Its active form, n-docosanol, is a saturated 22- carbon aliphatic alcohol which is inhibitory to numerous viruses [77]. The compound functions by targeting the cell membrane and modifying the regions that many viruses utilize for entry. Through this modification, the fusion between a virus and its host cells is inhibited. Docosanol not only functions as an entry inhibitor, but it also interferes with the replication of HSV-1 and some other viruses [76]. It is also administered in topical form and is mainly used for treating herpes labialis [78].
NEED FOR NEW THERAPIES
Despite the continued high prevalence of HSV-1 infection in the global community, there have been few major advancements in anti-HSV-1 therapy since ACV came onto the market in the 1980s and its subsequent derivates [79]. While the current therapies have played substantial roles in the fight against HSV-1, there are convincing reasons to invest in new antiviral agents targeting HSV-1. The development of resistance in specific populations is one concern. This resistance to the antiviral agent occurs via mutations in viral genes that encode either thymidine kinase or DNA polymerase [4, 80, 81]. Although the incidence rate of HSV-1 resistance to ACV in the general population is relatively low at 0.1%–0.7%, it is significantly higher in the immunocompromised population with rates between 4%–14%. In the corneas of those with herpetic keratitis, the resistance rate is 6.4% [82]. A population study of immunocompromised patients, such as those with autoimmune systemic immunodeficiencies or those on immunosuppressant therapy post-transplant, illustrates this resistance well. These patients often have recurrent HSV-1 lesions due to their immune status and can be seen to develop a resistance to ACV following multiple sequential treatments [83, 84]. Another concern is viral latency. For the most part, the currently available pharmaceuticals against HSV-1 exert their actions only after the virus has infected the host cell. Thus, drugs like ACV are essentially only effective at suppressing recurrent HSV-1 infections. So even though antivirals have decreased the incidence of viral reactivation and that of asymptomatic viral shedding, they have not cured infection and are unable to do so [85].
GLYCOPROTEIN-RECEPTOR TARGETING: POTENTIAL ROLE IN HSV-1 THERAPY
In a sense, the best types of new antiviral agents would include those that are effective against resistant virus variants, but at the same time those that pose low toxicity to the host [86]. To prevent latency, treatments that are virucidal in nature [87] and those that prevent the initial viral infection would be of great utility. From the authors’ perspective, one of the most attractive candidates to assume this greater role in antiviral therapy is the targeting of glycoprotein-receptor interactions to inhibit the initial steps of viral entry, attachment, and fusion. An examination of the structural components involved in these initial steps is necessary before a presentation of the possible antivirals that target their functions. The HSV-1 virion consists of four main components: an electron dense core containing the viral DNA, an icosahedral capsid that displays 162 protein units known as capsomeres, tegument proteins (lipids, viral proteins, polyamines and glycosylated and nonglycosylated viral protein) and a lipid bilayer envelope which contains 16 membrane proteins. Amongst the 16 proteins found on the envelope, 12 contain oligosaccharide (glycan) chains attached to their side chains and have been classified as glycoproteins B (gB), C (gC), D (gD), E (gE), G (gG), H (gH), I (gI), L (gL), K (gK), L (gL), M (gM), and N (gN) [88–91]. Many of these glycoproteins play key roles in mediating the initial steps of viral entry into cells and the cell-to-cell spread of the virus [92, 93].
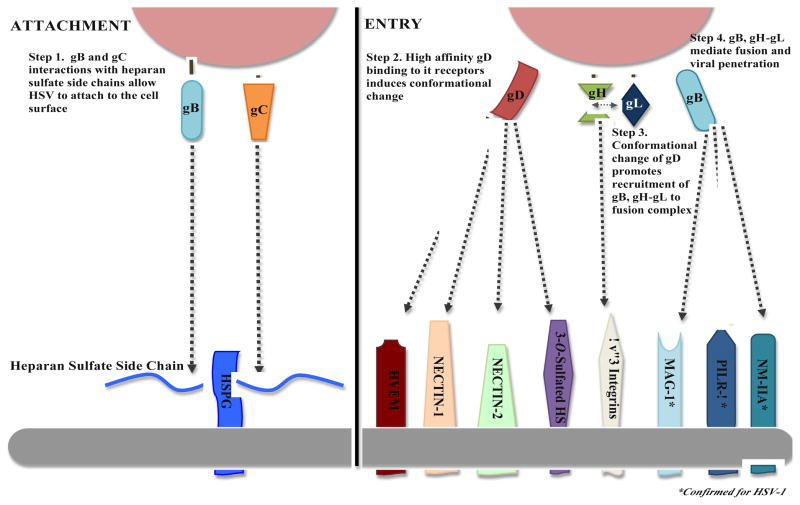
HSV-1 entry into cells is a multistep process that can be grouped into two glycoprotein-mediated phases, viral attachment and viral fusion (Figure 1). Of the twelve glycoproteins on the HSV-1 envelope, gB, gD, gH and gL are essential for the coordination of attachment and fusion of the virus with the cell (Table 1) [94]. Eisenberg et al. recently reported their model for HSV fusion, and this has helped refine our understanding on the roles of these essential glycoproteins in mediating HSV infection [95]. In their review, they reported that fusion of virus to host cell begins with gD binding to one of its host cell receptors, herpes virus entry mediator (HVEM) or nectin-1. Important to this process is the conformational change that gD assumes. This action allows for this gD-host-cell-receptor complex to interact with the heterodimer complex of gH/gL. gH/gL is able to then assume a form whereby it can bind to gB, allowing gB to bring the viral and host cell membrane together. Eventually, the two membranes fuse and the viral capsid is able to enter the cell [95]. Unclear in this model, however, is the role of 3-O sulfated heparan sulfate (3-OS HS), which is also a gD receptor. It is probably safe to predict that 3-OS HS also functions in the same way as the other gD receptors [96, 97].
Figure 1.
Viral Attachment and Entry. HSV-1 attachment to host cells is mediated by the binding of gB and/or gC to cell surface co-receptor, heparan sulfate. Next, the entry or penetration step is mediated through the collaborative efforts of glycoproteins gB, gD, gH, and gL. This requires binding of gD with one of its receptors, which facilitates a conformational change in gD that allows it to recruit gB, and gH-gL to form a fusion complex to facilitate capsid penetration into the host cytosol.
TABLE 1.
HSV-1 GLYCOPROTEINS THAT MEDIATE VIRAL ENTRY
| HSV GLYCOPROTEINS | FUNCTIONAL ROLE | HOST RECEPTOR |
|---|---|---|
| gB | Attachment Fusion Complex |
Heparan Sulfate Chondroitin Sulfate PILR-α NM-IIA MAG |
| gC | Attachment | Heparan Sulfate Chondroitin Sulfate |
| gD | Nectin-1 Recognition Nectin-2 Recognition 3-O-Sulfated Heparan Sulfate Recognition HVEM Binding |
Nectin-1 Nectin-2 HVEM 3-O-Sulfated Heparan Sulfate |
| gH-gL | Heterodimer Fusion Complex | αvβ3-Intergrin |
It is also important to note that the prior attachment phase of entry is heavily dependent on the interactions of gB and/or gC with heparan sulfate (HS) proteoglycans [98, 99]. In addition to HS, non-muscle myosin heavy chain IIA (NMHC-IIA) also functions as a HSV-1 gB receptor, allowing for viral entry [100]. By undergoing conformational changes (i.e. gB), interacting with each other and host cell proteins (i.e. gD), and forming heterodimers with other glycoproteins (i.e. gH-gL), the envelope glycoproteins harmoniously advance infectivity in cells.
Additionally, studies by Chowdhury et al. have elicited data that has been adapted and incorporated into Table 2 to reveal an amino acid variation in the gB sequence of KOS, McKrae, F, and H129 strains [101]. The gD amino acid sequence is also highly conserved [101, 102], which would potentially allow the administration of a glycoprotein targeted therapeutic to treat patients infected with multiple HSV-1 strains.
TABLE 2.
AMINO ACID SEQUENCE HOMOLOGY BETWEEN HSV-1 STRAINS
| HSV-1 Strain | Glycoprotein | Relative AA Homology to Other Strains |
|---|---|---|
|
| ||
| McKrae | gB | 96% |
| gC | 96% | |
| gD | 100% | |
| gH | 99% | |
| gL | 95% | |
|
| ||
| KOS | gB | 98% |
| gC | 99% | |
| gD | 100% | |
| gH | 96% | |
| gL | 95% | |
|
| ||
| F | gB | 97% |
| gC | 98% | |
| gD | 100% | |
| gH | 99% | |
| gL | 95% | |
Despite the important role of viral entry and fusion in HSV-1 pathogenesis, it is unfortunate that therapeutics directly targeting the glycoprotein interactions responsible for these initial processes in viral infection have not yet been introduced to combat this virus. This mode of inhibition is advantageous because it expands antiviral options that disrupt infection by interfering with entry. It also targets cell-associated spread and syncytia formation as well since membrane fusion is also vital for these processes. There is a great deal of information on HSV-1 glycoprotein interactions to give them a realistic potential to be targeted by new antivirals to control viral spread and transmission. Many unique properties of gB, gD, gH-gL, and their respective host receptors make them ideal targets for such a process. And while this has yet to be marketed clinically for individuals with HSV-1, the concept and feasibility of viral inhibition at the point of entry has been a topic of interest shared by many researchers and clinicians, a point which we will now examine.
gB-Receptor Interaction as an Antiviral Target
gB is one of the most highly conserved proteins among herpes viruses. Beginning with the virus attachment to cells, gB plays multiple essential roles in the HSV-1 lifecycle, including membrane fusion during entry, cell-to-cell spread, and egress of HSV-1 capsids from the nuclei. In a 1988 study by Cai et al., the authors established a vital role for gB in viral membrane fusion and cell-to-cell spread [103]. Their experiments demonstrated an impairment of the gB null mutants to form syncytial cells and a lack of the ability to enter host cells [103]. The recent discovery of the crystal structure for gB establishes its role as a class III fusion protein, which through interactions with gH-gL triggers viral penetration into cells [104]. Targeting any of the required functions of gB is likely to have significant effects on HSV-1 infectivity. As stated earlier, fusion is not limited to the merging of the virus and the cell membrane, but also includes cell-to-cell fusion, which is required for spread of HSV-1 from an infected cell to an uninfected cell. Due to strong and essential interactions among gB, gD, gH,-gL, and the impairment of fusion noted in the presence of antibodies against gB [105], it is likely that targeting gB with small molecule inhibitors or interfering with its gene via RNAi will neutralize the ability of the virions to infect cells.
Recent studies are a testament to the potential effectiveness of using peptides to interfere with gB-receptor interactions. Galdiero et al. demonstrated that peptides homologous to specific regions of HSV-1 gB were able to prevent viral infection [106]. Although most peptide-centered studies have utilized regions of the target proteins that are more well-known, newer approaches targeting distinct regions may produce even more selective peptides with greater efficiency. Using such methods, Akkarawongsa et al. synthesized two gB-targeting peptides, gB122 and gB131, that inhibited HSV-1 entry into the host cell [107]. Furthermore, gB122 and an additional peptide against gB, gB94, were observed in pretreatment assays to provide host cell protection from viral infection also by inhibiting viral entry. This gB94 was also found to have virucidal activity, whereas the other two peptides did not. These studies not only provide insights into the ability of novel peptides to impair HSV-1 entry into host cells, but also suggest an innovative method by which sequences against which these peptides can be synthesized and used for antiviral targeted therapy [107]. One caveat in the use of these peptides lies in their own ability for cell entry. As a generalization, peptides are not effective at entering cells and are limited to topical use [107], a route of administration that is generally not effective as oral-intake regimens [108]. Further studies investigating parameters such as half-life would give more reasonable expectations for clinical use. As for the virucidal activity of one the peptides synthesized, this may provide options to prevent viral latency.
There are other peptides that regulate HSV-1 infection, possibly via targeting gB-receptor interactions. Yasin et al. studied the effect of synthetic theta defensins, specifically retrocyclin 2 (RC-2), on inhibiting HSV entry and adhesion [109]. Although the role of gB in this mechanism has not been confirmed, studies have indicated a strong affinity of RC-2 for gB2, the glycoprotein B of HSV-2 [109]. Given the strong homology between gB and gB-2, it is possible that RC-2 mediates its effects partly though interfering with gB-receptor interactions. Although no virucidal activity was detected for this peptide, preincubation with RC-2 was observed to inhibit HSV-1 KOS in vitro. In terms of time of action relative to the HSV-1 lifecycle, studies with either virus preincubated with RC-2 or corneas with peptide application prior to viral infection showed significantly decreased viral titers. These results were not obtained when peptides were applied to a herpes keratitis model following infection. Thus, the utility of such peptides would appear to fall under prophylactic measures [110].
In addition to peptides, Shogan et al. demonstrated that oligonucleotides also have potential as antiviral agents [111]. The GT rich regions of oligodeoxynucleotides (ODNs) are thought to be important mediators of their antiviral mechanism. In relation to HSV-1, these authors showed that ODNs target the gB of the virus, a process that is crucial for its antiviral effect. Although the specific ODN, phosphorothioate oligonucleotide, ISIS 5652, did not appear to inhibit viral attachment and entry, it did possess virucidal activity. The authors of that study have suggested two hypotheses for this particular mechanism of action. One is that there is possibly a conformational change in gB upon interaction with the ODN that makes it no longer able to infect. Another is that this ODN might be interacting with another virion component, one that interacts directly with gB. In terms of clinical applications for this ODN with virucidal activity, it should be noted that there would be limitations for its use at this time, such as concern regarding its size, cost, and delayed length of activity. However, Shogan et al. proposed that assessing this compound for its virucidal activity may prove to be of more clinical benefit as these studies may help spawn the development of other antivirals with virucidal potential [111].
As briefly mentioned earlier, monoclonal antibodies directed against gB also show clinical promise. One of first studies that explored the effectiveness of monoclonal antibodies as a protectant against HSV infections was preformed in vivo by Dix et al.[112]. In this study, monoclonal antibodies HC1 and HD1, directed against HSV-1 glycoproteins gC and gD, were evaluated for their ability to passively immunize mice against acute virus-induced neurological disease.[112]. From their investigation they found passively transferred mouse monoclonal antibody directed against glycoproteins gC or gD reduced virus spread and severity of acute neurologic disease in HSV infected mice [112]. Dix later provided the first in vivo evidence that gB expresses both type-common and type-specific determinants as H233 and H368 antibodies provided significant neutralization in vitro which correlated to in vivo protection [113]. In a study done by Eis-Hubinger et al., a monoclonal antibody specific to gB, MAb 2C, was shown to have HSV-1 neutralizing effects in both in vitro and in vivo models [114]. A more recent study by Krawczyk et al. in 2011 showed that MAb 2C is able to block HSV-1 entry into host cells by cross-linking gB trimers, a process that prevents gB from emitting its fusogenic signal. Severely immunodeficient mice were protected by this MAb 2C from a viral challenge test of lethal dose. Additionally, even those animals with HSV-1 already in their peripheral nervous systems were able to benefit from this MAb 2C, as lethal encephalitis was prevented [115].
In addition to targeting the glycoprotein itself, approaches are being developed to target the host cell receptor to which gB binds. One of these receptors is the NMHC-IIA, which is a subunit of non-muscle myosin IIA, that helps to facilitate HSV-1 entry via interactions with gB. Arii et al demonstrated that inhibition of myosin light chain kinase, a phosphorylator of non-muscle myosin IIA (NM-IIA), effectively decreased HSV-1 infection leading to herpes stromal keratitis in both cell culture and murine models [100]. Drugs targeting these regulators of HSV-1 entry may have high prophylactic and therapeutic potential [100].
gD-Receptor Interaction as an Antiviral Target
Of the four essential glycoproteins that aid in HSV-1 entry, gD has been the most well studied. Its cellular receptors have been well defined, and gD has been found to have a strong binding affinity for these receptors [116–118]. Through crystal structure studies, it has been shown that gD contains a V-like core that is wrapped by two distinct extensions on the N-terminus and the C-terminus. These two distinctive regions allow site-specific receptor binding and trigger viral fusion [119]. The wealth of information on this important molecule makes it an ideal target for anti-HSV-1 therapies.
Through rational drug design, a small molecule inhibitor, oligonucleotides, or peptides can be designed and tested against the virus. Similar to that done against gB in previously mentioned studies, small molecules can be screened against purified gD and tested for inhibition of HSV-1 infectivity. We have recently reported the discovery of small peptides that block 3-O-S-HS, which is a host cell receptor for HSV-1 gD [120]. The peptides show strong ability to block HSV-1 infection in the murine eye by impairing the glycoprotein gD – host cell receptor interaction [120]. A possible advantage of such peptides that target host cell components essential in viral infection may be an avoidance of the originof resistant virus strains. In addition, although the main focus of this paper is on HSV-1 anti-virals, targeting components found on host cells may provide a wider spectrum of coverage against many other viruses as well [121].
In addition to blocking the receptor to which gD binds, there are methods to block gD itself. Previously, Krummenacher et al. and Nicola et al. had obtained results showing that virus-neutralizing, monoclonal antibodies are able to block gD from binding to both HVEM and nectin-1 [122, 123]. In later studies, Lazear et al. took advantage of the notion that the function of gD can be separated into two roles, receptor binding and post-receptor-binding actions [124]. These authors hypothesized that a conformational change in the C-terminus of gD was the event that effectively separated these two functions in time. They reported the discovery of two distinct monoclonal antibodies that are able to interact with gD in the presence of either HVEM or nectin-1. These antibodies did not show any impairment in receptor binding by gD, but they did render the viral infection ineffective. Because receptor binding was not impaired, and in the presence of one of the monoclonal antibodies receptor binding was actually enhanced, it was postulated that these antibodies exert their antiviral effect by preventing gD from undergoing the conformational change that is necessary for its post-receptor-binding effects, actions which normally activate gH-gL and allow gB to exert its effects on cell-to-cell fusion [124]. Another gD-receptor targeting potential antiviral is human lactoferrin (hLf). Knowing that hLf is a component of the mucosal innate defense system, Välimaa et al. decided to study its mechanism of action in neutralizing HSV-1 infection [125]. HSV-1 virions preincubated with hLf were directly correlated with decreased HSV-1 infection. Interestingly, HSV-1 mutants with mutated gD had infectivity that was unchanged with hLf preincubation, thus suggesting either a direct interaction with gD or its host cell receptors. The inhibition was seen at various levels, including post-attachment inhibition that was gD-associated and also the inhibition of viral cell-to-cell spread. In addition to its role in preventing cell-to-cell spread, hLf could have significant effects on regulating the infectivity of the subsequent progeny virions that are released within the host. In addition to gD interactions, it is possible that the effects of hLf on HSV-1 infectivity are mediated partly through interactions with gC and/or its host cell receptors [126].
Other gD targets arise from the group of synthetic rigid amphiphiles, the rigid amphipathic fusion inhibitors (RAFIs). In a study by St. Vincent et al., it was found that although this group of molecules does little to impair complete attachment of the virus to the host cells, they are able to inhibit viral fusion as their name would suggest [127]. The most potent of these was found to impair the subsequent gD interactions with its receptors that include HVEM and nectin-1. For the purposes of a discussion on new antivirals to HSV-1, perhaps what is most striking is the efficiency of this particular RAFI against mutant forms of HSV-1 that are resistant to currently available pharmaceuticals such as phosphonoacetic acid and acyclovir. The infectivity of released virions in cells treated with this particular RAFI was found to be impaired by approximately 350,000-fold [127]. As mentioned earlier, viral resistance, particularly that of ACV is significant in certain populations. Thus, further study and possible clinical implementation of anti-viral agents that remain effective against HSV-1 strains resistant to current therapies would be prudent.
Another recent possible antiviral directed against the gD-host receptor interaction are the zinc oxide (ZnO) micro-nano structures (MNSs). These take advantage of the fact that HSV-1 utilizes ionic interactions when positively charged viral glycoproteins bind to host cell surface HS, which are negatively charged. Studies by Mishra et al. using human corneal fibroblasts showed impaired HSV-1 entry when the cells were treated with ZnO-MNSs [87]. Furthermore, ultraviolet illuminated ZnO-MNSs showed increased antiviral activity, likely due to the increased negative charge this provides. Not only was viral entry inhibited, ZnO MNSs were also found to prevent the HSV-1 glycoprotein mediated cell-to-cell fusion [87]. Clinical significance of this particle was also investigated by the authors of that study. The entry of previously described clinically-relevant strains of HSV-1, including F, G, and MP [128] were also found to be blocked in cells pretreated with ZnO-MNSs. Even in vivo studies showed the potential for these structures as antiviral agents, as blocked entry of HSV-1 was also seen in zebrafish embryos pretreated with these ZnO-MNSs. The fact that this was a prophylactic administration is crucial, as one of the reasons mentioned for new therapies was the need for appropriate prophylaxis. Also important when gauging its potential clinical use is the fact that the concentrations used in these studies were all within the nontoxic range [87].
Non-specific Glycoprotein-Receptor Targets
Other studies have provided strong evidence for the potential use of antiviral options that do not have specific glycoprotein targets. Previous application of cyanovirin-N (CV-N) in HIV infected cells from studies by three separate authors showed CV-N to target high mannose oligosaccharides on envelope glycoproteins, thus leaving open the possibility that this is the case for HSV-1 as well [129–131]. More recently, Tiwari et al. reported that CV-N inhibits not only HSV-1 entry, but also the virus mediated cell-to-cell fusion that normally occurs via glycoprotein mediation in vitro[132]. CV-N was also found to inhibit DNA replication. Important to the possible future therapeutic application of this protein, these effects were attained at low nanomolar concentrations of the protein, levels that are non-cytotoxic to the host cells [132]. Also critical for its application clinically is the fact that these results would seem to be true in vivo as well, particularly owing to the fact that this protein impairs membrane fusion, a phenomenon which is necessary for both viral entry into the host cell and subsequent spread to neighboring, uninfected cells. Furthermore, Tsai et al. demonstrated the in vivo utility of this protein as a gel form in vaginal models of female macaques [133]. These studies were without detrimental effects either to the cell or clinically [133]. However, the specific target of CV-N within the host cell is still unknown [132] and warrants further investigation.
Hydrolyzable tannins are another class of potential antivirals without specific glycoprotein-receptor targets. Lin et al. identified two important members of this family, chebulagic acid (CHLA) and punicalagin (PUG) [134]. These compounds are able to inhibit the interactions between various HSV-1 glycoproteins and the cell surface glycosaminolgycans they normally bind to. By functioning as glycosaminoglycan competitors, these hydrolysable tannins are able to block the adsorption and penetration by HSV-1 and also are able to halt viral cell-to-cell spread [134].
FUTURE DIRECTION OF HSV-1 ANTIVIRALS
The putative antivirals mentioned throughout this article all hold high potential as clinical agents against HSV-1. As currently situated, these antivirals remain at various stages of testing and development in the drug pipeline. Many of these are still in the developmental stage, including anti-gB peptides, the theta defensin RC-2, ODNs, inhibitors of myosin light chain kinase, and anti-3-OS HS peptides. Those in the preclinical stage include the monoclonal antibodies against gB, hLf, ZnO MNSs, CV-N, and CHLA and PUG. It is difficult to predict at what rate these potential anti-HSV-1 agents will progress along the drug pipeline.
Nevertheless, these novel HSV-1 therapies have the potential to be successful in many ways. One such use might be as part of combination therapies. Models for successful combination therapies are found in cancer treatments as well as other pathologies stemming from infectious pathogens like HIV [135–141]. A potential caveat to combination therapies is that certain drug interactions do little to achieve greater therapy as a whole. However, since DNA replication inhibitors and entry inhibitors have proven to be synergistic in combating viruses such as HIV, it is hopeful that they will do the same for that against HSV-1. Another benefit to combination therapy is the effect on viral resistance. The resistance rate of ACV, while not high enough to render the drug irrelevant by any means, is substantial in certain populations as mentioned before. Combination therapy confers some sort of protection, as the risk of one drug in a combination regimen developing resistance may be lower than in the case of that drug as a single-agent therapy. Also, if resistance were to develop, because of the presence of multiple drugs in the regimen, the intended clinical outcome may be impacted to a lesser degree [142]
And while many of these novel therapeutics specific to HSV-1 glycoproteins have yet to be brought into clinical trials, investigators have made significant progress in identifying such inhibitors. It is hopeful that many of these anti-HSV-1 glycoprotein drugs will have translational benefit in the clinical field with the ultimate goal to provide alternative relief to patients suffering from chronic and recurrent problems associated with HSV-1.
ABBREVIATIONS
- 3-OS HS
3-O sulfated heparan sulfate
- ACV
acyclovir
- CHLA
chebulagic acid
- CS
chondroitin sulfate
- CMV
cytomegalovirus
- CTL
cytotoxic-T-lymphocyte
- CV-N
cyanovirin-N
- EBV
Epstein-Barr Virus
- FIV
feline immunodeficiency virus
- hLf
human lactoferrin
- HSV-1
herpes simplex virus-1
- HS
heparin sulfate
- HVEM
herpes virus entry mediator
- MNSs
micro-nano structures
- NMHC-IIA
non-muscle myosin heavy chain IIA
- NM-IIA
non-muscle myosin IIA
- ODNs
oligodeoxynucleotides
- RAFIs
rigid amphipathic fusion inhibitorsl
- RC-2
retrocyclin 2
References
- 1.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 2.Rubicz R, Leach CT, Kraig E, Dhurandhar NV, Duggirala R, Blangero J, Yolken R, Goring HH. Genetic factors influence serological measures of common infections. Human heredity. 2011;72(2):133–141. doi: 10.1159/000331220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737–763. doi: 10.1016/j.jaad.2007.06.027. quiz 764–736. [DOI] [PubMed] [Google Scholar]
- 4.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrobial agents and chemotherapy. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. The Journal of infectious diseases. 2002;186 (Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 6.Bedadala GR, Palem JR, Graham L, Hill JM, McFerrin HE, Hsia SC. Lytic HSV-1 infection induces the multifunctional transcription factor Early Growth Response-1 (EGR-1) in rabbit corneal cells. Virol J. 2011;8:262. doi: 10.1186/1743-422X-8-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chayavichitsilp P, Buckwalter JV, Krakowski AC, Friedlander SF. Herpes simplex. Pediatr Rev. 2009;30(4):119–129. doi: 10.1542/pir.30-4-119. quiz 130. [DOI] [PubMed] [Google Scholar]
- 8.Stevens JG, Nesburn AB, Cook ML. Latent herpes simplex virus from trigeminal ganglia of rabbits with recurrent eye infection. Nature: New biology. 1972;235(59):216–217. doi: 10.1038/newbio235216a0. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JG, Cook ML. Latent infections induced by herpes simplex viruses. Cancer research. 1973;33(6):1399–1401. [PubMed] [Google Scholar]
- 10.Wechsler SL, Nesburn AB, Watson R, Slanina SM, Ghiasi H. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. Journal of virology. 1988;62(11):4051–4058. doi: 10.1128/jvi.62.11.4051-4058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perng GC, Dunkel EC, Geary PA, Slanina SM, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. Journal of virology. 1994;68(12):8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perng GC, Slanina SM, Yukht A, Ghiasi H, Nesburn AB, Wechsler SL. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. Journal of virology. 2000;74(4):1885–1891. doi: 10.1128/jvi.74.4.1885-1891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook ML, Bastone VB, Stevens JG. Evidence that neurons harbor latent herpes simplex virus. Infection and immunity. 1974;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwaagstra JC, Ghiasi H, Nesburn AB, Wechsler SL. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1) Virology. 1991;182(1):287–297. doi: 10.1016/0042-6822(91)90672-x. [DOI] [PubMed] [Google Scholar]
- 15.Rautemaa R, Helander T, Meri S. Herpes simplex virus 1 infected neuronal and skin cells differ in their susceptibility to complement attack. Immunology. 2002;106(3):404–411. doi: 10.1046/j.1365-2567.2002.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perng GC, Jones C. Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. Interdisciplinary perspectives on infectious diseases. 2010;2010:262415. doi: 10.1155/2010/262415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed M, Fraser NW. Herpes simplex virus type 1 2-kilobase latency-associated transcript intron associates with ribosomal proteins and splicing factors. Journal of virology. 2001;75(24):12070–12080. doi: 10.1128/JVI.75.24.12070-12080.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Dujaili LJ, Clerkin PP, Clement C, McFerrin HE, Bhattacharjee PS, Varnell ED, Kaufman HE, Hill JM. Ocular herpes simplex virus: how are latency, reactivation, recurrent disease and therapy interrelated? Future microbiology. 2011;6(8):877–907. doi: 10.2217/fmb.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel R. Antiviral agents for the prevention of the sexual transmission of herpes simplex in discordant couples. Curr Opin Infect Dis. 2004;17(1):45–48. doi: 10.1097/00001432-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sacks SL, Griffiths PD, Corey L, Cohen C, Cunningham A, Dusheiko GM, Self S, Spruance S, Stanberry LR, Wald A, et al. HSV-2 transmission. Antiviral Res. 2004;63 (Suppl 1):S27–35. doi: 10.1016/j.antiviral.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM, Jr, Paavonen J, Morrow RA, Beutner KR, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 22.Maclean CA. HSV Entry and Spread. Methods Mol Med. 1998;10:9–18. doi: 10.1385/0-89603-347-3:9. [DOI] [PubMed] [Google Scholar]
- 23.Dohner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, Vallee R, Sodeik B. Function of dynein and dynactin in herpes simplex virus capsid transport. Molecular biology of the cell. 2002;13(8):2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garber DA, Beverley SM, Coen DM. Demonstration of circularization of herpes simplex virus DNA following infection using pulsed field gel electrophoresis. Virology. 1993;197(1):459–462. doi: 10.1006/viro.1993.1612. [DOI] [PubMed] [Google Scholar]
- 25.Jackson SA, DeLuca NA. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc Natl Acad Sci U S A. 2003;100(13):7871–7876. doi: 10.1073/pnas.1230643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehmer PE, Nimonkar AV. Herpes virus replication. IUBMB life. 2003;55(1):13–22. doi: 10.1080/1521654031000070645. [DOI] [PubMed] [Google Scholar]
- 27.LaBoissiere S, O’Hare P. Analysis of HCF, the cellular cofactor of VP16, in herpes simplex virus-infected cells. Journal of virology. 2000;74(1):99–109. doi: 10.1128/jvi.74.1.99-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grondin B, DeLuca N. Herpes simplex virus type 1 ICP4 promotes transcription preinitiation complex formation by enhancing the binding of TFIID to DNA. Journal of virology. 2000;74(24):11504–11510. doi: 10.1128/jvi.74.24.11504-11510.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long MC, Leong V, Schaffer PA, Spencer CA, Rice SA. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. Journal of virology. 1999;73(7):5593–5604. doi: 10.1128/jvi.73.7.5593-5604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer CA, Dahmus ME, Rice SA. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. Journal of virology. 1997;71(3):2031–2040. doi: 10.1128/jvi.71.3.2031-2040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice SA, Su LS, Knipe DM. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. Journal of virology. 1989;63(8):3399–3407. doi: 10.1128/jvi.63.8.3399-3407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai W, Schaffer PA. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. Journal of virology. 1992;66(5):2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong AD, Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liptak LM, Uprichard SL, Knipe DM. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures. J Virol. 1996;70(3):1759–1767. doi: 10.1128/jvi.70.3.1759-1767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukonis CJ, Burkham J, Weller SK. Herpes simplex virus type 1 prereplicative sites are a heterogeneous population: only a subset are likely to be precursors to replication compartments. J Virol. 1997;71(6):4771–4781. doi: 10.1128/jvi.71.6.4771-4781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Lehman IR. An initial ATP-independent step in the unwinding of a herpes simplex virus type I origin of replication by a complex of the viral origin-binding protein and single-strand DNA-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3024–3028. doi: 10.1073/pnas.061028298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makhov AM, Boehmer PE, Lehman IR, Griffith JD. The herpes simplex virus type 1 origin-binding protein carries out origin specific DNA unwinding and forms stem-loop structures. The EMBO journal. 1996;15(7):1742–1750. [PMC free article] [PubMed] [Google Scholar]
- 38.He X, Lehman IR. Unwinding of a herpes simplex virus type 1 origin of replication (Ori(S)) by a complex of the viral origin binding protein and the single-stranded DNA binding protein. Journal of virology. 2000;74(12):5726–5728. doi: 10.1128/jvi.74.12.5726-5728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang L, Godinez WJ, Kim IH, Tektonidis M, de Lanerolle P, Eils R, Rohr K, Knipe DM. Herpesviral replication compartments move and coalesce at nuclear speckles to enhance export of viral late mRNA. Proc Natl Acad Sci U S A. 2011;108(21):E136–144. doi: 10.1073/pnas.1103411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez TR, Lehman IR. Functional interaction between the herpes simplex-1 DNA polymerase and UL42 protein. The Journal of biological chemistry. 1990;265(19):11227–11232. [PubMed] [Google Scholar]
- 41.Gottlieb J, Marcy AI, Coen DM, Challberg MD. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. Journal of virology. 1990;64(12):5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song L, Chaudhuri M, Knopf CW, Parris DS. Contribution of the 3′- to 5′-exonuclease activity of herpes simplex virus type 1 DNA polymerase to the fidelity of DNA synthesis. The Journal of biological chemistry. 2004;279(18):18535–18543. doi: 10.1074/jbc.M309848200. [DOI] [PubMed] [Google Scholar]
- 43.Furman PA, de Miranda P, St Clair MH, Elion GB. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrobial agents and chemotherapy. 1981;20(4):518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauerbrei A, Deinhardt S, Zell R, Wutzler P. Testing of herpes simplex virus for resistance to antiviral drugs. Virulence. 2010;1(6):555–557. doi: 10.4161/viru.1.6.13806. [DOI] [PubMed] [Google Scholar]
- 45.Derse D, Cheng YC, Furman PA, St Clair MH, Elion GB. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981;256(22):11447–11451. [PubMed] [Google Scholar]
- 46.Engel JP, Englund JA, Fletcher CV, Hill EL. Treatment of resistant herpes simplex virus with continuous-infusion acyclovir. JAMA : the journal of the American Medical Association. 1990;263(12):1662–1664. [PubMed] [Google Scholar]
- 47.Beutner KR. Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antiviral research. 1995;28(4):281–290. doi: 10.1016/0166-3542(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 48.Ormrod D, Scott LJ, Perry CM. Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs. 2000;59(4):839–863. doi: 10.2165/00003495-200059040-00013. [DOI] [PubMed] [Google Scholar]
- 49.Granero GE, Amidon GL. Stability of valacyclovir: implications for its oral bioavailability. International journal of pharmaceutics. 2006;317(1):14–18. doi: 10.1016/j.ijpharm.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 50.Weller S, Blum MR, Doucette M, Burnette T, Cederberg DM, de Miranda P, Smiley ML. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clinical pharmacology and therapeutics. 1993;54(6):595–605. doi: 10.1038/clpt.1993.196. [DOI] [PubMed] [Google Scholar]
- 51.Sinko PJ, Balimane PV. Carrier-mediated intestinal absorption of valacyclovir, the L-valyl ester prodrug of acyclovir: 1. Interactions with peptides, organic anions and organic cations in rats. Biopharmaceutics & drug disposition. 1998;19(4):209–217. doi: 10.1002/(sici)1099-081x(199805)19:4<209::aid-bdd93>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 52.Chakrabarty A, Anderson NJ, Beutner R, Tyring SK. Valacyclovir for the management of herpes viral infections. Skin therapy letter. 2005;10(1):1–4. [PubMed] [Google Scholar]
- 53.Perry CM, Wagstaff AJ. Famciclovir. A review of its pharmacological properties and therapeutic efficacy in herpesvirus infections. Drugs. 1995;50(2):396–415. doi: 10.2165/00003495-199550020-00011. [DOI] [PubMed] [Google Scholar]
- 54.Degreef H, Andrejevic L, Aoki F, Arend J, Ashton R, Debacker W, Bartlett K, Vanblokland WB, Bishop S, Boon R, et al. Famciclovir, a New Oral Antiherpes Drug - Results of the First Controlled Clinical-Study Demonstrating Its Efficacy and Safety in the Treatment of Uncomplicated Herpes-Zoster in Immunocompetent Patients. Int J Antimicrob Ag. 1994;4(4):241–246. doi: 10.1016/0924-8579(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 55.Chakrabarty A, Tyring SK, Beutner K, Rauser M. Recent clinical experience with famciclovir--a “third generation” nucleoside prodrug. Antiviral chemistry & chemotherapy. 2004;15(5):251–253. doi: 10.1177/095632020401500503. [DOI] [PubMed] [Google Scholar]
- 56.Simpson D, Lyseng-Williamson KA. Famciclovir: a review of its use in herpes zoster and genital and orolabial herpes. Drugs. 2006;66(18):2397–2416. doi: 10.2165/00003495-200666180-00016. [DOI] [PubMed] [Google Scholar]
- 57.Kang SH, Chua-Gocheco A, Bozzo P, Einarson A. Safety of antiviral medication for the treatment of herpes during pregnancy. Canadian family physician Medecin de famille canadien. 2011;57(4):427–428. [PMC free article] [PubMed] [Google Scholar]
- 58.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrobial agents and chemotherapy. 2011;55(2):459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matthews T, Boehme R. Antiviral activity and mechanism of action of ganciclovir. Reviews of infectious diseases. 1988;10 (Suppl 3):S490–494. doi: 10.1093/clinids/10.supplement_3.s490. [DOI] [PubMed] [Google Scholar]
- 60.Castela N, Vermerie N, Chast F, Sauvageon-Martre H, Denis J, Godard V, Goldschmidt P, Pouliquen Y. Ganciclovir ophthalmic gel in herpes simplex virus rabbit keratitis: intraocular penetration and efficacy. Journal of ocular pharmacology. 1994;10(2):439–451. doi: 10.1089/jop.1994.10.439. [DOI] [PubMed] [Google Scholar]
- 61.Shiota H, Naito T, Mimura Y. Anti-herpes simplex virus (HSV) effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG) in rabbit cornea. Current eye research. 1987;6(1):241–245. doi: 10.3109/02713688709020098. [DOI] [PubMed] [Google Scholar]
- 62.Hoh HB, Hurley C, Claoue C, Viswalingham M, Easty DL, Goldschmidt P, Collum LM. Randomised trial of ganciclovir and acyclovir in the treatment of herpes simplex dendritic keratitis: a multicentre study. The British journal of ophthalmology. 1996;80(2):140–143. doi: 10.1136/bjo.80.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Majumdar S, Nashed YE, Patel K, Jain R, Itahashi M, Neumann DM, Hill JM, Mitra AK. Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: in vitro and in vivo evaluations. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2005;21(6):463–474. doi: 10.1089/jop.2005.21.463. [DOI] [PubMed] [Google Scholar]
- 64.Crumpacker CS. Mechanism of action of foscarnet against viral polymerases. The American journal of medicine. 1992;92(2A):3S–7S. doi: 10.1016/0002-9343(92)90329-a. [DOI] [PubMed] [Google Scholar]
- 65.Danve-Szatanek C, Aymard M, Thouvenot D, Morfin F, Agius G, Bertin I, Billaudel S, Chanzy B, Coste-Burel M, Finkielsztejn L, et al. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol. 2004;42(1):242–249. doi: 10.1128/JCM.42.1.242-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safrin S, Crumpacker C, Chatis P, Davis R, Hafner R, Rush J, Kessler HA, Landry B, Mills J. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1991;325(8):551–555. doi: 10.1056/NEJM199108223250805. [DOI] [PubMed] [Google Scholar]
- 67.Xiong X, Smith JL, Chen MS. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrobial agents and chemotherapy. 1997;41(3):594–599. doi: 10.1128/aac.41.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clinical microbiology reviews. 2003;16(4):569–596. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cihlar T, Chen MS. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Molecular pharmacology. 1996;50(6):1502–1510. [PubMed] [Google Scholar]
- 70.Mendel DB, Barkhimer DB, Chen MS. Biochemical basis for increased susceptibility to Cidofovir of herpes simplex viruses with altered or deficient thymidine kinase activity. Antimicrobial agents and chemotherapy. 1995;39(9):2120–2122. doi: 10.1128/aac.39.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muluneh B, Dean A, Armistead P, Khan T. Successful clearance of cutaneous acyclovir-resistant, foscarnet-refractory herpes virus lesions with topical cidofovir in an allogeneic hematopoietic stem cell transplant patient. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2012 doi: 10.1177/1078155212448408. [DOI] [PubMed] [Google Scholar]
- 72.Kriebs JM. Understanding herpes simplex virus: transmission, diagnosis, and considerations in pregnancy management. Journal of midwifery & women’s health. 2008;53(3):202–208. doi: 10.1016/j.jmwh.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 73.Baker DA. Consequences of herpes simplex virus in pregnancy and their prevention. Current opinion in infectious diseases. 2007;20(1):73–76. doi: 10.1097/QCO.0b013e328013cb19. [DOI] [PubMed] [Google Scholar]
- 74.ACOG practice bulletin: Clinical management guidelines for obstetrician-gynecologists, number 57, November 2004. Gynecologic herpes simplex virus infections. Obstetrics and gynecology. 2004;104(5 Pt 1):1111–1118. [PubMed] [Google Scholar]
- 75.Wojcechowskyj JA, Doms RW. A Potent, Broad-Spectrum Antiviral Agent that Targets Viral Membranes. Viruses. 2010;2(5):1106–1109. doi: 10.3390/v2051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pope LE, Marcelletti JF, Katz LR, Lin JY, Katz DH, Parish ML, Spear PG. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antiviral Res. 1998;40(1–2):85–94. doi: 10.1016/s0166-3542(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 77.Katz DH, Marcelletti JF, Khalil MH, Pope LE, Katz LR. Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex. Proc Natl Acad Sci U S A. 1991;88(23):10825–10829. doi: 10.1073/pnas.88.23.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung DT, Sacks SL. Docosanol: a topical antiviral for herpes labialis. Expert Opin Pharmacother. 2004;5(12):2567–2571. doi: 10.1517/14656566.5.12.2567. [DOI] [PubMed] [Google Scholar]
- 79.Wilson SS, Fakioglu E, Herold BC. Novel approaches in fighting herpes simplex virus infections. Expert Rev Anti Infect Ther. 2009;7(5):559–568. doi: 10.1586/eri.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frobert E, Ooka T, Cortay JC, Lina B, Thouvenot D, Morfin F. Herpes simplex virus thymidine kinase mutations associated with resistance to acyclovir: a site-directed mutagenesis study. Antimicrobial agents and chemotherapy. 2005;49(3):1055–1059. doi: 10.1128/AAC.49.3.1055-1059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frobert E, Ooka T, Cortay JC, Lina B, Thouvenot D, Morfin F. Resistance of herpes simplex virus type 1 to acyclovir: thymidine kinase gene mutagenesis study. Antiviral Res. 2007;73(2):147–150. doi: 10.1016/j.antiviral.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16(1):114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Russell DB, Tabrizi SN, Russell JM, Garland SM. Seroprevalence of herpes simplex virus types 1 and 2 in HIV-infected and uninfected homosexual men in a primary care setting. J Clin Virol. 2001;22(3):305–313. doi: 10.1016/s1386-6532(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 84.Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135(11):1393–1397. doi: 10.1001/archderm.135.11.1393. [DOI] [PubMed] [Google Scholar]
- 85.Hu K, He X, Yu F, Yuan X, Hu W, Liu C, Zhao F, Dou J. Immunization with DNA vaccine expressing herpes simplex virus type 1 gD and IL-21 protects against mouse herpes keratitis. Immunol Invest. 2011;40(3):265–278. doi: 10.3109/08820139.2010.534219. [DOI] [PubMed] [Google Scholar]
- 86.Yu H, Liu ZT, Lv R, Zhang WQ. Antiviral activity of recombinant cyanovirin-N against HSV-1. Virol Sin. 2010;25(6):432–439. doi: 10.1007/s12250-010-3131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mishra YK, Adelung R, Rohl C, Shukla D, Spors F, Tiwari V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Res. 2011;92(2):305–312. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Reviews in medical virology. 2008;18(1):35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 89.Mettenleiter TC. Budding events in herpesvirus morphogenesis. Virus research. 2004;106(2):167–180. doi: 10.1016/j.virusres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 90.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Reviews in medical virology. 2000;10(5):305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 91.Rao P, Pham HT, Kulkarni A, Yang Y, Liu X, Knipe DM, Cresswell P, Yuan W. Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. Journal of virology. 2011;85(16):8093–8104. doi: 10.1128/JVI.02689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chouljenko DV, Kim IJ, Chouljenko VN, Subramanian R, Walker JD, Kousoulas KG. Functional hierarchy of herpes simplex virus 1 viral glycoproteins in cytoplasmic virion envelopment and egress. J Virol. 2012;86(8):4262–4270. doi: 10.1128/JVI.06766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frampton AR, Jr, Goins WF, Nakano K, Burton EA, Glorioso JC. HSV trafficking and development of gene therapy vectors with applications in the nervous system. Gene Ther. 2005;12(11):891–901. doi: 10.1038/sj.gt.3302545. [DOI] [PubMed] [Google Scholar]
- 94.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72(1):873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4(5):800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99(1):13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 97.Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276(24):7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerber SI, Belval BJ, Herold BC. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology. 1995;214(1):29–39. doi: 10.1006/viro.1995.9957. [DOI] [PubMed] [Google Scholar]
- 99.Shieh MT, WuDunn D, Montgomery RI, Esko JD, Spear PG. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116(5):1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, Minowa A, Akashi H, Arase H, Kawaoka Y, et al. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature. 2010;467(7317):859–862. doi: 10.1038/nature09420. [DOI] [PubMed] [Google Scholar]
- 101.Chowdhury S, Naderi M, Chouljenko VN, Walker JD, Kousoulas KG. Amino acid differences in glycoproteins B (gB), C (gC), H (gH) and L (gL) are associated with enhanced herpes simplex virus type-1 (McKrae) entry via the paired immunoglobulin-like type-2 receptor alpha. Virology journal. 2012;9:112. doi: 10.1186/1743-422X-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. The Journal of infectious diseases. 2003;187(4):542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 103.Cai WH, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J Virol. 2011;85(13):6175–6184. doi: 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ. Bimolecular complementation defines functional regions of Herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J Virol. 2010;84(8):3825–3834. doi: 10.1128/JVI.02687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galdiero S, Vitiello M, D’Isanto M, Falanga A, Cantisani M, Browne H, Pedone C, Galdiero M. The identification and characterization of fusogenic domains in herpes virus glycoprotein B molecules. Chembiochem. 2008;9(5):758–767. doi: 10.1002/cbic.200700457. [DOI] [PubMed] [Google Scholar]
- 107.Akkarawongsa R, Pocaro NE, Case G, Kolb AW, Brandt CR. Multiple peptides homologous to herpes simplex virus type 1 glycoprotein B inhibit viral infection. Antimicrobial agents and chemotherapy. 2009;53(3):987–996. doi: 10.1128/AAC.00793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82(9):1075–1082. [PubMed] [Google Scholar]
- 109.Yasin B, Wang W, Pang M, Cheshenko N, Hong T, Waring AJ, Herold BC, Wagar EA, Lehrer RI. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J Virol. 2004;78(10):5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brandt CR, Akkarawongsa R, Altmann S, Jose G, Kolb AW, Waring AJ, Lehrer RI. Evaluation of a theta-defensin in a Murine model of herpes simplex virus type 1 keratitis. Invest Ophthalmol Vis Sci. 2007;48(11):5118–5124. doi: 10.1167/iovs.07-0302. [DOI] [PubMed] [Google Scholar]
- 111.Shogan B, Kruse L, Mulamba GB, Hu A, Coen DM. Virucidal activity of a GT-rich oligonucleotide against herpes simplex virus mediated by glycoprotein B. J Virol. 2006;80(10):4740–4747. doi: 10.1128/JVI.80.10.4740-4747.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dix RD, Pereira L, Baringer JR. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infection and immunity. 1981;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dix RD. Glycoprotein gB of herpes simplex virus expresses type-common and type-specific antigenic determinants in vivo. Journal of medical virology. 1990;30(3):192–195. doi: 10.1002/jmv.1890300309. [DOI] [PubMed] [Google Scholar]
- 114.Eis-Hubinger AM, Mohr K, Schneweis KE. Different mechanisms of protection by monoclonal and polyclonal antibodies during the course of herpes simplex virus infection. Intervirology. 1991;32(6):351–360. doi: 10.1159/000150219. [DOI] [PubMed] [Google Scholar]
- 115.Krawczyk A, Krauss J, Eis-Hubinger AM, Daumer MP, Schwarzenbacher R, Dittmer U, Schneweis KE, Jager D, Roggendorf M, Arndt MA. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J Virol. 2011;85(4):1793–1803. doi: 10.1128/JVI.01924-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nicola AV, Willis SH, Naidoo NN, Eisenberg RJ, Cohen GH. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol. 1996;70(6):3815–3822. doi: 10.1128/jvi.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Subramanian RP, Geraghty RJ. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc Natl Acad Sci U S A. 2007;104(8):2903–2908. doi: 10.1073/pnas.0608374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Whitbeck JC, Peng C, Lou H, Xu R, Willis SH, Ponce de Leon M, Peng T, Nicola AV, Montgomery RI, Warner MS, et al. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71(8):6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whitbeck JC, Muggeridge MI, Rux AH, Hou W, Krummenacher C, Lou H, van Geelen A, Eisenberg RJ, Cohen GH. The major neutralizing antigenic site on herpes simplex virus glycoprotein D overlaps a receptor-binding domain. J Virol. 1999;73(12):9879–9890. doi: 10.1128/jvi.73.12.9879-9890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tiwari V, Liu J, Valyi-Nagy T, Shukla D. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J Biol Chem. 2011;286(28):25406–25415. doi: 10.1074/jbc.M110.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krepstakies M, Lucifora J, Nagel CH, Zeisel MB, Holstermann B, Hohenberg H, Kowalski I, Gutsmann T, Baumert TF, Brandenburg K, et al. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. The Journal of infectious diseases. 2012;205(11):1654–1664. doi: 10.1093/infdis/jis273. [DOI] [PubMed] [Google Scholar]
- 122.Krummenacher C, Rux AH, Whitbeck JC, Ponce-de-Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty RJ, Spear PG, et al. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol. 1999;73(10):8127–8137. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol. 1998;72(5):3595–3601. doi: 10.1128/jvi.72.5.3595-3601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol. 2012;86(3):1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Valimaa H, Tenovuo J, Waris M, Hukkanen V. Human lactoferrin but not lysozyme neutralizes HSV-1 and inhibits HSV-1 replication and cell-to-cell spread. Virol J. 2009;6:53. doi: 10.1186/1743-422X-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Valori CF, Ning K, Wyles M, Azzouz M. Development and applications of non-HIV-based lentiviral vectors in neurological disorders. Curr Gene Ther. 2008;8(6):406–418. doi: 10.2174/156652308786848030. [DOI] [PubMed] [Google Scholar]
- 127.St Vincent MR, Colpitts CC, Ustinov AV, Muqadas M, Joyce MA, Barsby NL, Epand RF, Epand RM, Khramyshev SA, Valueva OA, et al. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc Natl Acad Sci U S A. 2010;107(40):17339–17344. doi: 10.1073/pnas.1010026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dean HJ, Terhune SS, Shieh MT, Susmarski N, Spear PG. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology. 1994;199(1):67–80. doi: 10.1006/viro.1994.1098. [DOI] [PubMed] [Google Scholar]
- 129.O’Keefe BR, Shenoy SR, Xie D, Zhang W, Muschik JM, Currens MJ, Chaiken I, Boyd MR. Analysis of the interaction between the HIV-inactivating protein cyanovirin-N and soluble forms of the envelope glycoproteins gp120 and gp41. Mol Pharmacol. 2000;58(5):982–992. doi: 10.1124/mol.58.5.982. [DOI] [PubMed] [Google Scholar]
- 130.Bolmstedt AJ, O’Keefe BR, Shenoy SR, McMahon JB, Boyd MR. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol Pharmacol. 2001;59(5):949–954. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- 131.Shenoy SR, O’Keefe BR, Bolmstedt AJ, Cartner LK, Boyd MR. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J Pharmacol Exp Ther. 2001;297(2):704–710. [PubMed] [Google Scholar]
- 132.Tiwari V, Shukla SY, Shukla D. A sugar binding protein cyanovirin-N blocks herpes simplex virus type-1 entry and cell fusion. Antiviral Res. 2009;84(1):67–75. doi: 10.1016/j.antiviral.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20(1):11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 134.Lin LT, Chen TY, Chung CY, Noyce RS, Grindley TB, McCormick C, Lin TC, Wang GH, Lin CC, Richardson CD. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J Virol. 2011;85(9):4386–4398. doi: 10.1128/JVI.01492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Allendoerfer R, Marquis AJ, Rinaldi MG, Graybill JR. Combined therapy with fluconazole and flucytosine in murine cryptococcal meningitis. Antimicrobial agents and chemotherapy. 1991;35(4):726–729. doi: 10.1128/aac.35.4.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arikan S, Lozano-Chiu M, Paetznick V, Rex JH. In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrobial agents and chemotherapy. 2002;46(1):245–247. doi: 10.1128/AAC.46.1.245-247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barchiesi F, Di Francesco LF, Compagnucci P, Arzeni D, Giacometti A, Scalise G. In-vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J Antimicrob Chemother. 1998;41(1):59–65. doi: 10.1093/jac/41.1.59. [DOI] [PubMed] [Google Scholar]
- 138.Barchiesi F, Gallo D, Caselli F, Di Francesco LF, Arzeni D, Giacometti A, Scalise G. In-vitro interactions of itraconazole with flucytosine against clinical isolates of Cryptococcus neoformans. J Antimicrob Chemother. 1999;44(1):65–70. doi: 10.1093/jac/44.1.65. [DOI] [PubMed] [Google Scholar]
- 139.Sondak VK, Korn EL, Kern DH. In vitro testing of chemotherapeutic combinations in a rapid thymidine incorporation assay. Int J Cell Cloning. 1988;6(6):378–391. doi: 10.1002/stem.5530060603. [DOI] [PubMed] [Google Scholar]
- 140.Sondak VK, Korn EL, Morton DL, Kern DH. Testing chemotherapeutic combinations in the Human Tumor Colony--Forming Assay. J Surg Oncol. 1988;37(3):156–160. doi: 10.1002/jso.2930370304. [DOI] [PubMed] [Google Scholar]
- 141.van Moorsel CJ, Pinedo HM, Veerman G, Bergman AM, Kuiper CM, Vermorken JB, van der Vijgh WJ, Peters GJ. Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. Br J Cancer. 1999;80(7):981–990. doi: 10.1038/sj.bjc.6690452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pirrone V, Thakkar N, Jacobson JM, Wigdahl B, Krebs FC. Combinatorial approaches to the prevention and treatment of HIV-1 infection. Antimicrobial agents and chemotherapy. 2011;55(5):1831–1842. doi: 10.1128/AAC.00976-10. [DOI] [PMC free article] [PubMed] [Google Scholar]