Abstract
Background
The possible mechanism(s) of inotropic and chronotropic effects of the extract from Rosa damascena (R. damascena) on heart was examined.
Methods
Inotropic and chronotropic effects of four concentrations of the extract from R. damascena and isoprenaline were examined in isolated guinea-pig hearts perfused through aorta in a Langendorff model. All measurements were performed in three different groups: 1) In the presence and absence of propranolol, 2) In the presence and absence of methacholine and 3) In the presence of diltiazem (n = 12 for each group).
Results
In all groups both isoprenaline and the extract caused an increase in heart rate and contractility (p < 0.05 to p < 0.001). Only in group 1, the final concentration of isoprenaline in the absence of propranolol caused significant greater increase in heart rate compared to the extract (207.6 ± 11.0 compared to 162.6 ± 11.8, p < 0.01). The percent increase in heart contractility due to the final concentration of the extract in the absence (362.4 ± 36.9 compared to 227.7 ± 31.6, p < 0.01) and presence of propranolol (577.1 ± 62.9 compared to 357.5 ± 45.6, p < 0.001) in group 1 and absence (403.7 ± 42.1 compared to 244.8 ± 18.9, p < 0.005) and presence of methcholine (499.88 ± 64.64 compared to 323.90 ± 44.49, p < 0.05) in groups 2 was significantly greater than the increase caused by isoprenaline.
Conclusions
The results of this study suggest that inotropic and chornotropic effect of R. damascena is possibly due to the stimulatory effect of this plant on beta-adrenoceptors.
Keywords: Rosa damascena, Inotropic effect, Chornotropic effect, β-adrenoceptor, Isolated heart
Background
Heart failure is a major cardiovascular problem, a syndrome with various etiologies that results in impaired quality of life and shortened life expectancy [1]. Positive inotropic drugs have therapeutic value in heart failure [2] but currently available classes of cardiotonic drugs limit their clinical usefulness [3]. Major available inotropic drugs include sympathetic stimulants, phosphodiesterase (PDE) inhibitors, digitalis glycosides and calcium channels openers [1].
Heart rate reflects the dynamic balance between sympathetic and parasympathetic divisions of autonomic nervous system. Sympathetic stimulators or parasympathetic inhibitory agents are able to increase heat rate. Chronotropic incompetence (CI) is the inability of heart to increase its rate commensurate with increased activity or demand and is common in patients with cardiovascular disease with particular emphasis on its prominent role in HF. Therefore positive chrontropic agents could be of therapeutic values in these patients [4].
R. damascena is cultivated in all over the world including Iran (especialy in Kashan) for visual beauty and its scent [5]. Flowers of this plant are large, showy and colorful. This plant contains carboxylic acid [6], terpene, myrcene, and vitamin C [7]. Kaemfrol and glycoside are two other constituents of this plant [8]. The plant also containe phenolic compound which could be used as anti-oxidant [8].
Therapeutic effects such as treatment of abdominal and chest pain, strengthening heart [9], treatment of menstrual bleeding, digestive problems [10], and anti inflammation has been described for R. damascena. A decoction of the root of R. damascena as a cough remedy to ease children’s cough [5] and the plant as a gentle laxative are also used [11]. The anti HIV [12], hypnotic [13,14], antispasmodic [15], anti-inflammatory, analgesic [16], antioxidant, hepatoprotective, antidiabetic [17,18] and antidepressant [19] effects for this plant were also reported. The antitussive [20] and relaxant effect of R. damascena[21] and its fractions [22] on guinea pig trachea were also demonstrated. In a recent review article, different pharmacological effect of R. damascena was summerized [23]. Cardiotonic effect was previously described for R. damascena[24]. In a recent study, the effect of the plant on heart rate and contractility was demonstrated [25].
In the present study, the possible mechanism(s) of ionotropic and chronotropic effects of the aqueous-ethanolic extract from R. damascena was examined.
Methods
Plant and extracts
R. damascena was the same plant used in our previous study [25]. A voucher specimen was preserved in the Herbarium of the school of Pharmacy, Mashhad University of Medical Sciences (Herbarium No: 254-1804-01). The Aqueous-ethanolic extract was prepared as previously described for R. damascena and other plants [21,25,26]. The plant ingredient concentration in the final extract was 10 g%.
Preparation of the isolated hearts
Dunkin Hartley guinea pigs of either sex, with a body weight of 400 - 500 g, were used in the present study (Razi Institute, Mashhad, Iran). Preparation of isolated heart was carried out exactly as previously described [25,27,28]. The hearts were perfused with K-H buffer solution (37°C, pH 7.4, saturated with 95% O2 and 5% CO2) through aorta on a modified Langendorff apparatus at a constant perfusion pressure of 70 mmHg [25]. The K-H buffer solution contained the following ingredients (in mMol/L): NaCl 118, NaHCO3 25.0, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, and glucose 11.0 (Merck, Germany) and equilibrated with 95% O2 + 5% CO2 at 37°C. All the hearts were first perfused with K-H solution for 20-30 min for stabilization in a Langendorff apparatus and then the effects of extract from R. damascena and also isoprenaline were studied.
Protocol of experiments
Heart rate and heart contractility were measured in the presence of four different concentrations of aqueous-ethanolic extract from R. damascena (0.1, 0.2, 0.4 and 1.0 mg% from the extract), isoprenaline sulphate (Sigma Chemical Co. Ltd UK), (1, 10 nM, 0.1 and 1 μM) and compared to baseline values. Each concentration of the solutions was given as one-minute intracoronary infusion and its inotropic and chornotropic effects were recorded in last 30 sec, similar to previous studies [25,27]. For infusion of each concentration of the extract or isoprenaline, Krebs solution containing that concentration was infused instead of Krebs solution alone. Both heart rate and contractility in the absence of pharmacological intervention were reproducible which were served as its own control [25,27]. The effects of different solutions were tested with three different experimental designs (n = 12 for each group) as follows: In the presence and absence of 1 μM propranolol hydrochloride (Sigma Chemical Co. Ltd UK) (group 1), in the presence and absence of 1 μM methacholine hydrochloride (Sigma Chemical Co. Ltd UK) (group 2), and in the presence of 10 μM diltiazem (group 3).
Three different series of animal hearts were used for examination of three groups of experiments. In each heart, the effects of the aqueous-ethanolic extract and isoprenaline were evaluated randomly with a 30-min resting period while the heart is perfused with Krebs solution. The heart rate (HR) and contractility were recorded on a kymograph (ET8 G-Boulitt, Paris) and measured after fixation similar to previous studies [25,27]. The local Animal Research Committee of Mashhad University of Medical Sciences approved the experimental procedures used in the present study. Animal care procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Statistical analysis
The data were expressed as mean ± SEM. The data of each concentration of the extract in each group were compared with those of isoprenaline using paired "t" test. The percent increase in heart rate and contractility due to final concentration of the extract and isoprenaline in each group were also compared using paired "t" test. The data obtained in the presence of different concentrations and baselines were compared using ANOVA test in each group. The effect of the aqueous extract and isoprenaline were related to the concentrations of the solutions using least square regression. Significance was accepted at p < 0.05.
Results
The effect of the extract and isoprenaline on heart rate
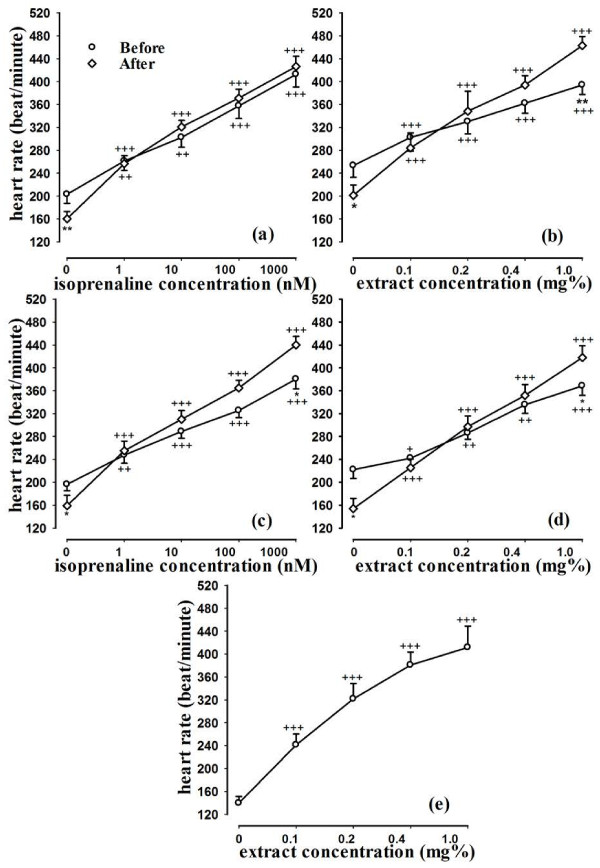
In experimental group 1, both isoprenaline and the extract caused concentration dependent and significant increase in heart rate (p < 0.001 for all cases). In addition, all concentrations of isoprenaline and the extract significantly reversed the effect of propranolol on heart rate (p < 0.001 for all concentrations), (Figure 1). In experimental group 2, also isoprenaline and the extract caused concentration dependent increase in heart rate (p < 0.001 for all concentrations). All concentrations of isoprenaline and the extract significantly reversed the effect of methacholine on heart rate (p < 0.001 for all concentrations), (Figure 1). In experimental group 3, all concentrations of the extract significantly reversed the effect of diltiazem on heart rate (p < 0.001 for all concentrations), (Figure 1). In group 1, the percent increase in heart rate due to the final concentration of isoprenaline (1 μM) in the absence of propranolol was significantly greater than that of the extract (p < 0.01), (Table 1). In group 2, there was no significant difference between the percent increase in heart rate caused by the final concentration of isoprenaline and extract both in absence and presence of methacholine (Table 1).
Figure 1.
Concentration response curves of aqueous-ethanolic extract from R. damascena and isoprenaline, on heart rate of guinea pigs in group 1 (in the presence and absence of propranolol), (a for the isoprenaline and b for the extract), group 2 experiments (in the presence and absence of methacholine), (c for the isoprenaline and d for the extract) and group 3 (in the presence diltiazem), (e for the extract). Statistical differences between the data in the presence and absence of propranolol and methacholie; *; p < 0.05, **; p < 0.01. Statistical differences between the data of each concentration of extract or isoprenaline compared to baseline value; +; p < 0.05, ++; p < 0.01, +++; p < 0.001. (n = 12 for each group).
Table 1.
Increased heart rate and contractility due to the final concentration pof the extract and isoprenaline (precent propertion to baseline values in group 1 and 2 experiments and the statistical differences between the extract and isoprenaline)
| Experimental design | Isoprenaline | Extract | St.Dif | Isoprenaline + An. | Extract + An. | St.Dif | |
|---|---|---|---|---|---|---|---|
| Group 1 |
Rate |
207.6 ± 11.0 |
162.6 ± 11.8 |
p < 0.01 |
281.8 ± 21.9 |
248.6 ± 21.0 |
NS |
| Contractility |
227.7 ± 31.6 |
362.4 ± 36.9 |
p < 0.01 |
357.5 ± 45.6 |
577.1 ± 62.9 |
p < 0.01 |
|
| Group 2 |
Rate |
192.8 ± 12.9 |
204.2 ± 10.2 |
NS |
308.7 ± 31.0 |
303.4 ± 36.6 |
NS |
| Contractility | 244.8 ± 18.9 | 403.7 ± 42.1 | p < 0.005 | 323.9 ± 44.5 | 499.9 ± 64.6 | p < 0.05 | |
Data were expressed as mean ± SEM. An.: antagonist. St.Di: Statistical difference.
The effect of the extract and isoprenaline on heart contractility
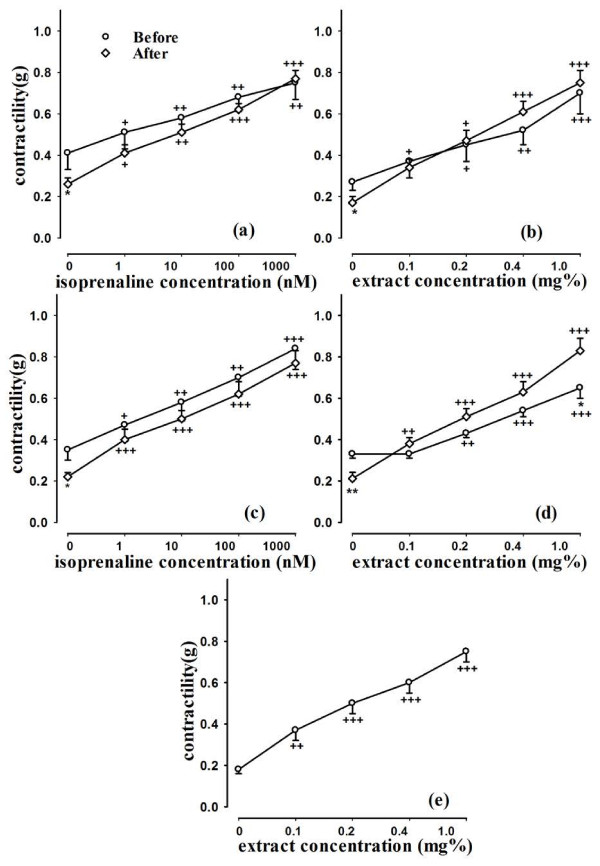
In experimental group 1, both isoprenaline and the extract caused concentration dependent and significant increase in heart contractility (p < 0.05 to p < 0.001). All concentrations of isoprenaline and the extract significantly reversed the effect of propranolol on heart contractility (p < 0.05 to p < 0.001), (Figure 2). In experimental group 2 also isoprenaline and the extract caused concentration dependent increase in heart contractility (p < 0.05 to p < 0.001). All concentrations of isoprenaline and the extract significantly reversed the effect of methacholine on heart contractility (p < 0.005 to p < 0.001), (Figure 2). In experimental group 3, all concentrations of the extract significantly reversed the effect of diltiazem on heart contractility (p < 0.05 to p < 0.001), (Figure 2). The percent increase in heart contractility due to the final concentration of the extract (1.0 mg%) in the absence and presence of propranolol was significantly greater than that of isoprenaline (p < 0.01 for both cases) in group 1 (Table 1). The percent increase in heart contractility due to the final concentration of the extract in the absence and presence of methacholine was also significantly greater than that of isoprenaline (p < 0.05 and p < 0.001 for the absence and presence of methacholine), (Table 1).
Figure 2.
Concentration response curves of aqueous-ethanolic extract from R. damascena and isoprenaline, on heart contractility of guinea pigs in group 1 (in the presence and absence of propranolol), (a for the isoprenaline and b for the extract), group 2 experiments (in the presence and absence of methacholine), (c for the isoprenaline and d for the extract) and group 3 (in the presence diltiazem), (e for the extract). Statistical differences between the the data in the presence and absence of propranolol and methacholie; NS: non-significant difference, *; p < 0.05, **; p < 0.01. Statistical differences between the data of each concentration of extract or isoprenaline compared to baseline value; +; p < 0.05, ++; p < 0.01, +++; p < 0.001. (n = 12 for each group).
Differences in the heart rate and contractility before and after propranolol, methacoline and diltiazem
Propranolol, methacholine and diltiazem caused significant reduction in heart rate (p < 0.05 for propranolol and methacholine and p < 0.005 for diltiazem) and its contractility (p < 0.05 for all cases). However, the increase in heart rate and contractility due to the final concentration of isopranaline and the extract in the presence of propranolol and methacholine was greater than the increase in absence of them in most cases which were significant in some cases (p < 0.01 for heart rate due to the extract after propranolol, p < 0.05 for heart rate due to isoprenaline and the extract after methacholine and p < 0.05 for heart contractility due to the extract after methacholine), (Figures 1 and 2).
Relationship between concentration and the effect of the aqueous-ethanolic extract and diltiazem
Significant correlations were seen between both heart rate and heart contractility and concentration of isoprenaline and the extract in all three groups of experiments (P < 0.01 to p < 0.001), (Table 2).
Table 2.
Correlation between the effects of aqueous-ethanolic extract from R. damascene and isoprenaline on heart rate and contractility of isolated guinea pig heart with concentrations in three groups of experiments
|
Groups |
|
Extract |
Extract + An.: antagonist. |
Isoprenaline |
Isoprenaline + An.: antagonist |
||||
|---|---|---|---|---|---|---|---|---|---|
| R | P value | R | P value | r | P value | r | p value | ||
| Group 1 |
HR |
0.4 |
p < 0.005 |
0.6 |
p < 0.001 |
0.7 |
p < 0.001 |
0.7 |
p < 0.001 |
| Cont |
0.4 |
p < 0.01 |
0.6 |
p < 0.001 |
0.3 |
p < 0.05 |
0.7 |
p < 0.001 |
|
| Group 2 |
HR |
0.6 |
p < 0.001 |
0.6 |
p < 0.001 |
0.7 |
p < 0.001 |
0.7 |
p < 0.001 |
| Cont |
0.7 |
p < 0.001 |
0.7 |
p < 0.001 |
0.3 |
p < 0.05 |
0.6 |
p < 0.001 |
|
| Group 3 |
HR |
|
|
0.5 |
p < 0.001 |
|
|
|
|
| Cont | 0.5 | p < 0.001 | |||||||
For abbreviations see Table 1.
Discussion
Concentration dependent increase in both heart rate and heart contractility due to the aqueous-ethanolic extract of R. damascena was observed in the present study with prominent effect of the extract on heart contractility (ionotropic effect).
The effect of the extract was also examined on pre-treated heart with propranolol, methacholine and diltiazem to explore the possible mechanism(s) for inotropic and chornotropic effects of the plant. The effect of propranolol on both heart rate and contractility was significantly and in a dose dependent manner reversed by the extract which was more prominent on heart contractility. In fact, propranolol led to a significant reduction in heat rate and contractility. Even low concentration of isoprenalie and the extract caused less increase in heat rate and contractility in presence of propranolol compared to its absence. However, inceasing the concentration of isoprenaline and the extract overcome the effect of propranolol simillary which is a well known characteristic of competitive antagonist and agonist interaction. In pre-treated heart with propranolol, the effect of the extract on heart rate was lower but its effect on contractility was greater than that of isoprenaline. In experimental group 2, the extract, significantly and in a dose dependent manner, reversed the effect of methacholine on both heart rate and contractility with a greater effect on heart contractility rather than heart rate. The effect of the extract on contractility of pre-treated heart with methacoline was also greater than that of isoprenaline. In experimental group 3 also the extract, significantly and in a dose dependent manner, reversed the effect of diltiazem on both heart rate and contractility. In this group the effect of the extract on contractility of pre-treated heart with diltiazem was also greater than the effect on heart rate.
The results of the present study increased heart rate and contractility as well as significant correlation between concentrations of the extract and its effects on heart rate and heart contractility and its similar effect to isoprenaline support the choronotropic and inotropic effect of hydro-ethanolic extract of R. damascena found in our previous study [25]. The results also showed that the extract reversed the effects of pre-treated heart with propranolol, methacholine and diltiazem. These findings suggest that the possible mechanisms of action of the extract from R. damascena on heart are β-adrenoceptor stimulatory, calcium channel opening activity or inhibitory effect on cholinergic receptors [29]. The findings on pre-treated heart with propranolol were also supported by our previous study [25]. However, if the hydro-ethanolic extract has a blockade effect on cholinergic receptors, it should increase heart rate more than contractility. The results of the present study showed that the extract affects heart contractility more than heart rate. Therefore the inhibitory effect of the extract on cholinergic receptors of the heart is excluded. Although the extract reversed the effect of diltiazem on both heart rate and contractility which may indicate an opening effect of the extract on calcium channels. With regard to the greater effect of calcium channel opener drugs on heart contractility than heart rate which is similar to the results of the present study, the extract of R. damascena may have an opening effect on calcium channels of heart.
However, the most possible mechanism of action of hydro-ethanolic extract on heart is suggested to be the stimulatory effect on β-adrenoceptor because the extract increased both heart rate and contractility. In addition, if the extract has a stimulatory effect on β-adrenoceptors, it can reverse the effect of both methacholine and diltiazem in a functional antagonism manner. However, the inhibitory effect of the extract on cholinergic receptors and its opening effect on calcium channels could not be fully excluded because the hydro-ethanolic extract is composed of several constituents and could have several mechanisms of action. In fact a muscarinic receptor inhibitory effect for R. damascena was shown in a recent study [15]. Therefore, the inhibitory effect of the plant on muscarinic receptors and its opening effect on calcium channels should be investigated in more detailed studies. In addition, the effect of different fractions of the extract also needs to be studied on heart activities and the mechanism(s) of their actions should be explored in further studies.
The other possible mechanism of action of the plant is an increased cAMP level like phosphodiestrase III inhibition [30] or forskolin-like action [31] of R. damascena. These possible mechanisms of action of the plant also should be examined in further studies. Because of the solution used for heart perfusion, contained ca2+ ion it is possible that the extract or drug could interact with calcium ion and calcium chelating is formed. However if such interactions had happened, the decrease in heart rate and specially contractility should have been observed. The results showed heart rate and contractility increasing effects for isoprenaline and the extract which suggest the abscence of interactions between the extract or drug and calcium ion.
Choronotropic and inotropic effect of the extract of R. damascenaobserved in the present study with a possible stimulatory effect on β-adrenoceptors may represent a pharmacological action and a therapeutic value in various cases of cardiac impairment such as lack of activator of calcium (e.g: hypocalcemia), [32,33].
Conclusions
In conclusion, this study supports a potent inotropic and chornotropic effect for R. damascena on isolated guinea pig heart with possible stimulatory effect on β-adrenoceptor of isolated guinea pig heart. In addition the results may also suggest an opening effect on calcium channels and/or an inhibitory property for the plant on muscarinic reptors of isolated heart.
Competing interest
The authors declare that they have no competing interest.
Authors’ contributions
BMH: study design, supervision of experiments, help in statistical analysis, preparation of manuscript. VA: performance of experiment, help in statistical analysis and manuscript preparation. PH: help in study design and supervision of experiments. BM: help in statistical analysis, preparation of Tables and Figures. All authors read and approved the final manuscript.
Contributor Information
Mohammad Hossein Boskabady, Email: boskabadymh@mums.ac.ir.
Alaleh Vatanprast, Email: Vatanprasta@gmail.com.
Haydar Parsaee, Email: Parsaeeh@mums.ac.ir.
Morteza Boskabady, Email: boskabadym851@mums.ac.ir.
Acknowledgements
This study was financially supported by the Vice chancellor of Research of Mashhad University of Medical sciences. This paper is the results of an MSc thesis.
References
- Skrabal MZ, Stading JA, Behmer-Miller KA, Hilleman DE. Behmer-Miller. Advances in the Treatment of Congestive Heart Failure: New Approaches for an Old Disease. Pharmacother. 2000;20:787–804. doi: 10.1592/phco.20.9.787.35195. [DOI] [PubMed] [Google Scholar]
- Erdmann E. The value of positive inotropy in acute and chronic heart failure. J Cardiovasc Pharmacol. 1989;14(3):S36–S41. doi: 10.1097/00005344-198914003-00008. [DOI] [PubMed] [Google Scholar]
- Packer M. Vasodilator and inotropic drugs for the treatment of chronic heart failure: distinguishing hype from hope. J Amer Coll Cardiol. 1988;12:1299–1317. doi: 10.1016/0735-1097(88)92615-0. [DOI] [PubMed] [Google Scholar]
- Tardif JC. Heart rate as a treatable cardiovascular risk factor. Br Med Bull. 2009;90:71–84. doi: 10.1093/bmb/ldp016. [DOI] [PubMed] [Google Scholar]
- Libster M. Delmars integrative herb guide for nurses. Delmar; 1999. pp. 360–370. [Google Scholar]
- Green M. The Rose. Aromatic Thymes. 1999;7:11–15. [Google Scholar]
- Buckle J. Clinical aromatherapy in nursing. London: Arnold, co published by singular; 1997. [Google Scholar]
- Schiber A, Mihalev K, Berardini N, Mollov P, Carle R. Flavonol glycosides from distilled petals of Rosa damascena Mill. Z Naturforsch C. 2005;60:379–384. doi: 10.1515/znc-2005-5-602. [DOI] [PubMed] [Google Scholar]
- Avicenna. Law in Medicine. Interpreter: Sharafkhandy A. Tehran: Ministry of Guidance publication; 1990. pp. 129–131. [Google Scholar]
- Wood G, Bache F. The dispensatory of the United States of America. 4. Philadelphia: Griggand Elliot; 1839. [Google Scholar]
- Zargari A. Medicinal plants. 5. Tehran: Tehran University publications; 1992. pp. 281–284. Volume 2. [Google Scholar]
- Mahmood N, Piacenet S, Pizza C, Bruke A, Khan A, Hay A. The anti -HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem Biophys Res Commun. 1996;229:73–79. doi: 10.1006/bbrc.1996.1759. [DOI] [PubMed] [Google Scholar]
- Rakhshandah H, Hosseini M. Potentiation of pentobarbital hypnosis by Rosa damascena in mice. Indian J Exp Biol. 2006;44:910–912. [PubMed] [Google Scholar]
- Rakhshandah H, Shakeri MT, Ghasemzadeh MR. Comparative hypnotic effect of Rosa damascena fractions and Diazepam in Mice. Iran J Pharm Res. 2007;6:193–197. [Google Scholar]
- Dolati K, Rakhshandeh H, Shafei MN. Effect of aqueous fraction of Rosa damascena on ileum contractile response of guinea pigs. Avicenna J Phytomed. 2013;3:248–253. [PMC free article] [PubMed] [Google Scholar]
- Rakhshandah H, Vahdati mashhadian N, Dolati K, Hosseini M. Antinoceptive effect of Rosa Damascena in mice. J Biol Sci. 2008;8:176–180. [Google Scholar]
- Shahriari S, Yasa N, Mohammadirad A, Khorasani Rand Abdollahi M. In vivo antioxidant potentials of Rosa damascena petal extract from Guilan, Iran, comparable to a-tocopherol. Int J Pharmacol. 2007;3:510–514. [Google Scholar]
- Ozkan G, Baydar NG, Baydar H. Note: Antioxidant and Antibacterial Activities of Rosa Damascena Flower Extracts. Food Sc Technol Int. 2004;10:277–281. doi: 10.1177/1082013204045882. [DOI] [Google Scholar]
- Dolati K, Rakhshandeh H, Shafei MN. Evaluation of antidepressant effect of ethanolic extract of Rosa damascena using forced swimming test. Avicenna J Phytomed. 2012;2:46–51. [Google Scholar]
- Shafei MN, Boskabady MH. Antitussive effect of Rosa damascena in guinea pigs. Iran J Pharmac Res. 2003;2:231–234. [Google Scholar]
- Boskabady MH, Kiani S, Rakhshandeh H. Relaxant effects of Rosa damascena on guinea pig tracheal chains. J Ethnopharmacol. 2006;106:377–382. doi: 10.1016/j.jep.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Rakhshandah H, Boskabady MH, Mossavi Z, Gholami M, Saberi Z. The Differences in the relaxant effects of different fractions of Rosa damascena on guinea pig tracheal smooth muscle. Iran J Basic Med Sci. 2010;13:126–132. [Google Scholar]
- Boskabady MH, Shafei MN, Saberi Z, Amini A. Pharmacological Effects of Rosa Damascena. Iran J Basic Med Sci. 2011;14:295–307. [PMC free article] [PubMed] [Google Scholar]
- Penckofer S, Schwertz D, Florczak K. Oxidative stress and cardiovascular disease in type 2 diabetes: the role of antioxidants and pro-oxidants. J Cardiovasc Nurs. 2002;16:68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Boskabady MH, Vatanprast A, Parsee H, Ghasemzadeh M. Effect of Aqueous-Ethanolic Extract from Rosa damascena on Guinea Pig Isolated Heart. Iran J Basic Med Sci. 2011;14:116–121. [Google Scholar]
- Boskabady MH, Sheiravi N. Inhibitory effect of Nigella sativa on histamine (H1) receptors of isolated guinea pig tracheal chains. Pharmac Biol. 2002;40:596–602. doi: 10.1076/phbi.40.8.596.14653. [DOI] [Google Scholar]
- Boskabady MH, Shafei MN, Shakiba A, Sang SH. Effect of aqueous-ethanol extract from Crocus sativus (saffron) on guinea-pig isolated heart. Phytother Res. 2008;22:330–334. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]
- Chlopicki S, Kozlovski VI, Geygewski RJ. Clonidine-induced coronary vasodilation in isolated guinea pig heart is not mediated by endothelial a2 adrenoceptors. J Physiol Pharmacol. 2003;54:511–521. [PubMed] [Google Scholar]
- Gilani AH, Janbaz KH, Aziz N, Herzig JU, Kazemi M, Choudhary MI, Herzig JW. Possible mechanism of selective inotropic activity of the n-butanolic fraction from berberis aristata fruit. Gen Pharmacol. 1999;33:407–444. doi: 10.1016/S0306-3623(99)00035-X. [DOI] [PubMed] [Google Scholar]
- Sys SU, Boels PJ, Brutsaert DL. Positive inotropic and vasodilating effects of amrinone and milrinone in isolated canine heart. J Cardiovasc Pharmacol. 1987;10:445–449. doi: 10.1097/00005344-198710000-00010. [DOI] [PubMed] [Google Scholar]
- England PJ, Shahid M. Effects of forskolin on contractile response and protein phosphorylation in the isolated perfused rat heart. Biochem J. 1987;246:687–695. doi: 10.1042/bj2460687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RM, Fellner SK, Neumann A, Bushinsky DA, Borow KM. Left Ventricular contractility varies directly with blood-ionized calcium. Ann Intern Med. 1988;108:524–529. doi: 10.7326/0003-4819-108-4-524. [DOI] [PubMed] [Google Scholar]
- Francis GS, Cohn J. Heart failure: mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J. 1990;4:3068–3075. doi: 10.1096/fasebj.4.13.2210153. [DOI] [PubMed] [Google Scholar]