Abstract
Objective
To delineate the plasma pharmacokinetics and determine the corneal uptake of valine based stereoisomeric dipeptide prodrugs of acyclovir (ACV) in rats.
Methods
Male Sprague-Dawley rats were used for the study. Pharmacokinetics of ACV, L-valine-acyclovir (LACV), L-valine-D-valine-acyclovir (LDACV) and D-valine-L-valine acyclovir (DLACV) prodrugs were delineated. These compounds were administered intravenously as a bolus via jugular vein cannula and orally by gavage. Samples were purified by protein precipitation method and analyzed by LC-MS/MS. Pertinent pharmacokinetic parameters were obtained by using WinNonlin. Corneal uptake studies of LDACV and LACV were studied following oral administration.
Results
Following i.v. administration, the area under the curve (AUC) in μM*min of generated ACV was in the order of LACV > LDACV > DLACV indicating their rate of metabolism. The AUC values of total drug obtained in the systemic circulation after oral administration LACV and LDACV were 1077.93 ± 236.09 and 1141.76 ± 73.67 μM*min, respectively. DLACV exhibited poor oral absorption. Cmax (μM) and AUC of the intact prodrug obtained in the systemic circulation following oral administration of LDACV were almost 4–5 times higher than LACV. Moreover, concentrations achieved in the cornea after oral administration of LDACV were almost two times of LACV.
Conclusions
LDACV increased both the oral bioavailability and subsequent in vivo corneal uptake of ACV. Hence, LDACV can be considered as the most promising drug candidate for delivery of ACV, in treatment of both genital herpes and ocular herpes keratitis after oral administration.
Keywords: acyclovir, dipeptide, prodrugs, PEPT, cornea
Introduction
Infection with herpes simplex virus (HSV) can be classified as orofacial herpes, genital herpes and herpes keratitis depending on the site of infection. Acyclovir (ACV) is an approved drug of choice for infections caused by both HSV-1 and HSV-2. The current mode of treatment for herpes keratitis includes use of topical trifluorodothymidine (TFT), idoxuridine (IDU) and vidarabine. These agents are highly cytotoxic, thus limiting their usage for long term treatment. Even though, ACV is considered safe, currently, there is no topical formulation of ACV available for the treatment of herpes keratitis in United States. Genital herpes is usually treated with ACV and its analogs/prodrugs administered intravenously or orally. Following initial episode of herpes, chance of recurrence is fairly high due to various factors including fever, trauma, stress, light, immunosuppressive agents and exposure to UV radiation. There is approximately 50% chance of recurrence of herpes keratitis within 2 years of initial episode.1 Hence continuous suppression rather than intermittent dosing is suggested as the preferred therapeutic intervention.
Systemic drug delivery (intravenous or oral) is potentially an effective route to treat various systemic as well as ocular disorders. However, drugs administered by this route must cross the intestine to reach the systemic circulation and subsequently the blood ocular barriers (BOB) to reach the inner ocular tissues. BOB is comprised of blood aqueous and blood retinal barriers. Transport of hydrophilic molecules like acyclovir and ganciclovir from systemic circulation into anterior chamber tissues like cornea is restricted by blood aqueous barrier (BAB). BAB is a selectively permeable barrier between systemic circulation and ocular aqueous fluids formed by the tight junctions of nonpigmented layer of the ciliary body epithelium and the endothelium of iridial blood vessels. It mostly regulates the inward movement of the compounds from blood into the eye.2
Limited therapeutic efficacy of ACV against herpes infections following oral administration is due to its poor permeability across oral mucosa. Among various strategies that have been investigated to improve cellular permeability of ACV, prodrugs have been found to be effective and most promising. Transporter targeted prodrug delivery has emerged successfully due to its ability to deliver the drug to targeted tissues and also translocate it intracellularly at a higher rate.3–7 A wide variety of ester prodrugs of acyclovir were designed to target nutrient transporters including carriers for amino acids and peptides. 15,16 Amongst them, peptide transporters (PEPT) are very useful due to their high capacity and wide substrate specificity which provide more flexibility in designing a prodrug.8–10 Increased oral bioavailability of valacyclovir (VACV), a valine ester prodrug of acyclovir is due its recognition and translocation by peptide transporters present on intestinal mucosa.11–14 A series of dipeptide esters of ACV were evaluated for corneal and systemic bioavailability following topical and oral administrations.15,16,21
A functionally active PEPT was identified on both the blood aqueous and blood retinal barriers.17,18 PEPT at BAB can be utilized to improve drug delivery of ACV after i.v. or oral administration. Targeting PEPT at BAB using dipeptide prodrugs to improve the ACV absorption into aqueous chamber has been already reported.17 However, targeting PEPT at BAB following oral or systemic administration requires prolonged presence of intact prodrug at the site of absorption. Hence it requires the absorption of intact prodrug into systemic circulation after oral administration. Amino acid and dipeptide prodrugs of ACV that have been evaluated before were found to be metabolized rapidly and completely to the parent drug during first pass following oral administration.15,19,21 They have resulted in improved oral bioavailability of acyclovir. However, as described previously regenerated ACV has to cross BAB to permeate into the anterior chamber ocular tissues. So there is a necessity to design prodrugs such that their hydrolytic rate can be modulated to generate higher amounts of intact prodrug in the systemic circulation resulting from improved oral bioavailability. This can be achieved by incorporation of D-isomers into a dipeptide conjugate. L-amino acid ester prodrugs are natural substrates of esterases. Incorporation of a D-isomer will modulate the hydrolytic rates by both amidases and esterases the two groups of enzymes primarily responsible for hydrolysis of amino acid and dipeptide conjugates.
Hence enzymatically stable dipeptide conjugates of ACV were synthesized and evaluated for interaction with PEPT, uptake/transport across Caco-2 cells and metabolism in various tissue homogenates.20 In the same study, the effect of incorporation of a D-isomer upon rate of prodrug hydrolysis was also evaluated. Stereoisomeric dipeptide conjugates had different hydrolytic rates depending on the position of a D-isomer in a dipeptide and also the number of D-isomers. One important observation in that study was that the dipeptide conjugates with one D-isomer did not lose their affinity towards PEPT and also possess enhanced metabolic stability.
Hence, it is important to evaluate the stereoisomeric dipeptide prodrugs in vivo to examine whether this strategy could improve systemic absorption as well as in vivo uptake into the cornea. Hence, the objective of this work is to delineate the pharmacokinetics and in vivo corneal uptake of ACV following oral administration of the valine based stereoisomeric dipeptide prodrugs of ACV in rats. ACV and VACV were also included in the study. The disposition of all the prodrugs was studied following i.v. administrations to rat.
Materials and Methods
Materials
VACV was a gift from GlaxoSmithKline, (Research Triangle Park, NC). L-valine-D-valine-acyclovir-(LDACV) and D-valine-L-valine acyclovir (DLACV) were synthesized in our laboratory according to a standard established procedure.20 Heparin and heparin coated tubes were obtained from Fisher scientific. Saline was purchased from Sigma chemical Co (St. Louis, MO). All other chemicals were purchased from Sigma chemical Co and were used as received without further purification.
Animals
Jugular vein cannulated male Sprague-Dawley rats weighing between 200 to 250 g were obtained from Charles River Laboratories (Wilmington, MA). Animal care and treatment employed in this research were in compliance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Pharmacokinetic studies
Prodrugs of ACV equivalent to 20 mg/kg of ACV were administered orally by gavage to rats. VACV, LDACV and DLACV were administered intravenously as a bolus via jugular vein cannula at a dose level of 10 mg/kg (3–4 animals per route). For oral studies, animals were fasted overnight (10–12 h) with free access to water. Freshly prepared drug/prodrug solutions were given by oral gavage (0.8 ml). For intravenous dosing 200 μl of drug containing sterilized saline followed by 200 μl of blank sterile heparanized saline were administered via jugular vein cannula. Blood samples (200 μl) were collected from the jugular vein into heparin coated tubes and replaced with same volume of heparanized saline (100 U/ml) at predetermined time intervals over a period of 8 hrs. Blood was immediately centrifuged (7500 g for 5 minutes) to separate the plasma which was stored at −80 °C until further analysis. At the end of an experiment, animals were euthanized with 10 mg per 100 g body weight of sodium pentobarbital administered through jugular vein cannula.
Corneal uptake studies
For determining the corneal uptake, equimolar doses of prodrugs LACV and LDACV equivalent to 30 mg/kg of ACV were administered to rats by oral gavage. At the time of plasma Tmax (15 min), animals were euthanized with 10 mg per 100 g body weight of sodium pentobarbital. Immediately the eyes balls were proptosed, enucleated carefully and washed with ice-cold with Dulbecco’s phosphate buffered saline (DPBS) pH 7.4 to remove any traces of blood and immediately frozen. Subsequently, a small incision was made in the sclera under the magnifying glass and the cornea was carefully excised ensuring that no trace of sclera was present. Corneas were washed with DPBS pH 7.4, dried and weighed.
The cornea was homogenized with a tissue homogenizer (Tissue tearer model 985-370). The homogenate was centrifuged, and the supernatant was collected and frozen at −80 °C. For preparing the calibration standards, blank tissues were collected and treated in a similar manner.
Sample preparation for LC-MS/MS
Samples were purified by protein precipitation method. Ganciclovir (GCV) was used as an internal standard. Plasma and corneal tissue samples were thawed at room temperature. Five μl of 10 μg/ml GCV (500 ng/ml) and 200 μl of acetonitrile were added to 100 μl of sample. The mixture was vortexed vigorously for 2–3 min and centrifuged at 5000 g for 10 min at 4 °C. The supernatant was collected and the solvent was evaporated to dryness with a Speedvac (SAVANT Instruments, Inc., Holbrook, NY). The dry residue was reconstituted in 100 μl water, vortexed for 2 min and further centrifuged (5000 g, 4 min). The obtained supernatant was analyzed using LC-MS/MS. Appropriate calibration standards of ACV and its prodrugs were prepared by spiking a known analyte concentrations to blank plasma and corneal homogenate obtained from untreated rats. The calibration standards were also subjected to identical treatment as samples using a blank corneal matrix and a calibration curve was generated.
LC/MS/MS
A validated LC-MS/MS method for the analysis of dipeptide prodrugs of ACV was established.22 Briefly, a linear Ion Trap Quadrople LC/MS/MS mass spectrometer (AB Sciex instruments) with electrospray ionization on Turbo Spray ion source (API 2000; Applied Biosystems, Foster City, CA, USA) coupled to an Agilent 1100 binary pump, degasser, and an autosampler was used. Data was collected in multiple reactions monitoring (MRM) mode. Typical ion source parameters were tabulated (Table 1). The entrance potential (EP) was 6 V. The nebulizer gas was set at 10 (arbitrary units), the curtain gas at 8, the collision gas at 4, the ionization voltage at 4500 V and the source temperature was at 500 °C. The compounds were separated with Grace Alltima C8 (150 mm × 2.1 mm, 5 μm pore size) column attached to a guard column. The flow rate was 0.15 ml/min. The prodrugs and their respective metabolites were quantified by the ratio of analyte to the internal standard (ganciclovir).
Table 1.
Mass spectrometric conditions for the LC-MS/MS analysis of ACV and its prodrugs in plasma.
| Analyte | Q1 mass | Q3 mass | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|
| ACV | 226.10 | 152.10 | 36 | 18 | 6 |
| LACV/DACV | 325.10 | 152.10 | 36 | 18 | 6 |
| LDACV/DLACV | 424.2 | 174.20 | 36 | 18 | 6 |
Abbreviations: DP, declustering potential; CE, collision energy; CXP, collision cell exit potential.
Pharmacokinetic data analysis
All the relevant pharmacokinetic parameters were calculated by noncompartmental analyses of plasma concentration—time curves with a pharmacokinetic software package WinNonlin, version 5.0 (Pharsight, Mountain View, CA). Cmax, Tmax, Clast and Tlast values were obtained from the log plasma concentration—time curves. Concentration at zero time point (C0) after i.v. administration was obtained by extrapolation of plasma–time curve of prodrugs and their metabolites to zero time point. The slopes of the terminal phase of plasma profiles were estimated by log-linear regression and the terminal rate constant (λz ) was derived from the slope of terminal elimination phase. AUC from zero to the last measurable concentration (AUC0-t ) was estimated by the linear trapezoidal method. AUC from time zero to infinity (AUC0-inf) was calculated as AUC0-inf = AUC0-t + Ct/λz.
Ct is the last quantitable concentration at time t. Total body clearance (ClT) and volume of distribution (Vz) after i.v. bolus administration were calculated as a ratio of dose/AUC and ClT/λz respectively.
Results
Pharmacokinetics of LACV and ACV following i.v. and oral administrations of LACV
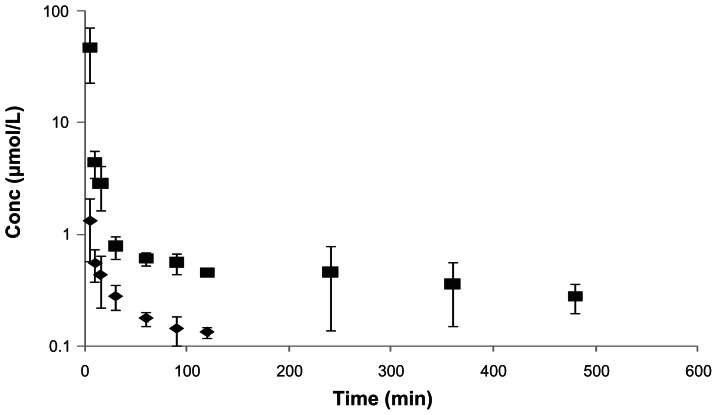
Following i.v. administration of LACV, both LACV and the regenerated ACV were observed for a period of 120 and 480 min, respectively. Mean plasma concentration—time profiles of LACV and ACV after i.v. administration of LACV are shown in Figure 1. Over the entire duration of the experiment, ACV concentration was significantly higher than LACV. Plasma concentrations of both the species declined biexponentially. All relevant pharmacokinetic parameters for LACV and ACV are tabulated in Table 2. C0 and AUC values were in the order of ACV > LACV. The systemic clearance (0.07 L/min/kg) of LACV was significantly higher than of ACV (0.01 L/min/kg).
Figure 1.
Plasma concentration—time profiles of LACV, and the regenerated ACV following i.v. administration of LACV in rats (◆-LACV, ■-ACV). Each value is represented as mean ± SD (n = 3–4).
Table 2.
Plasma pharmacokinetic parameters of LACV and the regenerated ACV following i.v. administration of LACV in rats. (Values are represented as mean ± S.D, n = 3–4).
| pK parameter | LACV | ACV |
|---|---|---|
| λz (min−1) | 0.002 ± 0.0008 | 0.0053 ± 0.0039 |
| C0 (μM) | 0.78 ± 0.15 | 120.6 ± 13.7 |
| Tlast (Min) | 240 | 480 |
| Clast (μM) | 0.11 ± 0.01 | 0.33 ± 0.13 |
| AUC0-t (min*μM) | 53.2 ± 13.4 | 760.8 ± 466.6 |
| AUC0-inf (min*μM) | 114.1 ± 36.72 | 833.6 ± 461.1 |
| Vz (L/kg) | 33.4 ± 5.72 | 2.44 ± 1.6 |
| Cl (L/min/kg) | 0.07 ± 0.02 | 0.01 ± 0.004 |
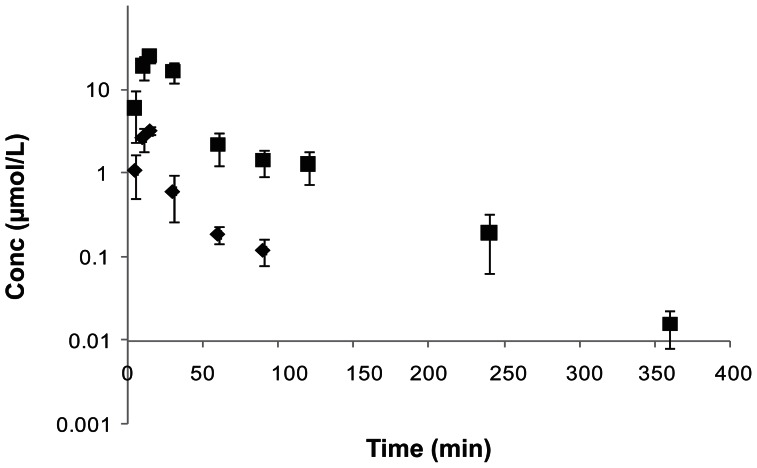
Like in i.v. administration, both LACV and the regenerated ACV appeared in the systemic circulation following oral administration of LACV. Both the species were observed from the initial time point (5 min) indicating rapid absorption of the prodrug. LACV was not detected in the plasma 90 min post dosing. Mean plasma concentration—time profile observed after oral administration of LACV is depicted in Figure 2. At all the time points, amounts of ACV were significantly higher than LACV, which indicates rapid and complete in vivo hydrolysis of LACV by esterases. After the peak plasma concentration was reached (Cmax), concentrations of both LACV and ACV declined rapidly in a biphasic manner. All pertinent pharmacokinetic parameters for LACV and ACV are tabulated in Table 3. As expected, the Cmax and AUC were in the order of ACV > LACV.
Figure 2.
Plasma concentration—time profiles of LACV, and the generated ACV following oral administration of LACV in rats (◆-LACV, ■-ACV). Each value is represented as mean ± SD (n = 3–4).
Table 3.
Plasma pharmacokinetic parameters of LACV and the regenerated ACV following oral administration of LACV in rats. (Values are represented as mean ± S.D, n = 3–4).
| pK parameter | LACV | ACV |
|---|---|---|
| Cmax (μM) | 3.38 ± 0.13 | 26.5 ± 4.15 |
| Tmax (min) | 13.7 ± 2.5 | 15 |
| Clast (μM) | 0.16 ± 0.04 | 0.016 ± 0.007 |
| AUC0-t (min*μM) | 86.3 ± 22.4 | 958.9 ± 106.9 |
| AUC0-inf (min*μM) | 91.2 ± 21.06 | 959.8 ± 107.4 |
Pharmacokinetics following i.v. and oral administrations of LDACV
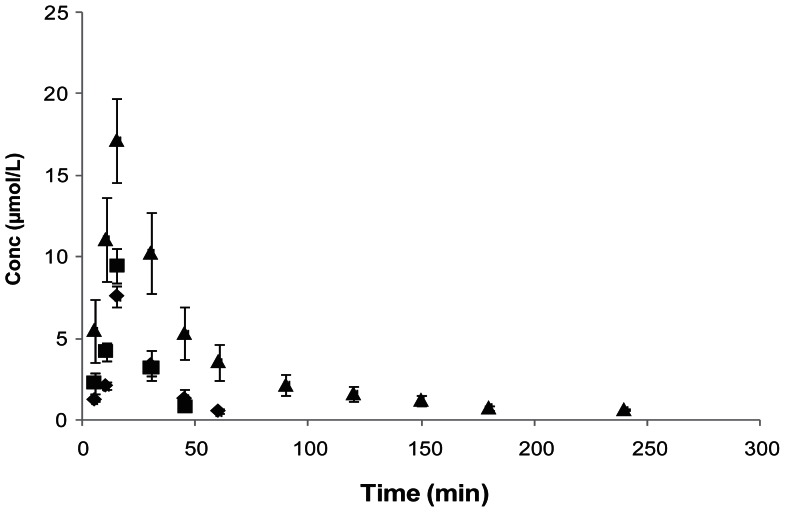
Following i.v. administration of LDACV, all the three species LDACV, intermediate DACV and the parent drug ACV appeared in plasma from the first sampling time point (5 min). Mean plasma concentration—time profiles are illustrated in Figure 3. Plasma profiles of generated DACV and ACV appeared to be identical. Relevant pharmacokinetic parameters are tabulated in Table 4. The C0 and AUC were in the order of LDACV > ACV > DACV. LDACV, DACV and ACV were not detected in the plasma after 15, 30 and ~34 minutes, respectively. Systemic clearance was in the order of DACV > ACV > LDACV.
Figure 3.
Plasma concentration—time profiles of LDACV, and the generated DACV and ACV following i.v. administration of LDACV in rats (◆-LDACV, ■-DACV, ▲-ACV). Each value is represented as mean ± SD (n = 3–4).
Table 4.
Plasma pharmacokinetic parameters of LDACV and the generated DACV and ACV following i.v. administration of LDACV in rats. (Values are represented as mean ± S.D, n = 3–4).
| pK parameter | LDACV | DACV | ACV |
|---|---|---|---|
| λz (min−1) | 0.277 ± 0.051 | 0.044 ± 0.004 | 0.046 ± 0.008 |
| C0 (μM) | 111.8 ± 47.1 | 1.36 ± 0.22 | 1.82 ± 0.39 |
| Tlast (min) | 15 | 30 | 33.75 ± 7.5 |
| Clast (μM) | 1.44 ± 0.73 | 0.2 ± 0.05 | 0.24 ± 0.14 |
| AUC0–t (min*μM) | 411.1 ± 109.9 | 15.4 ± 1.35 | 22.74 ± 2.80 |
| AUC0–inf (min*μM) | 416.8 ± 106.7 | 20.2 ± 3.04 | 29.4 ± 6.28 |
| Vz (L/kg) | 0.21 ± 0.099 | 25.1 ± 1.98 | 18.2 ± 2.65 |
| Cl (L/min/kg) | 0.055 ± 0.014 | 1.12 ± 0.18 | 0.77 ± 0.149 |
Similar to i.v. administration, oral administration of LDACV, generated all the three species LDACV, DACV and ACV in plasma from the beginning (5 min). Mean plasma concentration—time profiles of all the three species are shown in Figure 4. As observed earlier, the regenerated ACV from LDACV declined in a biexponential manner. Pertinent pharmacokinetic parameters are tabulated in Table 5. The Cmax and AUC values were in the order of ACV > DACV > LDACV, indicating enzymatic hydrolysis of LDACV and the generated DACV in the intestine or liver during absorption. LDACV, DACV and ACV were not detected in the plasma after 45, 60 and 240 minutes, respectively.
Figure 4.
Plasma concentration—time profiles of LDACV, the generated DACV and ACV following oral administration of LDACV in rats (◆-DLACV, ■-ACV). Each value is represented as mean ± SD (n = 3–4).
Table 5.
Plasma pharmacokinetic parameters of LDACV and the generated DACV and ACV following oral administration of LDACV in rats. (Values are represented as mean ± S.D, n = 3–4).
| pK parameter | LDACV | DACV | ACV |
|---|---|---|---|
| λz (min−1) | 0.0063 ± 0.0044 | 0.083 ± 0.0083 | 0.010 ± 0.004 |
| Tmax (min) | 15 | 15 | 15 |
| Cmax (μM) | 7.56 ± 0.642 | 9.46 ± 1.04 | 17.2 ± 2.56 |
| Clast (μM) | 0.52 ± 0.162 | 0.79 ± 0.155 | 0.605 ± 0.058 |
| AUC0-t (min*μM) | 165.4 ± 26.6 | 180.3 ± 11.04 | 765.4 ± 120.6 |
| AUC0-inf (min*μM) | 173.7 ± 29.5 | 190.04 ± 8.32 | 833.9 ± 102.2 |
Pharmacokinetics following i.v. and oral administrations of DLACV
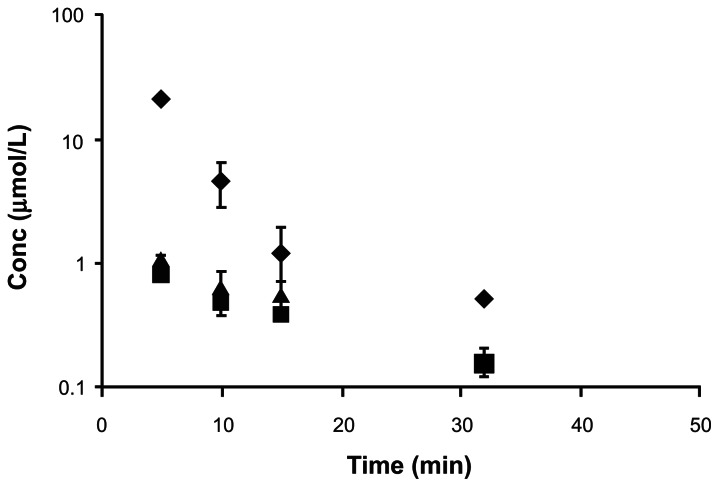
Following i.v. administration of DLACV, only two species DLACV and the regenerated ACV were detected in the plasma. The intermediate LACV was not detected at any time point. Mean plasma concentration—time profile is shown in Figure 5. DLACV and the regenerated ACV were not detected in the plasma after 30 and 15 min, respectively. At all time points, the observed concentrations of DLACV were much higher than ACV. Pertinent pharmacokinetic parameters are tabulated in Table 6. The C0 and AUC values were in the order of DLACV > ACV. The systemic clearance of DLACV was lower than regenerated ACV.
Figure 5.
Plasma concentration—time profiles of DLACV, and the generated ACV following i.v. administration of DLACV in rats (◆-DLACV, ■-ACV). Each value is represented as mean ± SD (n = 3–4).
Table 6.
Plasma pharmacokinetic parameters of DLACV and the generate ACV following i.v. administration of DLACV in rats. (Values are represented as mean ± S.D, n = 3–4).
| pK parameter | DLACV | ACV |
|---|---|---|
| λz (min−1) | 0.225 ± 0.027 | 0.07 ± 0.014 |
| C0 (μM) | 46.3 ± 5.23 | 0.79 ± 0.213 |
| AUCall (min*μM) | 196.48 ± 18.59 | 9.95 ± 2.59 |
| AUCinf (min*μM) | 202.4 ± 17.66 | 12.9 ± 2.25 |
| Vz (L/kg) | 0.50 ± 00.21 | 18.04 ± 6.96 |
| CL (L/min/kg) | 0.077 ± 0.006 | 1.22 ± 0.19 |
Following oral administration of DLACV, only DLACV and ACV were detected at a very low concentration for a period of 15 and 10 min, respectively. Pharmacokinetic parameters were not calculated since the drug was not detected after the initial few time points. Maximum concentration of DLACV detected in the systemic circulation was 0.53 ± 0.04 μM, which might be due to its poor absorption across intestinal mucosa.
Comparison
Cmax, Tmax and AUC of total drug (prodrug + drug) in the systemic circulation following oral administration of LACV and LDACV were tabulated and compared (Table 7). Based on the Cmax and AUC values, it is evident that both LACV and LDACV have certainly improved the oral bioavailability of ACV. The Cmax and AUC of LDACV are comparable to those of LACV.
Table 7.
Comparison of pharmacokinetic parameters of LACV and LDACV following oral administration to rats. (Each value represents mean ± SD, n = 3–4).
| pK parameter | LACV | LDACV |
|---|---|---|
| Cmax (μM) | 28.6 ± 4.48 | 32.08 ± 2.81 |
| Tmax (min) | 15 | 15 |
| AUC0-inf (min*μM) | 1077.9 ± 236.09 | 1141.8 ± 73.7 |
Moreover, the amount of prodrug (dipeptide + amino acid intermediate) observed in the systemic circulation following oral administration of LACV and LDACV were also tabulated and compared (Table 8). Both Cmax and AUC values were in the order of LDACV > LACV. The Cmax and AUC of the intact prodrug resulted following oral administration of LDACV is almost 5 and 4 fold higher than compared to LACV.
Table 8.
Comparison of pharmacokinetic parameters of intact prodrug (dipeptide + amino acid metabolite) obtained following oral delivery of LACV and LDACV. (Each value represents mean ± SD, n = 3–4).
| pK parameter | LACV | LDACV |
|---|---|---|
| Cmax (μM) | 3.37 ± 0.13 | 15.06 ± 2.68 |
| Tmax (min) | 15 | 15 |
| AUC0-inf (Min*μM) | 91.2 ± 21.06 | 336.6 ± 20.09 |
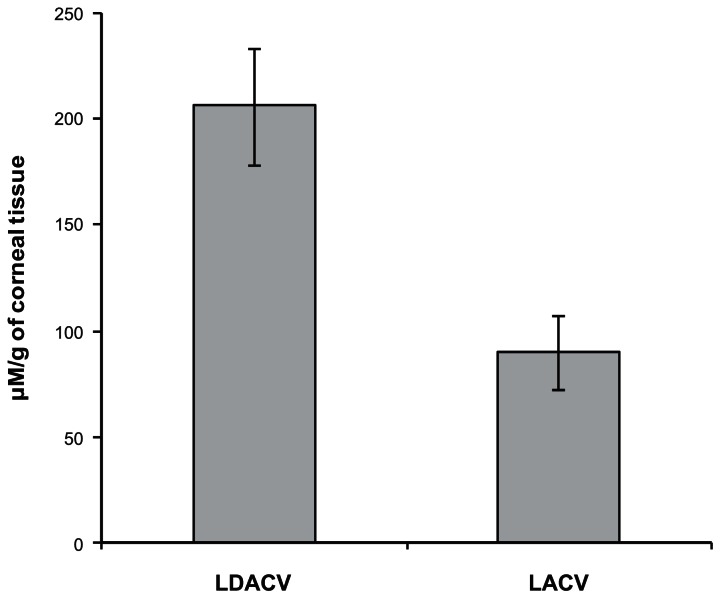
Corneal uptake of ACV following oral administration of LACV and LDACV
The amount of total drug that was detected in cornea following oral administration of LACV and LDACV was compared (Fig. 6). LDACV resulted in almost 2 times higher corneal concentration of ACV compared to LACV. The corneal homogenate was analyzed for all the species. However, only LDACV and ACV were detected in the corneal homogenate with significantly higher concentrations of ACV compared to LDACV in both the eyes (Fig. 7).
Figure 6.
Corneal uptake of ACV following oral administration of LDACV and LACV in rats. (Each value represents mean ± SD).
Figure 7.
Corneal concentrations of LDACV and ACV following oral administration of LDACV at a molar dose equivalent to 30 mg/kg of acyclovir in rats (
 -ACV, ■-LDACV ). Values are represented as mean ± SD, n = 3–4) [R-right eye, L-left eye].
-ACV, ■-LDACV ). Values are represented as mean ± SD, n = 3–4) [R-right eye, L-left eye].
Discussion
The concept of dipeptide prodrugs of acyclovir targeting the peptide transporters on the intestinal mucosa and rabbit cornea has been previously reported from our laboratory.15,16,21 All the dipeptide prodrugs were based on L-amino acid isomers which are natural substrates for hydrolyzing enzymes. However, a recent report from the authors has shown that the incorporation of a D-isomer into a dipeptide can modulate the enzymatic stability of the prodrugs as well as retain the affinity towards PEPT.20 The effect of rate of prodrug hydrolysis on cellular permeability was also studied in the previous report using valine based stereoisomeric dipeptide prodrugs of acyclovir. In this research, the stereoisomeric dipeptide prodrug concept was extended to in vivo in rats. Hence this study is focused on the pharmacokinetics and corneal uptake of stereoisomeric dipeptide prodrugs in vivo in rats. LACV was included in the study. L-valine-L-valine-acyclovir (LLACV) was not included as its pharmacokinetics was studied earlier.15,21,22 D-valine-D-valine-acyclovir (DDACV) was also not included as its affinity towards PEPT was lost and found to be highly stable without any hydrolysis to release ACV even in rat intestinal and liver homogenates.20
Following i.v. administration of LACV, higher Cmax and AUC values of generated ACV clearly indicate its rapid hydrolysis (Table 2, Fig. 1). However, in the pharmacokinetic profiles observed after i.v. administration of LDACV and DLACV, the concentration of prodrug was much higher than ACV at all the time points (Figs. 3 and 5). In previous studies with LLACV, the concentrations of the generated ACV were significantly higher than the prodrug.15,21,22 These results demonstrate that the incorporation of one D-valine has certainly enhanced the metabolic stability of prodrugs against hydrolyzing enzymes. DLACV appears to be more stable resulting in higher concentrations of intact dipeptide conjugate and generated only the parent drug ACV without any intermediate LACV following i.v. administration (Fig. 5). These results are in complete agreement with our earlier in vitro data. The clearance of the generated ACV following i.v. administration of DLACV was slightly higher than the ACV generated from LDACV (Tables 4 and 5).
Oral administration of LACV resulted in both the prodrug and drug. However, Cmax and AUC of ACV were significantly higher than LACV (Table 3). This rapid and complete hydrolysis of LACV is in agreement with published literature. Unlike the results obtained after i.v. administration of LDACV, Cmax and AUC of the regenerated ACV were higher than of intact prodrug following oral administration (Tables 4 and 5). However, due to the enhanced stability of prodrug against hydrolyzing enzymes, a significant amount of intact prodrug was also observed in the systemic circulation. This rapid pre-systemic hydrolysis observed following oral dosing compared to i.v. dosing can be attributed to hydrolysis by proteases and/or esterases in either intestine and/or liver during absorption. Earlier studies have shown that LDACV was hydrolyzed in both rat intestine and liver tissue homogenates with rapid hydrolytic rate in liver homogenate.20 Following oral administration of DLACV, small amounts of DLACV and ACV were detected without any LACV. This might be due to its poor absorption across intestinal mucosa coupled with slow hydrolytic rate. Similar results were observed in our transport studies across Caco-2 with DLACV exhibiting the lowest cellular permeability.20 Our in vitro studies with Caco-2 had indicated that DLACV seems to have good affinity towards peptide transporter (PEPT). However, it had poor permeability across Caco-2. Even in this study, it exhibited poor oral bioavailability. Hence we speculate that even though DLACV was recognized by PEPT, it is not translocated across the membrane resulting in poor absorption.20 More in vitro studies have to be performed to confirm this hypothesis.
Cmax and AUC values observed after oral delivery of LACV and LDACV are tabulated and compared (Table 7). Both LACV and LDACV have enhanced the oral bioavailability of ACV by 5–6 folds. The Cmax and AUC values observed after oral delivery of LDACV were comparable to that of LACV. However, the amount of intact prodrug (dipeptide + amino acid metabolite) resulting with LDACV was almost 4–5 fold higher than of LACV (Table 8). Thus, LDACV has not only improved the oral bioavailability of ACV but also resulted in significant concentrations of intact prodrug. Since the ultimate objective is to evaluate whether the resulted intact prodrug in the systemic circulation would aid in enhancing the corneal absorption of ACV, corneal concentrations were analyzed for total drug following oral dosing of LDACV. LACV was used as a control. The concentrations in the cornea following LDACV was 2 fold higher than those resulted after administration of LACV (Fig. 6). Furthermore, in the cornea, LDACV has resulted in both LDACV and ACV. However the concentrations of ACV were much higher than LDACV in both the eyes indicating the hydrolytic capability of ocular tissues (Fig. 7). The higher corneal concentrations of ACV observed after LDACV might be due to the recognition and translocation of the prodrug from the systemic circulation into cornea by PEPT expressed on the blood aqueous barrier (BAB) followed by hydrolysis by proteases and/or esterases in iris-ciliary body, aqueous humor and cornea. The ability of ocular tissues to hydrolyze these prodrugs was reported.15,16,21 This clearly indicates that the intact prodrug concentrations in the systemic circulation would be helpful to improve the concentrations of the drugs in cornea by targeting the transporters at BAB.
In summary, of all the stereoisomeric dipeptide prodrugs evaluated, LDACV improved both the systemic and corneal absorption of acyclovir after oral administration. Thus it is a promising candidate for further preclinical and clinical studies for the treatment of both genital herpes and corneal keratitis. Pharmacokinetics of stereoisomeric dipeptide prodrugs provided significant insight into their metabolism and absorption across intestinal mucosa and BAB. This information will aid in designing various other dipeptide promoieties of other therapeutic molecules. This concept can be further extended to deliver drugs to the posterior segment of the eye following oral administration.
Acknowledgements
The authors would like to acknowledge Dr. Swapan K. Samanta for synthesizing the prodrugs. We would like to thank GlaxoSmithKline for the generous supply of Valacyclovir. This work was supported by NIH grants RO1 EY09171-14 and RO1 EY10659-12.
Footnotes
Disclosures
The authors report no conflicts of interest.
References
- 1.McGill J, Fraunfelder FT, Jones BR, et al. Current and proposed management of ocular herpes simplex. Surv Ophthalmol. 1976;20:358–65. doi: 10.1016/s0039-6257(96)90004-1. [DOI] [PubMed] [Google Scholar]
- 2.Cunha-Vaz JG. The blood-ocular barriers: past, present, and future. Doc Ophthalmol. 1997;93:149–57. doi: 10.1007/BF02569055. [DOI] [PubMed] [Google Scholar]
- 3.Oh DM, Han HK, Amidon GL, et al. Drug transport and targeting. Intestinal transport. Pharm Biotechnol. 1999;12:59–88. doi: 10.1007/0-306-46812-3_3. [DOI] [PubMed] [Google Scholar]
- 4.Han HK, Amidon GL. Targeted prodrug design to optimize drug delivery. AAPS Pharm Sci. 2000;2(1):E6. doi: 10.1208/ps020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sai Y, Tsuji A. Transporter-mediated drug delivery: recent progress and experimental approaches. Drug Discov Today. 2004;9:712–20. doi: 10.1016/S1359-6446(04)03198-8. [DOI] [PubMed] [Google Scholar]
- 6.Anand BS, Dey S, Mitra AK, et al. Current prodrug strategies via membrane transporters/receptors. Expert Opin Biol Ther. 2002;2:607–20. doi: 10.1517/14712598.2.6.607. [DOI] [PubMed] [Google Scholar]
- 7.Kim RB. Transporters and drug discovery: why, when, and how. Mol Pharm. 2006;3:26–32. doi: 10.1021/mp050084o. [DOI] [PubMed] [Google Scholar]
- 8.Yang CY, Dantzig AH, Pidgeon C, et al. Intestinal peptide transport systems and oral drug availability. Pharm Res. 1999;16:1331–43. doi: 10.1023/a:1018982505021. [DOI] [PubMed] [Google Scholar]
- 9.Brandsch M, Knütter I, Bosse-Doenecke E, et al. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60:543–85. doi: 10.1211/jpp.60.5.0002. [DOI] [PubMed] [Google Scholar]
- 10.Terada T, Inui K. Peptide transporters: structure, function, regulation and application for drug delivery. Curr Drug Metab. 2004;5:85–94. doi: 10.2174/1389200043489153. [DOI] [PubMed] [Google Scholar]
- 11.Han H, de Vrueh RL, Rhie JK, et al. 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998;15:1154–9. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 12.Balimane PV, Tamai I, Guo A, et al. Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem Biophys Res Commun. 1998;250:246–51. doi: 10.1006/bbrc.1998.9298. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson MA. Valaciclovir (BW256U87): the L-valyl ester of acyclovir. J Med Virol. 1993;Suppl 1:150–3. doi: 10.1002/jmv.1890410529. [DOI] [PubMed] [Google Scholar]
- 14.Soul-Lawton J, Seaber E, On N, et al. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39:2759–64. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand BS, Katragadda S, Mitra AK, et al. Pharmacokinetics of novel dipeptide ester prodrugs of acyclovir after oral administration: intestinal absorption and liver metabolism. J Pharmacol Exp Ther. 2004;311:659–67. doi: 10.1124/jpet.104.069997. [DOI] [PubMed] [Google Scholar]
- 16.Anand BS, Katragadda S, Gunda S, Mitra AK, et al. In vivo ocular pharmacokinetics of acyclovir dipeptide ester prodrugs by microdialysis in rabbits. Mol Pharm. 2006;3:431–40. doi: 10.1021/mp0498998. [DOI] [PubMed] [Google Scholar]
- 17.Dias C, Nashed Y, Atluri H, Mitra AK, et al. Ocular penetration of acyclovir and its peptide prodrugs valacyclovir and val-valacyclovir following systemic administration in rabbits: An evaluation using ocular microdialysis and LC-MS. Curr Eye Res. 2002;25:243–52. doi: 10.1076/ceyr.25.4.243.13488. [DOI] [PubMed] [Google Scholar]
- 18.Atluri H, Anand BS, Patel J, Mitra AK, et al. Mechanism of a model dipeptide transport across blood-ocular barriers following systemic administration. Exp Eye Res. 2004;78:815–22. doi: 10.1016/j.exer.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Katragadda S, Jain R, Kwatra D, Hariharan S, Mitra AK, et al. Pharmacokinetics of amino acid ester prodrugs of acyclovir after oral administration: interaction with the transporters on Caco-2 cells. Int J Pharm. 2008;362:93–101. doi: 10.1016/j.ijpharm.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talluri RS, Samanta SK, Gaudana R, Mitra AK, et al. Synthesis, metabolism and cellular permeability of enzymatically stable dipeptide prodrugs of acyclovir. Int J Pharm. 2008;361:118–24. doi: 10.1016/j.ijpharm.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand BS, Katragadda S, Nashed YE, Mitra AK, et al. Amino acid prodrugs of acyclovir as possible antiviral agents against ocular HSV-1 infections: interactions with the neutral and cationic amino acid transporter on the corneal epithelium. Curr Eye Res. 2004;29:153–66. doi: 10.1080/02713680490504614. [DOI] [PubMed] [Google Scholar]
- 22.Talluri RS, Gaudana R, Hariharan S, Jain R, Mitra AK. Disposition kinetics of a dipeptide ester prodrug of acyclovir and its metabolites following intravenous and oral administrations in rat. Clinical Research and Regulatory Affairs. 2009;26:65–72. doi: 10.1080/10601330903200684. [DOI] [PMC free article] [PubMed] [Google Scholar]