Abstract
Background
Several β-adrenergic receptor (βAR) antagonists have been shown to have neuroprotective effects against cerebral ischemia. However, clenbuterol, a β2AR agonist, was shown to have neuroprotective activity by increasing nerve growth factor expression. We used β2AR knockout mice and a β2 selective antagonist to test the effect of loss of β2ARs on outcome from transient focal cerebral ischemia.
Methods
Ischemia was induced by the intraluminal suture method, for 60 min of middle cerebral artery occlusion (MCAO) followed by 24 h reperfusion. Neurological score was determined at 24 h reperfusion and infarct size was determined by cresyl violet or 2,3,5-triphenyltetrazolium chloride staining. β2AR knockout mice and wild-type congenic FVB/N controls were studied, as well as 2 groups of wild type mice given either ICI 118,551 (0.2 mg/kg) or 0.9% saline intraperitoneally 30 min before MCAO (n = 10 per group). Changes in expression of heat shock protein (Hsp)72 after ischemia were examined by immunohistochemistry and western blots.
Results
Compared with wild type littermates, infarct volume was decreased by 22.3% in β2AR knockout mice (39.7 ± 10.7 mm3 vs 51.0 ± 11.4 mm3, n = 10/group, P = 0.034) after 60 min of MCAO followed by 24 h reperfusion. Pretreatment with a β2AR selective antagonist, ICI 118,551, also decreased infarct size significantly, by 25.1%, compared with the saline control (32.8 ± 11.9 mm3 vs 43.8 ± 10.3 mm3, n = 10/group, P = 0.041). Neurological scores were also significantly improved in mice lacking the β2AR or pretreated with ICI 118,551. After cerebral ischemia, total levels of Hsp72 and the number of Hsp72 immunopositive cells were greater in mice lacking β2 AR.
Conclusion
Brain injury is reduced and neurological outcome improved after MCAO in mice lacking the β2AR, or in wild type mice pretreated with a selective β2AR antagonist. This is consistent with a shift away from prosurvival signaling to prodeath signaling in the presence of β2AR activation in cerebral ischemia. Protection is associated with higher levels of Hsp72, a known antideath protein. The effect of β2AR signaling in the setting of cerebral ischemia is complex and warrants further study.
Implications
Brain injury is reduced and neurological outcome improved after middle cerebral artery occlusion in mice lacking the β2AR or in wild type mice pretreated with a selective β2AR antagonist. This is consistent with a shift away from prosurvival signaling to prodeath signaling in the presence of β2AR activation in cerebral ischemia.
β-Adrenergic receptors (βARs) are seven transmembrane helix G-protein-coupled receptors. While all three known subtypes, β1AR, β2AR, and β3AR, are found in brain, the β3AR subtype has limited expression.1–3 Important differences between β1AR and β2AR signaling and cellular effects have been described. In cardiac myocytes, β1ARs couple only to stimulatory G-protein (Gs), while β2ARs first couple to Gs, but with several minutes of continuous stimulation switch to inhibitory G-protein (Gi) coupling.4 Gi-mediated β2AR signaling in myocytes has been shown to be protective against some insults associated with ischemic stress.5 However, there are likely subtype-specific differences in other cell types, and these receptors likely couple to different responses in different cell types. Mice lacking the β2AR were found to have normal resting cardiovascular physiology, no change in β1AR expression, and an increased tolerance to acute stress shown by greater total exercise capacity,6 but a greater vulnerability to chronic myocardial stress.7
Whether β1AR or β2AR activation protects the brain against ischemia is the topic of debate. Both in experimental animal models of cerebral ischemia and in the clinical setting of cardiac surgery, several βAR antagonists (d- and l- propranolol, carvedilol, esmolol, and landiolol) have been shown to protect the brain from ischemia.8–13 However, these agents vary in that some are mixed β1AR/β2AR antagonists, others are relatively β1AR-specific, some have antioxidant properties, and some influence signaling pathways not typically impacted by βAR antagonists. In contrast to these studies showing protection with antagonists, the β2AR agonist clenbuterol provided neuroprotection from forebrain ischemia and focal ischemia by increasing nerve growth factor (NGF) expression and activating astrocytes.14,15 Activation of both β1AR and β2ARs was associated with astrocyte activation and protection against excitotoxicity in mixed hippocampal cultures.16 Reduced infarct volume was observed with β2 activation in a mouse permanent focal ischemia model,16 while others reported protection with β1 antagonists and nonspecific βAR blockade.10 It is thus difficult to ascribe either beneficial or deleterious effects to the blockade of either β1ARs or β2ARs based upon the published studies.
Heat shock protein72 (Hsp72), the highly stress-inducible member of the Hsp70 family, contains an ATF-like element in its promoter region, making its induction sensitive to cyclic adenosine monophosphate (cAMP) as well as other promoter elements responsive to heat and other stresses. Agents that increase cAMP increase Hsp72,17 and a βAR agonist elevated Hsp72 levels in bronchial epithelial cells.18 Hsp72 blocks both necrotic and apoptotic cell death and reduces ischemic brain injury.19,20 Since β1 and β2AR differentially affect cAMP due to the ability of β2AR to switch from Gs coupling, which increases cAMP to Gi coupling which does not, we also tested whether levels of induction of Hsp72 differed between wild type (WT) and β2AR knockout (KO) mice.
In this study, we readdress the possible role of the β2AR subtype in modulating infarct size and neuroscore after transient middle cerebral artery occlusion (MCAO) with β2KO mice and ICI 118,551, a βAR antagonist that is 100-fold more selective for the β2AR than the β1AR.21 A secondary goal was to evaluate the effect loss of β2AR activation on production of Hsp72. We provide evidence that transient focal ischemic brain injury is attenuated in mice lacking β2ARs, with similar reduction of injury observed with a highly selective β2AR antagonist. This protection was associated with greater induction of Hsp72.
Methods
Transient Focal Ischemia
All experiments were performed according to a protocol approved by the Stanford Institutional Animal Care and Use Committee. Male adult β2AR-deficient FVB/N (KO) mice and congenic FVB/N (WT) mice weighing 25 to 30 g were used. The KO mice are those produced by targeted gene disruption.6 Transient focal ischemia was induced by the intraluminal suture method of MCAO originally described by Longa et al.22 Briefly, mice were anesthetized with isoflurane (3% initial, 1.0%–1.5% maintenance) and 60% nitrous oxide in oxygen with spontaneous respiration via mask. Under the operating microscope, the left common carotid artery (CCA), internal carotid artery, and external carotid artery were exposed through a midline neck incision. The proximal portions of the left CCA and the external carotid artery were ligated and a 6–0 silicon-coated nylon suture was introduced into the CCA and advanced about 9 mm beyond the carotid bifurcation for transient occlusion of the middle cerebral artery (MCA). Animals were allowed to awaken from anesthesia during MCAO and returned to their cages. After 60 min, reperfusion was obtained by withdrawal of the suture. During all surgical procedures, animals were maintained normothermic (37°C ± 0.5°C) by means of a servo controlled heating blanket (Harvard Apparatus, Holliston, MA) with rectal temperature monitoring. Pulse oximetry (Spo2), heart rate, and respiratory rate were monitored continuously (STARR life sciences Corp., Allison Park, PA). Plasma glucose concentration was measured before MCAO, 5 min and 24 h after reperfusion. Animals with no observable deficits immediately after ischemia, those that died before 24 h, generally 10%–15%, and those with subarachnoid hemorrhage at the time of death were excluded from analysis. Sham-treated animals received all surgical procedures but the filament was not inserted into the MCA.
For animals pretreated with either ICI 118,551 (0.2 μg/g, Sigma-Aldrich, Milwaukee, MI) or saline, the active drug or carrier was administered intraperitoneally to FVB mice 30 min before MCAO (n = 10 per group, WT FVB/N mice). Additional mice were used to assess the effect of ICI 118,551 treatment on Hsp72 levels. Brains were collected at 2 times after injection (n = 2 each at 5 h and 24 h after injection, for both drug and vehicle treatment groups) for immunoblotting to detect Hsp72 expression.
After 24 h reperfusion, neurological evaluation was performed by the method of Garcia et al.23 This consisted of six tests: spontaneous activity, symmetry in the movement of four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch. The score given to each mouse at the completion of the evaluation is the sum of all six individual test scores. The maximum is 18 in a normal animal. Both surgeon and neurologic scorer were blinded to the genotype or pharmacological treatment group.
Determination of Infarct Volume
At 24 h after MCA occlusion and after neurological assessment, the mice were deeply anesthetized with isoflurane, and brains were harvested rapidly after perfusion with cold phosphate buffered saline and cold 4% paraformaldehyde. For histological analysis, brains were cut into 40 micron coronal sections with a vibratome. Coronal sections taken at rostral 2.34 mm, 1.34 mm, 0.26 mm, and caudal −0.70 mm, −1.70 mm, and −2.70 mm to bregma were assessed by cresyl violet staining. The infarct area was measured by a blinded observer using digital imaging and image analysis software (Image J 1.37v, Wayne Rasband, available through National Institutes of Health). Infarct area was corrected for edema using the method of Swanson et al.24 The person assessing infarct areas was blinded to the genotype of each brain. Cresyl violet staining allows the same brains to be used for additional histological analysis.
For ICI 118,551-treated mice and vehicle controls brains were removed, sectioned, and incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) in saline for 20 min at 37°C. To determine infarct volume by TTC staining, six slices per rat were analyzed by a blinded observer using the National Institutes of Health Image program as previously described.24,25
Immunohistochemistry and Immunoblot
Immunohistochemistry was performed to assess Hsp72 expression after 24 h reperfusion. Free-floating sections were incubated in primary antibody to Hsp72 (1:500, SPA-810, StressGen Biotechnologies, Victoria, Canada) overnight at 4°C, followed by fluorescein isothiocyanate-conjugated secondary antibody at room temperature for 1 h. Images were viewed with an epifluorescence microscope (Zeiss Axiovert 200M, Carl Zeiss, Goettingen, Germany) and brain regions were selected for cell counting based on a previously published procedure.26 For Hsp72 staining, positive cells were counted in five randomly selected nonoverlapping fields within the cortex adjacent to the outer boundary of the infarct. Cell counts were performed by an investigator blinded to the conditions of the experiment.
Western blotting was performed to quantify Hsp72 expression. The ipsilateral hemisphere was homogenized in lysis buffer (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 1 mM dithiothreitol) plus 0.2% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 4 mM benzamidine, 10 mg of leupeptin per mL, and 10 mg of aprotinin per mL, kept on ice for 10 min, sonicated for 30 s, and centrifuged at 10,000g for 30 min. The supernatant was collected as the cytoplasmic fraction. Protein concentrations were determined by the bicinchoninic acid method (BCA™ Protein Assay Kit, Pierce, Rockford, IL). Samples containing equal amounts of protein (80 μg) were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Catalog No. 161-1155, Bio-Rad Laboratories, Hercules, CA). The proteins were transferred to a polyvinylidinene fluoride membrane (IPVH00010, Millipore, Bedford, MA), probed with anti-Hsp72 primary antibody (1:1000, SPA-810, StressGen Biotechnologies, Victoria, Canada) and visualized by horseradish peroxidase-conjugated antimouse immunoglobulin G (sc-7076, 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) and chemiluminescence (Amersham ECL Western Blotting Analysis System, Buckinghamshire, UK). Membrane was stripped and reprobed with anti-β-actin antibody (sc-1616, 1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). Densitometric analysis of the bands was performed using image J software. Hsp72 band intensity was normalized to β-actin.
Statistics
Data are presented as mean ± sd. For physiologic data, differences between groups were determined by t-test for unpaired data and differences between time points in each group were determined using analysis of variance followed by post hoc Fisher's least significant difference test. All other data were evaluated by t-test for unpaired data. All statistical analyses were performed on a personal computer with the SPSS program. Differences were considered significant at P < 0.05.
Results
β2AR Knockout Reduced Infarct Volume and Reduced Neurological Deficits
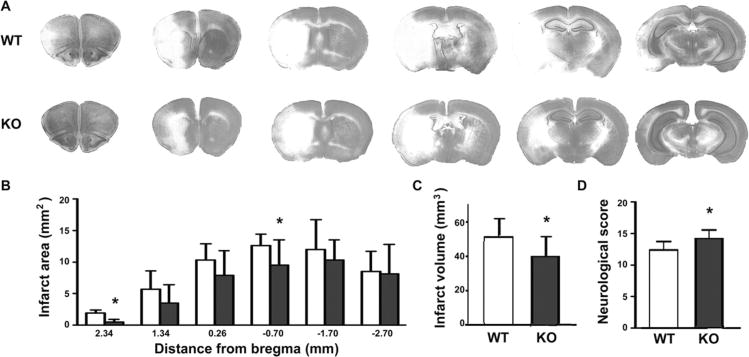
No significant differences were observed between WT and KO mice in respiratory rate, Spo2, body temperature, blood glucose at baseline, and 24 h after reperfusion. At the end of the ischemic period heart rate was higher in KO mice than in WT (471 ± 42 vs 529 ± 43, P = 0.007) and blood glucoses were increased slightly (106 ± 9 vs 114 ± 7, P = 0.043) (Table 1). Animals in both groups lost body weight by the 24 h time point after injury (16.5% ± 5.2% in WT vs 14.2% ± 4.6% in KO, difference not significant). Infarct volume was assessed by cresyl violet staining after 24 h reperfusion. As shown in Figure 1A, brain sections had smaller infarct areas in KO mice than WT mice. Infarct area measurements were significantly lower at individual levels of 2.34 and −0.70 mm from bregma (Fig. 1B), and total infarct volume was significantly decreased by 22.3% in KO mice compared to WT littermates (39.7 ± 10.7 mm3 vs 51.0 ± 11.4 mm3, n = 10/group, P = 0.034, Fig. 1C). The neurological scores were modestly but significantly better in KO mice compared with WT (Fig. 1D).
Table 1. Physiologic Values in β2AR Knockout (KO) and Wild-Type Mice (WT).
| WT (n = 10) | KO (n = 10 | |
|---|---|---|
| Before MCAO | ||
| Heart rate (bpm) | 490 ± 19 | 489 ± 28 |
| Spo2(%) | 97.7 ± 0.8 | 99.0 ± 0.9 |
| Temperature (°C) | 36.8 ± 0.2 | 36.5 ± 0.3 |
| Glucose (mg/dL) | 97 ±8 | 102 ±9 |
| During MCAO | ||
| Heart rate (bpm) | 471 ± 42 | 529 ± 43* |
| Spo2(%) | 97.6 ± 0.3 | 97.8 ± 1.4 |
| Temperature (°C) | 37.2 ± 0.4 | 37.3 ± 1.1 |
| Glucose (mg/dL) | 106 ±9 | 114 ± 7† |
| After 24 h reperfusion | ||
| Temperature (°C) | 37.0 ± 0.5 | 37.2 ± 0.7 |
| Glucose (mg/dL) | 112 ± 10 | 107 ± 12 |
Values are means ± SD.
Before middle cerebral artery occlusion (MCAO) = 5 min before MCAO; During MCAO = at the end of 60 min MCAO; Spo2 = oxygen saturation.
P = 0.007.
(P = 0.043) compared with WT.
Figure 1.
β2AR knockout reduced infarct size and improved neurological deficits after transient focal ischemia. (A) Histology of cresyl violet stained brain sections was assessed at 2.34 mm, 1.34 mm, and 0.26 mm rostral, and −0.70 mm, −1.70 mm, and −2.70 mm caudal from bregma. Representative sections from brains of wild type (WT) (upper row) and knockout (KO) (lower row) mice are shown. Pale areas indicate injured tissue. (B) Analysis of infarct area at each of the 6 coronal levels in WT versus KO mice after 24 h reperfusion. (C) Total infarct volume was decreased by 22.3% in KO mice compared with WT littermates (39.7 ± 10.7 mm3 vs 51.0 ± 11.4 mm3, P = 0.034). (D) Neurological deficit score was improved in KO mice compared with WT at 24 h reperfusion (12.9 ± 1.4 in WT versus 14.7 ± 1.5 in KO, P = 0.012; n = 10 per group). *Indicates significant difference from WT. Filled bars indicate KO, open bars WT.
Selective β2AR Antagonism Reduced Infarct Volume and Reduced Neurological Deficits
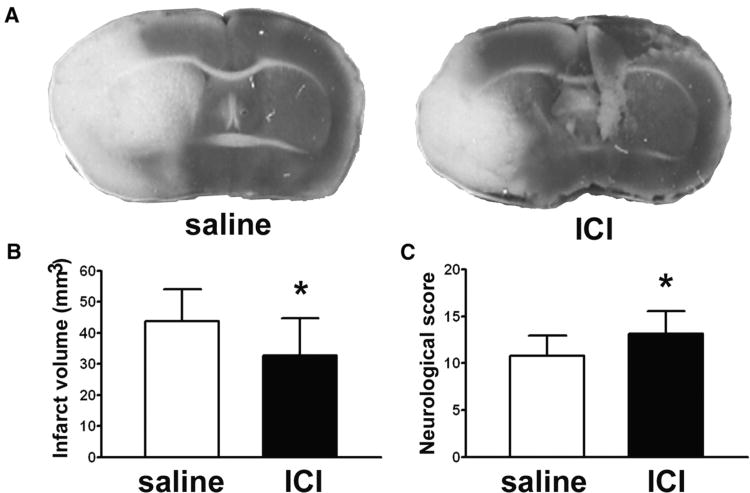
Because KO mice may have developed some compensation for the absence of β2 ARs during development, we also tested the effect of acutely blocking β2 AR in normal mice using the selective antagonist ICI 118,551. Physiologic variables were measured and were not significantly different between saline and drug-treated mice (Table 2). As shown in Figure 2A, a representative brain section from an ICI 118,551-treated mouse shows a smaller infarct area than that from a saline-treated control mouse. The infarct volume of ICI 118,551-treated mice assessed by TTC staining was significantly reduced, by 25.1%, compared to saline controls (32.8 ± 11.9 mm3 vs 43.8 ± 10.3 mm3, P = 0.041, Fig. 2B). Neurological scores were also significantly improved in ICI 118,551 treated mice (Fig. 2C). The extent of protection was similar in the drug treated mice compared to the genetically modified mice. ICI 118,551 treatment did not increase Hsp72 levels in brain assessed by immunoblot either 5 or 24 h following injection (data not shown).
Table 2. Physiologic Values in Wild lype Mice with or Without ICI 118,551 Treatment.
| Saline (n = 10) | ICI 118,551 (n = 10) | |
|---|---|---|
| Before MCAO | ||
| Heart rate (bpm) | 477 ± 37 | 461 ± 28 |
| Spo2(%) | 98.0 ± 0.8 | 97.8 ± 1.3 |
| Temperature (°C) | 36.8 ± 0.3 | 36.8 ± 0.2 |
| Glucose (mg/dL) | 105 ±9 | 112 ± 16 |
| During MCAO | ||
| Heart rate (bpm) | 488 ± 14 | 478 ± 28 |
| Spo2(%) | 97.9 ± 0.6 | 98.3 ± 1.2 |
| Temperature (°C) | 36.9 ± 0.2 | 37.0 ±1.5 |
| Glucose (mg/dL) | 113 ± 11 | 110 ± 17 |
| After 24 h reperfusion | ||
| Temperature (°C) | 36.9 ± 0.3 | 36.8 ± 0.3 |
| Glucose (mg/dL) | 95 ±31 | 118 ± 18 |
Values are means ± SD.
Differences between groups were not statistically significant.
Before middle cerebral artery occlusion (MCAO) = 5 min before MCAO; During MCAO = at the end of 60 min MCAO; Spo2 = oxygen saturation.
Figure 2.
A selective β2AR antagonist reduced infarct size and improved neurological deficits. (A) Representative TTC-stained brain sections show a smaller infarct (pale area) in an ICI 118,551 treated mouse compared with saline control. (B) The infarct volume of ICI 118,551 treated mice was reduced by 25.3% compared with saline control (32.8 ± 11.9 mm3 in saline vs 43.8 ± 10.3 mm3 in ICI 118,551, P = 0.041). (C) Neurological scores of ICI 118,551 treated mice were significantly improved at 24 h reperfusion compared with saline control (10.8 ± 2.1 in saline versus 13.1 ± 2.5 in ICI 118,551, n = 10 per group; P = 0.039).
β2AR Knockout Mice had a Greater Increase in Hsp72 Expression After Ischemia
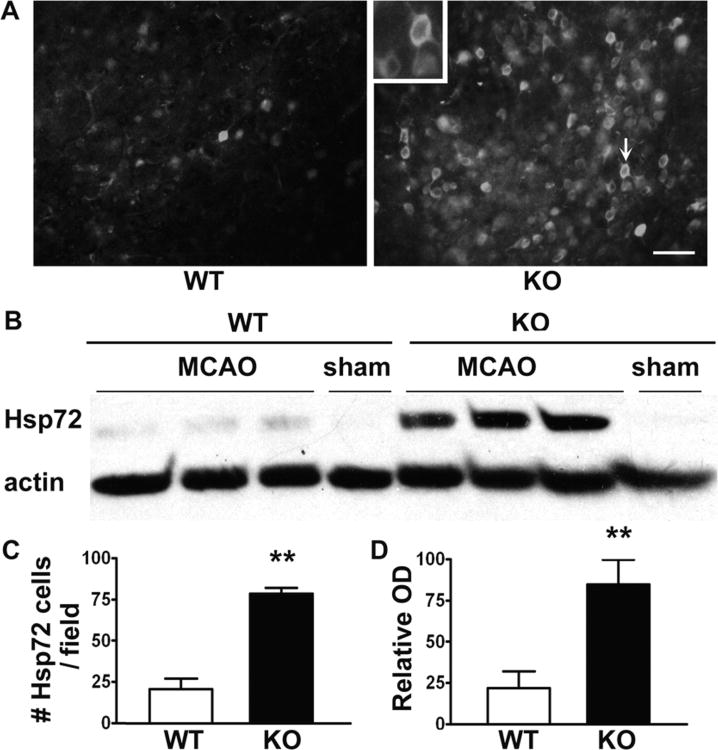
Hsp72 expression after ischemia was assessed by immunohistochemistry and Western blot. Sham brain sections showed no Hsp72 immunoreactivity except for faint background staining. After MCAO, the number of Hsp72 immunopositive cells in the penumbra increased to a significantly greater extent in KO mice compared to WT mice (Figs. 3A and B). Western blots showed that cytosolic Hsp72 expression was increased to a significantly greater extent in the ischemic hemisphere of KO mice compared to WT mice (Figs. 3C and D).
Figure 3.
Increased heat shock protein (Hsp) Hsp72 expression in mice lacking β2AR after 24 h ischemia. (A) Brain sections immunostained for Hsp72. (C) Following middle cerebral artery occlusion (MCAO) the number of Hsp72 positive cells in the penumbra increased significantly in knockout (KO) mice compared with wild type (WT) mice (21 ± 7 vs 78 ± 5, P < 0.001). (B, D) Western blots of cytosolic fractions show that Hsp72 is increased in the ischemic hemisphere of KO mice compared with WT (22.0% ± 9.0% vs 84.8% ± 13.2%, P < 0.001). Actin band intensity was used to normalize the Hsp72 band intensity. (n = 6 per group, scale bar = 40 μm).
Discussion
The exact role of the β-adrenergic system in modulating ischemic cerebral injury is unclear. Clenbuterol, a β2AR agonist, has been shown to have neuroprotective activity against transient forebrain ischemia and focal ischemia, an action that is dependent on induction of NGF expression.14–16,27,28 In contrast, others have found that propranolol, a nonspecific βAR blocker is neuroprotective against focal ischemia, and inhibits NGF synthesis induced by clenbuterol.10,29 Furthermore, induction of NGF observed with clenbuterol was limited to the cortex. Since βAR antagonists that block NGF induction by an agonist also provide protection, the involvement of βAR in stroke is complex. It is possible that changes in the regulation of multiple processes, including metabolism, apoptosis, inflammation, and other pathways activated in ischemic brain injury, are also influenced by βAR activation. Mice lacking the β2AR were previously shown to have greater exercise tolerance than WT, and this was associated with a lower respiratory exchange ratio.6 We can only speculate that related mechanisms may also be relevant in acute brain ischemia.
Studies in cardiac myocytes have shown that β1AR and β2AR possess distinct functions in response to identical stimuli. Because of the similarities between the β1AR and β2AR agonist binding sites, pharmacologic intervention at the agonist binding site does not clearly reveal subtype-specific properties. Thus, in this study, we chose to use mice lacking a β2AR. However, since permanent absence of these receptors in the KO mice may have affected other pathways, we also compared the findings in the β2KO mice with mice treated acutely with a relatively specific β2AR antagonist, ICI 118,551.
βAR signaling and subtype-specific effects on cell death and survival pathways have been studied in cardiac myocytes. While β1AR couples only to stimulatory G-protein (Gs), β2AR has been shown to couple initially to Gs but then switch to Gi. Inhibiting β2AR-Gi coupling, the βγ subunit of Gi (Gβγ), or phosphoinositide 3 kinase, converts β2AR signaling from prosurvival to proapoptotic in myocytes.5,30 In contrast, β1AR stimulation in the cardiac myocyte elicits a proapoptotic signal mediated by a protein kinase A-independent Ca2+/Calmodulin kinase II pathway.31–33 Even in myocytes, β2 signaling can generate both pro- and antiapoptotic signaling.30 Whether the same signaling pathways are activated by the βAR subtype in neuronal cells is currently unclear. Levels of cAMP were not measured in these studies, so we do not know if they are increased or decreased in KO mice. Several studies suggest that β2 AR signaling is highly localized due to its participation in a signaling complex, including observations in hippocampal neurons showing coupling to a L-type calcium channel, as well as both the kinases to produce cAMP and the counterbalancing phosphatase.34
Our data indicate greater induction of Hsp72 after cerebral ischemia in β2AR KO mice than in WT mice. Hsp72 inhibits both apoptotic and necrotic cell death, and over-expression of Hsp72 provides protection from cerebral ischemia and ischemia-like insults.25,35,36 Conversely, Hsp72 deficiency leads to worsened outcome after experimental stroke.37 After MCAO in the rat, Hsp72 is expressed both in the penumbra38 and in regions distant from sites of primary damage, but which may be affected by spreading depression.39 Our findings of greater Hsp72 expression in KO mice are consistent with better protection by a more robust stress response. However, the mechanism underlying this effect is currently unknown. Among signals for the induction of Hsp72, catecholamines play a key role. Catecholamines have been shown to upregulate intracellular and extracellular Hsp72 via a α1 AR pathway rather than through β2AR.40–43 As noted above, higher cAMP levels might contribute. Besides augmenting Hsp72 expression, it is likely that the loss of β2AR exerts neuroprotective effects by additional parallel mechanisms. For example, inflammatory mechanisms have recently been suggested to contribute both to exacerbation of ischemic brain injury and to recovery and repair. The potential contribution of modulation of inflammation after ischemia in β2AR KO mice merits further investigation.
The possibility that differences in hemodynamic response of β2KO and WT animals to ischemia might have contributed to the protection seen in the KO animals should also be considered. Although our heart rate data show greater increases in heart rate in the β2KO animals at the time of MCAO than WT animals, this was not seeing with ICI 118, 551. Given that mice treated with ICI 118,551 demonstrated equivalent cerebral protection as β2KO mice, our data do not suggest that the heart rate difference can fully account for the difference in infarct size in β2KO mice when compared to WT mice.
sEvidence suggests that β1AR and β2AR form heterodimers in mouse cardiac myocytes.44–46 The full physiologic consequence of β1/β2AR heterodimerization is unclear. Zhu et al.46 demonstrated that compared to expression of either βAR subtype alone, co-expression of β1/β2AR on mouse cardiac myocytes results in increased affinity for isoproterenol, and increased cAMP production. These results suggest that in cardiac myocytes, the βAR subtypes work in synergy to optimize sympathetic control of myocardial performance. If there were a similar mechanism of βAR synergism in the brain, one could postulate that if β1AR activity in the setting of cerebral ischemia is detrimental, inhibiting either β1AR or β2AR activity (pharmacologically or by transgenic methods) would be beneficial. This would be consistent with findings that nonselective βAR antagonists and relatively selective β1 antagonists show benefit in models of cerebral ischemia. Furthermore, manipulating the β2AR may have consequences for the other members of the adrenergic system as well. Several studies show that the β2AR also can heterodimerize with β3AR, α2AAR, and α1DAR in human embryonic kidney 293 cells.47–49 The physiologic consequences of these interactions are unknown. Nevertheless, the present work demonstrates the neuroprotective effect of both genetic deletion and blockade of β2AR against focal cerebral ischemia at 24 h survival, and its association with greater induction of Hsp72. These results provide more evidence for an important role of β2 AR in influencing the outcome from stroke, and warrant future studies of longer survival times using this strategy.
Acknowledgments
We thank Jenny Hu for assistance in manuscript preparation and Dr. Jim Wong for reviewing and providing helpful comments on the manuscript.
Supported in part by grants RO1 GM49831, PO1 NS37520 and P50 NS14543 to R.G.G. and grant K08 HL75519 to A.J.P.
References
- 1.Asanuma M, Ogawa N, Mizukawa K, Haba K, Hirata H, Mori A. Distribution of the beta-2 adrenergic receptor messenger RNA in the rat brain by in situ hybridization histochemistry: effects of chronic reserpine treatment. Neurochem Res. 1991;16:1253–6. doi: 10.1007/BF00966654. [DOI] [PubMed] [Google Scholar]
- 2.Shao YP, Sutin J. Noradrenergic facilitation of motor neurons: localization of adrenergic receptors in neurons and nonneuronal cells in the trigeminal motor nucleus. Exp Neurol. 1991;114:216–27. doi: 10.1016/0014-4886(91)90038-e. [DOI] [PubMed] [Google Scholar]
- 3.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–9. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–83. [PubMed] [Google Scholar]
- 5.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i) -dependent coupling to phosphatidylinositol 3′ -kinase. Circ Res. 2000;87:1172–9. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 6.Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 7.Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, Kobilka B. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–8. doi: 10.1097/01.ccm.0000120049.43113.90. [DOI] [PubMed] [Google Scholar]
- 8.Amory DW, Grigore A, Amory JK, Gerhardt MA, White WD, Smith PK, Schwinn DA, Reves JG, Newman MF. Neuroprotection is associated with beta-adrenergic receptor antagonists during cardiac surgery: evidence from 2,575 patients. J Cardiothorac Vasc Anesth. 2002;16:270–7. doi: 10.1053/jcan.2002.124132. [DOI] [PubMed] [Google Scholar]
- 9.Capraro JA, Reedy DP, Latchaw JP, Slugg RM, Stowe NT, Lesser RP, Little JR. Treatment of acute focal cerebral ischemia with propranolol. Stroke. 1984;15:486–91. doi: 10.1161/01.str.15.3.486. [DOI] [PubMed] [Google Scholar]
- 10.Goyagi T, Kimura T, Nishikawa T, Tobe Y, Masaki Y. Beta-adrenoreceptor antagonists attenuate brain injury after transient focal ischemia in rats. Anesth Analg. 2006;103:658–63. doi: 10.1213/01.ane.0000228859.95126.69. [DOI] [PubMed] [Google Scholar]
- 11.Latchaw JP, Little JR, Slugg RM, Lesser RP, Stowe N. Treatment of acute focal cerebral ischemia and recirculation with d-propranolol. Neurosurgery. 1985;16:18–22. doi: 10.1227/00006123-198501000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Savitz SI, Erhardt JA, Anthony JV, Gupta G, Li X, Barone FC, Rosenbaum DM. The novel beta-blocker, carvedilol, provides neuroprotection in transient focal stroke. J Cereb Blood Flow Metab. 2000;20:1197–204. doi: 10.1097/00004647-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Standefer M, Little JR. Improved neurological outcome in experimental focal cerebral ischemia treated with propranolol. Neurosurgery. 1986;18:136–40. doi: 10.1227/00006123-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Semkova I, Krieglstein J. Neuroprotection mediated via neurotrophic factors and induction of neurotrophic factors. Brain Res Brain Res Rev. 1999;30:176–88. doi: 10.1016/s0165-0173(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 15.Semkova I, Schilling M, Henrich-Noack P, Rami A, Krieglstein J. Clenbuterol protects mouse cerebral cortex and rat hippocampus from ischemic damage and attenuates glutamate neurotoxicity in cultured hippocampal neurons by induction of NGF. Brain Res. 1996;717:44–54. doi: 10.1016/0006-8993(95)01567-1. [DOI] [PubMed] [Google Scholar]
- 16.Junker V, Becker A, Huhne R, Zembatov M, Ravati A, Culmsee C, Krieglstein J. Stimulation of beta-adrenoceptors activates astrocytes and provides neuroprotection. Eur J Pharmacol. 2002;446:25–36. doi: 10.1016/s0014-2999(02)01814-9. [DOI] [PubMed] [Google Scholar]
- 17.Choi HS, Li B, Lin Z, Huang E, Liu AY. cAMP and cAMP-dependent protein kinase regulate the human heat shock protein 70 gene promoter activity. J Biol Chem. 1991;266:11858–65. [PubMed] [Google Scholar]
- 18.Hastie AT, Everts KB, Shaver JR, Cirelli R, Zangrilli J, Pollice MB, Fish JE, Peters SP. Beta 2-agonist-elevated stress response in human bronchial epithelial cells in vivo and in vitro. Lung. 1997;175:287–98. doi: 10.1007/pl00007575. [DOI] [PubMed] [Google Scholar]
- 19.Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and antiinflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 20.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Bilski AJ, Halliday SE, Fitzgerald JD, Wale JL. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551) J Cardiovasc Pharmacol. 1983;5:430–7. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 24.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Ouyang YB, Xu L, Chow AM, Anderson R, Hecker JG, Giffard RG. The carboxyl-terminal domain of inducible Hsp70 protects from ischemic injury in vivo and in vitro. J Cereb Blood Flow Metab. 2006;26:937–50. doi: 10.1038/sj.jcbfm.9600246. [DOI] [PubMed] [Google Scholar]
- 26.Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114:1081–90. doi: 10.1016/s0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- 27.Culmsee C, Junker V, Kremers W, Thal S, Plesnila N, Krieglstein J. Combination therapy in ischemic stroke: synergistic neuroprotective effects of memantine and clenbuterol. Stroke. 2004;35:1197–202. doi: 10.1161/01.STR.0000125855.17686.6d. [DOI] [PubMed] [Google Scholar]
- 28.Hayes VY, Isackson PJ, Fabrazzo M, Follesa P, Mocchetti I. Induction of nerve growth factor and basic fibroblast growth factor mRNA following clenbuterol: contrasting anatomical and cellular localization. Exp Neurol. 1995;132:33–41. doi: 10.1016/0014-4886(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 29.Follesa P, Mocchetti I. Regulation of basic fibroblast growth factor and nerve growth factor mRNA by beta-adrenergic receptor activation and adrenal steroids in rat central nervous system. Mol Pharmacol. 1993;43:132–8. [PubMed] [Google Scholar]
- 30.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–12. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–25. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–50. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 33.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 34.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 35.Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, Yenari MA, Steinberg GK. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 2002;52:160–7. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- 36.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, Whitaker S, Steinberg GK. Hu23F2G, an antibody recognizing the leukocyte CD11/CD18 integrin, reduces injury in a rabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153:223–33. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 2001;32:2905–12. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- 38.Kinouchi H, Sharp FR, Koistinaho J, Hicks K, Kamii H, Chan PH. Induction of heat shock hsp70 mRNA and HSP70 kDa protein in neurons in the ‘penumbra’ following focal cerebral ischemia in the rat. Brain Res. 1993;619:334–8. doi: 10.1016/0006-8993(93)91630-b. [DOI] [PubMed] [Google Scholar]
- 39.Kinouchi H, Sharp FR, Chan PH, Koistinaho J, Sagar SM, Yoshimoto T. Induction of c-fos, junB, c-jun, and hsp70 mRNA in cortex, thalamus, basal ganglia, and hippocampus following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1994;14:808–17. doi: 10.1038/jcbfm.1994.101. [DOI] [PubMed] [Google Scholar]
- 40.Chin JH, Okazaki M, Hu ZW, Miller JW, Hoffman BB. Activation of heat shock protein (hsp)70 and proto-oncogene expression by alpha1 adrenergic agonist in rat aorta with age. J Clin Invest. 1996;97:2316–23. doi: 10.1172/JCI118674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heneka MT, Gavrilyuk V, Landreth GE, O'Banion MK, Weinberg G, Feinstein DL. Noradrenergic depletion increases inflammatory responses in brain: effects on IkappaB and HSP70 expression. J Neurochem. 2003;85:387–98. doi: 10.1046/j.1471-4159.2003.01694.x. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M. Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J Appl Physiol. 2005;99:1789–95. doi: 10.1152/japplphysiol.00390.2005. [DOI] [PubMed] [Google Scholar]
- 43.Meng X, Brown JM, Ao L, Banerjee A, Harken AH. Norepinephrine induces cardiac heat shock protein 70 and delayed cardioprotection in the rat through alpha 1 adrenoceptors. Cardiovasc Res. 1996;32:374–83. doi: 10.1016/0008-6363(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 44.Lavoie C, Hebert TE. Pharmacological characterization of putative beta1-beta2-adrenergic receptor heterodimers. Can J Physiol Pharmacol. 2003;81:186–95. doi: 10.1139/y02-167. [DOI] [PubMed] [Google Scholar]
- 45.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hebert TE. Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem. 2002;277:35402–10. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 46.Zhu WZ, Chakir K, Zhang S, Yang D, Lavoie C, Bouvier M, Hebert TE, Lakatta EG, Cheng H, Xiao RP. Heterodimerization of beta1- and beta2-adrenergic receptor subtypes optimizes beta-adrenergic modulation of cardiac contractility. Circ Res. 2005;97:244–51. doi: 10.1161/01.RES.0000176764.38934.86. [DOI] [PubMed] [Google Scholar]
- 47.Breit A, Lagace M, Bouvier M. Hetero-oligomerization between beta2- and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties. J Biol Chem. 2004;279:28756–65. doi: 10.1074/jbc.M313310200. [DOI] [PubMed] [Google Scholar]
- 48.Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J Biol Chem. 2003;278:10770–7. doi: 10.1074/jbc.M207968200. [DOI] [PubMed] [Google Scholar]
- 49.Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with beta2-adrenergic receptors promotes surface expression and functional activity of alpha1D-adrenergic receptors. J Pharmacol Exp Ther. 2005;313:16–23. doi: 10.1124/jpet.104.079541. [DOI] [PubMed] [Google Scholar]