Abstract
Coronary artery anomalies may involve the origin, course, and structure of epicardial coronary arteries and occur in less than 1% of the general population. Clinical presentation ranges from being completely asymptomatic to sudden death. Multi-detector computed tomography has come a long way in the diagnosis of coronary artery anomalies since the introduction of 4 rows of detectors in scanners, considering its non invasive nature and the benefits of 3D reconstruction. Defining the coronary anatomy helps in clinical decision making and timely intervention. Since repeated angiographies may be required, low dose CT is an excellent investigation for diagnosis and post interventional follow up rather than repeated invasive catheter angiographies or high dose CT examination. We report two cases of clinically significant single coronary artery anomalies; a case of single Right coronary artery and another case of single Left coronary artery (Anomaly of origin & course).
Keywords: Coronary anomalies, coronary arteries, single coronary artery, MDCT in coronary artery imaging
CASE REPORT
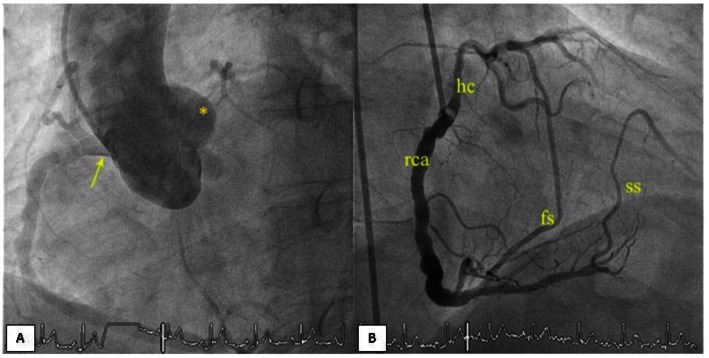
Case 1: A 62 year old male patient presented with complaints of jaw pain associated with sweating for 7 days. He had a history of dyslipidemia and smoking, and Troponin I was negative. His clinical history included 3 episodes of frank syncope for which he was investigated previously. Physical examination, including vitals, was normal. Catheter angiography was performed which raised the suspicion of a single coronary artery (Figure 2a, b). CT coronary angiography was further advised for confirmation and ruling out a complete block of the Left main coronary artery.
Figure 2.
62 year old male patient with single Right coronary artery.
(a) Cathether Aortogram (Philips FD10, LAO 45, CAU 1; WL 116, WW 239 ) shows a single ostium with a single Right coronary artery and absent Left coronary ostium.
(b) RCA cathether angiogram (Philips FD10, LAO 45, CAU 1; WL 116, WW 239) showing a single Right coronary artery (rca) with the hypertrophied conus branch (hc), first septal and second septal branches
The CT coronary angiogram was performed on a 128-slice Philips, Ingenuity Low Dose CT at a mean heart rate of 55beats/min. after stabilizing using a beta- blocker and an anxiolytic. 80ml of 350mgI/ml non-ionic contrast (Ioversol) was injected at a flow rate of 5.5ml/sec using a dual head Medrad injector followed by a saline chase of 30ml at the same rate. The scan was done at 120kv, 1000mAs, 0.4mm detector at a pitch of 0.2 with ECG- gated dose modulation and retrospective gating.
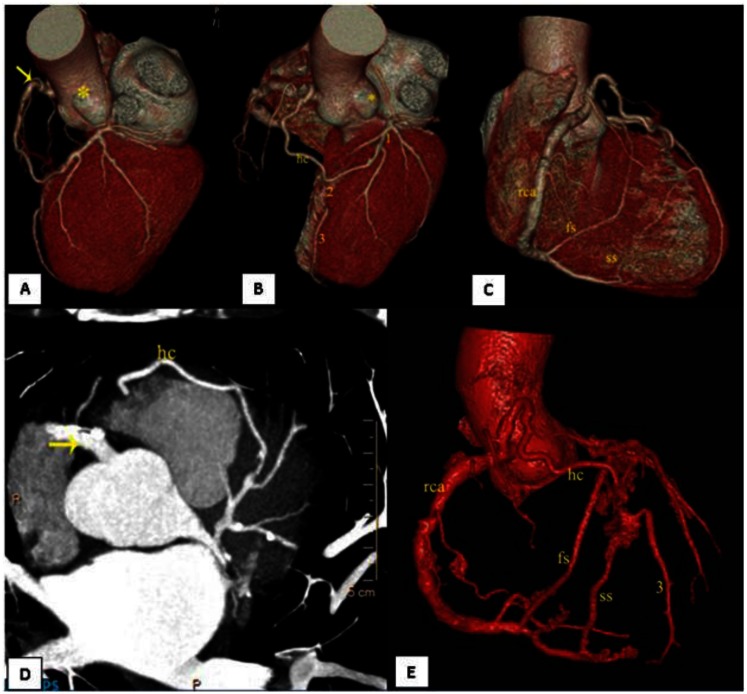
CT coronary angiography revealed a single Right coronary ostium and absent Left coronary ostium and LMA (Left main coronary artery) (Figure 1a). RCA (Right coronary artery) was a very large vessel (super-RCA). Multiple calcified-soft plaques were seen in the proximal, mid and distal RCA with areas of 20–30% luminal stenosis. The PDA (Posterior descending artery) and PLV (Posterolateral ventricular artery) were defined and appeared normal.
Figure 1.
62 year old male patient with single right coronary artery. VR reconstruction of CT coronary angiography (128-slice Philips, Ingenuity Low dose CT at a mean heart rate of 55beats/min. after stabilizing using a beta- blocker and an anxiolytic. 80ml of 350mgI/ml non- ionic contrast (Ioversol) was injected at a flow rate of 5.5ml/sec using a dual head Medrad injector followed by a saline chase of 30ml at the same rate. The scan was done at 120kv, 1000mAs, 0.4mm detector at a pitch of 0.2 with ECG- gated dose modulation and retrospective gating)
(a) single Right coronary ostium and absent Left coronary ostium (asterisk) and LMA (Left main artery). RCA was a very large vessel (super-RCA) (yellow arrow)
(b) Hypertrophied conus branch(hc) forming the proximal LAD(1). Distal LAD(3) is also shown. Mid LAD (2) is absent. Left coronary ostium and LMA is absent(asterisk).
(c) RCA with first septal and second septal branches from the proximal and distal PDA respectively, forming the proximal LAD and distal LAD.
(d) Post contrast axial thick slab MIP(14.8mm, WW 558, WC 242, 128-slice Philips, Ingenuity shows RCA (yellow arrow) with the hypertrophied conus branch coursing further to form the proximal LAD.
(e) VR reconstruction of CT coronary angiography shows single Right coronary artery (rca) with the hypertrophied conus (hc) branch which forms the proximal LAD along with the first septal (fs) branch of proximal PDA. The second septal (ss) branch from distal PDA forms the distal LAD (3).
The proximal LAD (Left anterior descending artery) was constituted through a hypertrophied Conus branch (also known as the ring of Vieussens’) with a thin intramuscular pre-pulmonic twig and the first septal branch (arising from proximal PDA). The mid LAD was absent. The distal LAD was constituted through a hypertrophied second septal branch (arising from the distal PDA) (Figure 1b). The LCX (Left circumflex) was seen as a continuation of proximal LAD in the left atrio ventricular groove. The LCX was a medium sized branch. Large first and second diagonal arteries were noted which divided into medial and lateral branches. Mixed plaques were seen in the coronary arteries with variable stenosis, most pronounced at the proximal LAD with 40–50% luminal narrowing.
The Calcium Score (Agatston Score) totaled 580.92 (LAD - 20.26, LCX - 21.85, RCA - 538.81). The Functional Analysis of the Left Ventricle on CT revealed an end - diastolic volume of 131.5ml, end - systolic volume of 32.7ml, stroke volume of 98.8ml and ejection fraction of 75%.
Case 2: A 79 years old, nonsmoker, diabetic and hypertensive male patient presented with complaint of breathlessness for 2–3 months, sore throat and hoarseness of voice. The patient also had pain bilaterally in the lower limbs. Routine clinical examination and investigations were normal. The echocardiogram revealed an ejection fraction of 65%. CECT Thorax revealed early interstitial lung disease and sub centimeter mediastinal lymph nodes. CT coronary angiography was performed on a Philips, Ingenuity CT at a stabilized mean heart rate of 60 beats/min. to rule out coronary artery disease. The same protocol was used as in the previous case.
CT coronary angiography revealed an absent Right coronary ostium (Figure 3a) and the Right coronary artery (RCA) arising from the Left main coronary artery (LMA) (Figure 3c,d). The RCA was then seen to course between the pulmonary artery and the aorta (interarterial course) (Figure 3b). It gave rise to conus, acute marginal, PDA1 and PDA2, and PLV branches. Concentric soft plaques were seen in the ostial and proximal PDA2 with 70–80% luminal stenosis. The LAD was short (Type I), the large first diagonal showed short segments of near complete stenosis and soft calcified plaques were seen in the LCX and proximal first obtuse marginal with 70–80% luminal narrowing.
Figure 3.
79 year old male patient with single Left coronary artery. VR reconstruction of CT coronary angiography (128-slice Philips, Ingenuity Low dose CT at a mean heart rate of 60 beats/min. after stabilizing using a beta- blocker and an anxiolytic. 80ml of 350mgI/ml non- ionic contrast (Ioversol) was injected at a flow rate of 5.5ml/sec using a dual head Medrad injector followed by a saline chase of 30ml at the same rate. The scan was done at 120kv, 1000mAs, 0.4mm detector at a pitch of 0.2 with ECG- gated dose modulation and retrospective gating)
(a) Absent Right coronary ostium (asterisk) and the RCA (yellow arrow) arising from the proximal part of the LMA.
(b) RCA (yellow arrow) arising from the LMA, and the RCA has an interarterial course.
(c) Post contrast axial MIP (0.8mm, WW-795, WC-374) of CT coronary angiography shows RCA (yellow arrow) arising from the proximal part of the LMA (red arrow)
(d) Post contrast axial curved MPR (1 mm, WW-812, WC-137) of CT coronary angiography shows RCA (yellow arrow) arising from the proximal portion of the LMA.
The Calcium Score (Agatston Score) totaled 362.11(LAD - 251.13, LCX - 0.47, RCA - 110.51). Functional analysis of the Left ventricle included an end diastolic volume of 108.7ml, end systolic volume of 59.7ml, stroke volume of 49.0ml and ejection fraction of 45%.
In light of the significant stenosis reported on CT angiography, the patient underwent a catheter angiogram which corroborated the CT findings - anomalous RCA from the left sinus, PDA ostial stenosis of 90%, 100% stenosis of the large D1 mid, and 70% stenosis of the LCX/OM1 (Figure 4). No interventions were performed, however.
Figure 4.
Catheter angiogram (Philips FD10)
(a) shows LMA dividing into LAD and LCX. (RAO 5, CAU 44; WL 116, WW 239)
(b) shows LMA (red arrow) giving rise to RCA (yellow arrow) (LAO 45, CAU 1; WL 116, WW 239)
(c) showing RCA (yellow arrow) arising from LMA (red arrow) and stenosed PDA (thick yellow arrow) (LAO 45, CAU 1; WL 116, WW 239)
DISCUSSION
The definition of a coronary artery anomaly is proposed by Angelini and colleagues as an unusual morphologic feature seen in less than 1% of an unselected population.[1,2] Anomalies of the coronary arteries may be found incidentally in 0.3%–1.0% of healthy individuals depending on whether the evaluation is on autopsy [3], catheter angiography [4], surgery [5] or CT angiography [6]. The anomalous course, origin, or proximal course anomalies are more common than those of fistulous terminations. Anomalies of the Left circumflex artery are more common and account for nearly two- thirds of the reported single coronary artery anomalies, especially the Left circumflex arising separately from the Right coronary sinus, followed by the Left circumflex arising from the proximal Right coronary artery [5].
The coronary arteries arise from a peritruncal ring of vasculature and establish contact with the aorta. Aberrations in embryonic cell lineage commitment, diversification, cell migration, transition and cell differentiation, vasculogenesis, neural crest cells, peripheral conduction system, alterations in growth factors and genes can result in coronary anomalies [7].
A higher incidence of coronary arteries anomalies is associated with an abnormal aortic valve [8] and is clinically significant in these patients in deciding aortic root surgery. The persistent truncus arteriosus [9] and pulmonary atresia [10] are other congenital anomalies which may be seen in association with a single coronary artery. In our cases, there were no associated cardiac defects.
Coronary artery anomalies maybe classified by clinical and functional significance as either hemodynamically significant (malignant) or insignificant (benign). They can be classified morphologically as major or minor, or as critical, severe, relevant, or benign. They can also be classified anatomically as anomalies of origination and course, intrinsic anatomy, or termination. A detailed classification of coronary anomalies by anatomy is provided by Angelini in normal human hearts [2].
A single coronary artery is an extremely rare congenital anomaly that is seen in only 0.0024%–0.044% of the population.[11] According to Lipton’s classification, single coronary artery can be described using a three-character coded nomenclature in which the first alphabet represents ostial location, second number represents anatomical distribution and third character represents the course of the transverse trunk. Ostial location can be either right or left of the sinus of Valsalva, represented by R or L. Anatomical distribution is represented by I, II or III. Type “I” refers to a solitary dominant vessel following the course of either a normal right or left coronary artery (RI or LI). Type “II” refers to one coronary artery arising from the proximal part of the normally located other coronary artery (RII or LII). In type “III,” the Left anterior descending and Left circumflex arise separately from a common trunk originating from the Right sinus of Valsalva (RIII). The third character denotes the course of the transverse trunk and is symbolized by A, B, P, S or C. A refers to “Anterior to the great vessels”, “B” is Between the aorta and pulmonary arteries, “P” is Posterior to the great vessels, “S” is ‘Septal type’: a part of the route passes through inter ventricular septum and “C” is ‘Combined type’: combination of diverse routes[12]. According to this Classification our first case is RIIC while the second case is LIIB.
Another Classification was given by Ogden and Goodyer as five patterns of distribution: (1) a single coronary artery supplying the entire myocardium, (2) a single coronary artery with 2 major branches, 1 of which has a retroaortic course, (3) 2 major branches, 1 of which has an interarterial course, (4) 2 major branches, 1 of which has a prepulmonic course, and (5) 3 equally dominant major branches [13]. Our cases are in pattern 4 and 3 respectively, according to this classification.
Such anomalies may remain clinically silent or may manifest as exertional chest pain, syncope, myocardial ischemia and infarction, malignant ventricular arrhthymias and even sudden cardiac death in affected individuals, especially young atheletes or exercising individuals [4]. Symptoms occur by compression of the intramural segment of the left coronary artery by the great vessels, usually during exercise or other stress. Other hypothetic mechanisms causing symptoms can be related to a decrease in coronary artery blood flow because of a kinking of the left coronary artery at its origin from the right coronary artery. Kinking may be caused by an increased angulation resulting from distention of the aorta during increased cardiac activity [14]. In our case, however, the patients were of an older age group and the detection of the anomalies was an incidental finding.
There is also a debate whether coronary anomalies have a positive or negative impact on development of atherosclerosis, but the general consensus is that the two conditions may simply coexist [15]. In our cases, there was association of the anomalies with significant atherosclerotic changes in the coronary vessels.
Earlier, the detection of these anomalies was picked up on coronary angiograms, which were invasive procedures. With the advent of MDCT, these anomalies are better characterized on CT coronary angiography which is relatively non invasive. Since these anomalies may infer a higher risk of complications, routine and repeated angiographies may be needed. Low dose CT with Iterative reconstruction, combined with prospective gating in patients with stable heart rates, confers a significant advantage as it can be repeated without significantly increasing the radiation risk. However, retrospective gating also provides functional assessment of the left ventricle, obviating the need for any other functional analysis study.
CT Coronary Angiography has often been the eye of controversy due to its high radiation dose (approx. 9.5 to 21.4 mSv on retrospectively gated single source- 64-slice CT) as compared to Conventional Catheter Angiography ( 3.1–9.4 mSv) for diagnostic studies. The radiation dose depends on the specific equipment and techniques employed for scanning. Currently the concept of ALARA (As Low As Reasonably Achievable) is advocated to address the issue of radiation dose reduction.
CT Coronary Angiography performed with retrospective gating results in higher radiation dose as the data is acquired throughout the cardiac cycle and a low pitch is needed with fast gantry rotation for high temporal resolution and acquisition of all phases of the heart. ECG- dependent tube current modulation is the most common method to reduce radiation dose by 20 – 50%. It works on the premise that the best image quality in Coronary CT Angiography is obtained in the mid-to end-diastole of the R-R interval, and hence the tube current and radiation dose can be lowered in the phases where the image quality is sub-optimal. Reduction in tube voltage and increasing the pitch also helps in reducing the radiation dose. [16]
In Prospective ECG triggering, the data is acquired at only a specific point in the R-R interval and a sequential (step-and-shoot) scan is acquired rather than in the helical mode. A dose reduction of up to 83% is achieved as compared to retrospective technique. The major disadvantage of this technique is lack of functional data and no additional phases are available for reconstruction, as the acquisition is only in the predefined phase. This technique can only be applied in patients with low and stable heart rates and good breath hold. The radiation dose may be decreased to as low as 1 mSv. [17]
The new iterative reconstruction technology or the iDose is being used as an alternative to the standard filtered back projection. iDose4 is a sophisticated and complex reconstruction algorithm that demands enormous computational power for interaction of information between the projection and image domains, and requires the support of elegant software and hardware architectures. Multiple components of the imaging chain have been enhanced to increase volume imaging speed, dose efficiency, and image quality, thereby enabling opportunities for lower dose scan protocols with up to 80% dose reduction while still maintaining the image quality.
We have used retrospective gating with ECG-dose modulation and iDose technology to reduce the radiation dose of the scans to approximately 8mSv.
MR Angiography is emerging as a non-invasive imaging modality for coronary arteries as it lacks ionising radiation, obviates the use of iodinated contrast, and with the new Breath-hold three dimensional ultra-fast sequences it is possible to produce excellent images due to short acquisition times. Paramagnetic contrast medium may be added to these techniques to increase contrast to noise ratio and improve visualization of the small vessels, especially the origins of anomalous arteries. [18]
Coronary artery anomalies need earlier detection to avoid untoward cardiac emergencies, particularly in young individuals. Our patients were over the age of 60 years, but remained asymptomatic for the anomalies in spite of these anomalies being clinically significant. The patient’s presenting symptoms were attributable to atherosclerosis.
TEACHING POINT
Single coronary artery is an extremely rare anomaly. Significant clinical variants may be picked up in patients with atherosclerosis. Recent technological advances in the form of Low Dose CT using retrospective gating with ECG-dependent current dose modulation, and/or prospective gating in conjunction with iterative reconstructions, lower the radiation risk in imaging these patients. Maximum intensity projections and volume rendered images delineate these anomalies better for interpretation and therapeutic decision making.
Table 1.
Lipton’s classification of single coronary artery
| Code | Description | |
|---|---|---|
| Ostial Location | R | Right Sinus of Valsalva |
| L | Left Sinus of Valsalva | |
| Anatomical Distribution | I | The solitary dominant vessel follows the course of either a normal right or left coronary artery (RI or LI) |
| II | One coronary artery arises from the proximal part of the normally located other coronary artery (RII or LII) | |
| III | LAD and LCX arise separately from a common trunk originating from the right sinus of Valsalva (RIII) | |
| Course of the transverse trunk | A | Anterior to the great vessels |
| B | Between the aorta and pulmonary arteries | |
| P | Posterior to the great vessels | |
| S | ‘Septal type’: a part of the route passes through the interventricular septum | |
| C | ‘Combined type’: combination of diverse routes |
Reprinted from Coronary angioplasty with stenting for acute coronary syndrome in patients with an isolated single coronary artery: a report of two cases Journal of Cardiovascular Medicine: July 2009 - Volume 10 - Issue 7)
Table 2.
Angelini classification of coronary artery anomalies (Continued on next page)
Anomalies of origination and course
|
If a single, common ostium is present, the pattern is considered to represent “single” coronary artery.
Table 3.
Summary table for single coronary artery
| Etiology | Congenital |
| Incidence | 0.0024%–0.044% |
| Gender ratio | No sex predilection |
| Findings on imaging | Single coronary artery with absent ostium of contralateral side |
Table 4.
Types of left anterior descending (LAD) artery
| Type I | Ends above the cardiac apex |
| Type II | Ends at the cardiac apex |
| Type III | Ends beyond the cardiac apex |
ABBREVIATIONS
- D1
Diagonal branch
- LAD
Left anterior descending
- LCX
Left circumflex artery
- LMA
Left main artery
- OM1
Obtuse marginal branch
- PDA
Posterior descending artery
- RCA
Right coronary artery
REFERENCES
- 1.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–2454. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 2.Angelini P, Fairchild VD, editors. Coronary Artery Anomalies: A Comprehensive Approach. Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 3.Frescura C, Basso C, Thiene G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998;29(7):689–95. doi: 10.1016/s0046-8177(98)90277-5. [DOI] [PubMed] [Google Scholar]
- 4.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21(1):28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 5.Click RL, Holmes DR, Jr, Vlietstra RE, et al. Anomalous coronary arteries: location, degree of atherosclerosis and effect on survival - a report from the Coronary Artery Surgery Study. J Am Coll Cardiol. 1989;13(3):531–7. doi: 10.1016/0735-1097(89)90588-3. [DOI] [PubMed] [Google Scholar]
- 6.Cadermartiri F, La Grutta L, Malago R, et al. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol. 2008;18(4):781–91. doi: 10.1007/s00330-007-0821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogers AJ, Gittenberger-de Groot AC, Poelmann RE, et al. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol(Berl) 1989;180(5):437–41. doi: 10.1007/BF00305118. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez MC, Duran AC, Real R, et al. Coronary anomalies and aortic valve morphology in the Syrian hamster. Lab Anim. 2000;34(2):145–54. doi: 10.1258/002367700780457545. [DOI] [PubMed] [Google Scholar]
- 9.de la Cruz MV, Cayre R, Angelini P, Noriega-Ramos N, Sadowinski S. Coronary arteries in truncus arteriosus. Am J Cardiol. 1990;66:1482–6. doi: 10.1016/0002-9149(90)90539-d. [DOI] [PubMed] [Google Scholar]
- 10.Calder AL, Co EE, Sage MD. Coronary arterial abnormalities in pulmonary atresia with intact ventricular septum. Am J Cardiol. 1987;59:436–42. doi: 10.1016/0002-9149(87)90952-0. [DOI] [PubMed] [Google Scholar]
- 11.Desmet W, Vanhaecke J, Vrolix M, et al. Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. Eur Heart J. 1992;13:1637–1640. doi: 10.1093/oxfordjournals.eurheartj.a060117. [DOI] [PubMed] [Google Scholar]
- 12.Lipton MJ, Barry WH, Obrez I, Silverman JF, Wexler L. Isolated single coronary artery: diagnosis, angiographic classification, and clinical significance. Radiology. 1979;130:39–47. doi: 10.1148/130.1.39. [DOI] [PubMed] [Google Scholar]
- 13.Ogden JA, Goodyer AV. Patterns of distribution of the single coronary artery. Yale J Biol Med. 1970;43(1):11–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37:593–597. doi: 10.1016/s0735-1097(00)01136-0. [DOI] [PubMed] [Google Scholar]
- 15.Ritagelli G, Gemelli M, Zamboni A, et al. Are coronary artery anomalies an accelerating factor for coronary atherosclerosis development? Angiology. 2004;55(1):29–35. doi: 10.1177/000331970405500105. [DOI] [PubMed] [Google Scholar]
- 16.Primak AN, McCollough CH, Bruesewitz MR, et al. Relationship between noise, dose, and pitch in cardiac multi-detector row CT. Radiographics. 2006 Nov-Dec;26(6):1785–94. doi: 10.1148/rg.266065063. [DOI] [PubMed] [Google Scholar]
- 17.Earls JP, Berman EL, Urban BA, et al. Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology. 2008 Mar;246(3):742–53. doi: 10.1148/radiol.2463070989. [DOI] [PubMed] [Google Scholar]
- 18.Van Geuns RJ, Wielopolski PA, de Bruin HG, et al. MR coronary angiography with breath-hold targeted volumes: preliminary clinical results. Radiology. 2000 Oct;217(1):270–7. doi: 10.1148/radiology.217.1.r00oc01270. [DOI] [PubMed] [Google Scholar]