Abstract
Castleman’s disease (CD) is a rare lymphoproliferative disease of uncertain etiology that affects lymph nodes. CD can be classified as a) unicentric vs. multicentric, based on clinical and radiological findings, b) hyaline vascular (80–90%) vs. plasmacytic (10–20%) vs. mixed cellularity variety based on histopathology. Unicentric disease is more common in the 3rd and 4th decade, whereas the multicentric form is more common in the 5th and 6th decade with no sex predilection. HIV seropositive individuals appear to be at an increased risk for multicentric castleman's disease (MCD) at a younger age due to the increased incidence of HHV- 8 infection. Diagnosis is usually based on histopathology features as imaging features show considerable overlap, thus posing diagnostic difficulties. Overall prognosis is good, particularly in the unicentric variety of disease. We have presented a case of the unicentric CD in a 40 year old male patient having abdominal pain and hematuria as chief complaints.
Keywords: Castleman's Disease, unicentric, idiopathic, CT, Computed Tomography, MRI, Magnetic Resonance Imaging
CASE REPORT
A 40 year old male patient, working in an iron factory, coming from a lower socio-economic status was admitted to our hospital, with chief complaints of abdominal pain for 3 months and hematuria for 3 days. The character of the pain was dull aching, intermittent and nonradiating. There was no significant past medical and surgical history. An insignificant family history with personal history of habitual tobacco chewing. Bowel and bladder habits were normal. The general and abdominal examination was within normal limits.
LABORATORY INVESTIGATIONS
Patient's Hb, total count, differential count, red blood cell count, platelet count, ESR were normal. His liver function tests and renal function tests were within normal limits. Serum protein, sugar and electrolytes were also normal. Mantoux test, HIV serology, HHV-8 serology and HHV-8 DNA PCR were negative.
ULTRASONOGRAPHY (USG) REPORT
A 4 × 3.5 cm sized, well-defined encapsulated hypo-echoic mass, with vascularity within it on doppler study was noted in relation to interpolar and lower pole of right kidney. Multiple enlarged lymphnodes in preaortic and paraaortic region with the largest measuring 2 ×1cm in size. There was no evidence of any abnormalities in other organs.
Computed Tomography (CT)
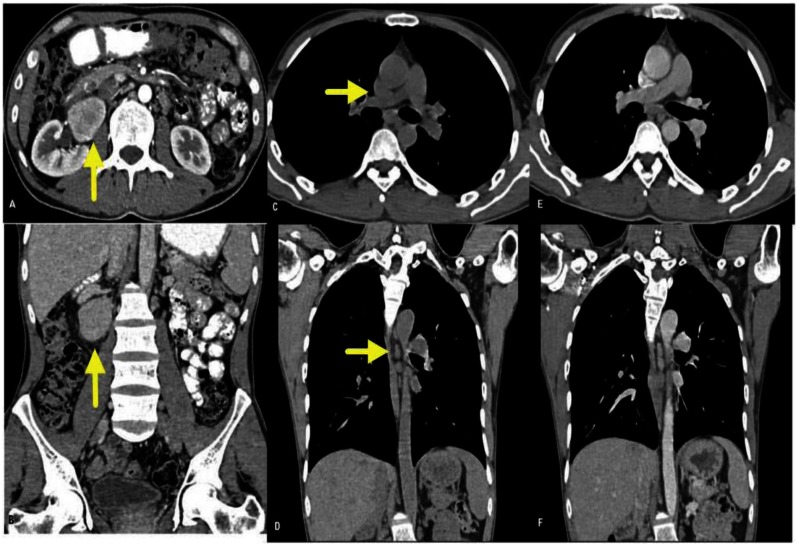
Approx. 48 × 40 × 37 mm sized, well-defined soft tissue density lesion with intense inhomogeneous post contrast enhancement was noted in relation to right renal hilum in the retroperitoneal region, displacing right renal vessels anteriorly. Multiple enlarged lymphnodes were noted in pre-aortic and para-aortic regions with the largest measuring 26 × 14 mm in size. There was no evidence of any abnormalities in other organs (figure 2). Findings were suggestive of paraganglioma (more likely) than a lymphnodal mass.
Figure 2.
40 year old male patient with unicentric Castleman's disease. 64 slice thickness MDCT SCAN with 1mm abdominal cuts, 250 mAs and 120 kVp with optiscan 120ml as contrast agent: A, B-Axial and coronal abdominal post contrast cuts showing well defined soft tissue density lesion with intense inhomogeneous enhancement noted in relation to the right renal hilum in the retroperitoneal region, displacing right renal vessels anteriorly with multiple enlarged lymphnodes in pre and para-aortic region. C to F-Axial and coronal thoracic pre and post contrast cuts showing few enlarged lymphnodes in thorax, which do not show the same intense enhancement.
Magnetic Resonance Imaging (MRI)
There was evidence of a 55 × 44 × 32mm size heterogeneously hyperintense retroperitoneal lesion antero-medial to right renal hilum displacing renal vessels antero-superiorly and compressing over the right renal pelvis on T2-weighted sequences. Lesion appeared isointense to muscles on T1–weighted images with intense inhomogeneous contrast enhancement. Multiple enlarged lymphnodes were noted in pre- and paraaortic region, aortocaval and precaval region, with the largest measuring 16 × 10mm in size (figure 1). There was no evidence of any abnormalities in other organs. Findings were suggestive of paraganglioma are more likely; nevertheless, the remote possibility of lymph nodal mass could not be excluded.
Figure 1.
40 year old male patient with unicentric Castleman's disease. MRI IMAGES, 1.5 T magnetic strength and Gadolinium 7cc as contrast agent: Lesion is heterogeneously hyperintense in the retroperitoneum, antero-medial to the right renal hilum, displacing renal vessels antero-superiorly and compressing the right renal pelvis (C, D) on T2-weighted images and isointense to muscles (A, B) on T1-weighted images showing intense inhomogeneous post contrast enhancement (E, F).
OPERATIVE DETAILS
Patient was operated for abdominal mass excision by right retroperitoneal approach and specimen was sent for histopathological examination.
HISTOPATHOLOGY
Gross/Macroscopic examination showed that specimen consists of irregular fibro fatty tissue measuring 8 × 5 × 3 cm. On cut section, a well circumscribed nodule measuring 5 × 5 × 3 cm is noted which is homogeneously yellowish white.
Microscopic examination showed large follicles scattered in lymphoid tissue. Follicles show marked vascular proliferation and hyalinization of germinal centres. Many large cells with vesiculous nuclei are present in hyaline centres. There is tight concentric laying of lymphocytes at periphery of follicles with mantle zone expansion. Intra-follicular stroma shows marked proliferation of vessels and hyalinisation (figure 3). Based on this, diagnosis of hyaline vascular type of Castleman's disease was given.
Figure 3.
40 year old male patient with Castleman's disease. Histology slides A, B with haematoxylin and eosin stain and 10 × magnifications showing marked vascular proliferation and hyalinization of germinal centres and many large cells with vesiculous nuclei.
On follow up after surgery, the patient's histopathological report confirmed our diagnosis of castleman's disease. No adjuvant treatment like monoclonal antibodies or radiotherapy was given. The patient did not present with any recurrence till date.
DISCUSSION
Castleman's disease (CD) is a rare lymphoproliferative disease that affects lymph nodes and other immune-cell structures of the body. CD represents a distinct clinico-pathological entity, first described by Dr. Benjamin Castleman in 1956 [1]. Castleman's Disease can be classified as a) unicentric vs. multicentric, based on clinical and radiological findings, b) hyaline vascular (80–90%) vs. plasmacytic (10–20%) vs. mixed cellularity variety based on histopathology and c) HIV negative versus HIV positive based on the HIV status of the patient.
There are two pathological types of CD, namely the hyaline vascular variant and the plasma cell variant [2]. The hyaline vascular variant (unicentric) exhibits prominent proliferation of small hyalinised follicles with marked interfollicular vascular proliferation, while the plasma cell variant (multicentric) exhibits hyperplastic germinal centres, sheets of plasma cells in the interfollicular region, proliferation of blood vessels, and persistent sinuses. It is postulated that 10 – 20 percent of all cases are of the plasma cell variant, with a small percentage being of mixed histological appearance.
The average age of people with unicentric castleman's Disease is around 3rd and 4th decade, whereas most patients with the multicentric form are in their age group of 50s and 60s. However, for those who are HIV (+), the disease presents at a younger age [3]. HIV seropositive individuals appear to be at an increased risk for multicentric castleman's disease (MCD) [4]. There is no sex predilection for the disease.
The exact cause of CD remains unknown. Infection by a virus called the human herpes virus 8 (HHV8) is associated with CD, and it is suspected that the virus may play a causal role, especially in MCD. This virus has also been linked to the development of Kaposi's sarcoma, a cancerous tumour of blood vessel walls.
The unicentric form of CD is asymptomatic in almost half of the cases. It is usually not associated with HHV-8 infection. It is often discovered during routine physical examination, chest x-ray, and abdominal sonography. In the other half of cases, when the lesion is large enough to cause compressive symptoms, the mode of presentation is usually chest pain or abdominal pain. The size of lesions varies widely (1–12 cm) with a mean size of 6 cm. The unicentric form remains localised to only one site, as the name suggests, with preferential involvement in decreasing order of frequencies including abdomen, peripheral lymph nodes and mediastinum. However, it can affect any other site as well. The usual signs and symptoms of the unicentric variety include fullness in the chest or abdomen, low-grade fever, anaemia, fatigue, weight loss, profuse sweating and skin rashes [2,5–8]
The multicentric form, on other hand, is often symptomatic [5]. The symptoms are mainly because of the elevated production of interleukin-6. Symptoms include fever, night sweats, loss of appetite, nausea and vomiting, weight loss, weakness or fatigue, anaemia, enlarged peripheral lymph nodes, usually around the neck, collarbone, underarm and groin areas, along with enlarged liver or spleen, and peripheral neuropathy. Peripheral polyadenopathy is quite common, with a mean of 4 sites being involved. Another clinical observation is the association of MCD with POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes) syndrome [8]. Some multicentric forms are associated with Kaposi's sarcoma (KS) as well. It has been reported that among HIV (+) patients with MCD who are infected with HHV-8, up to 70 percent will develop KS at some time in their clinical course [6,9,10,11]. MCD commonly results in a fatal outcome due to infectious complications, multi-organ failure, and development of malignancies such as lymphoma (Hodgkin's and non-Hodgkin) or Kaposi sarcoma [6,10]. The diagnosis of CD is based on clinical evaluation, which includes patient history, laboratory studies like IL-6, CRP and ESR, CBC, HIV serology testing with consent, HHV-8 serology, HHV-8 DNA PCR, but the correct diagnosis of this disease is achieved only by histological examination of excised lymphnodes.
In the case being reported, apart from abdominal pain and hematuria, there were no other complaints. Neurological and endocrine examinations were normal. Acute phase reactants, blood counts, and serological testing for HIV and HHV-8 were also unremarkable; hence a disease of uncertain etiology.
Imaging findings include non-enhanced CT where CD manifests as homogeneous or heterogeneous mass of soft tissue density [3]. Calcification is rare and typically central and coarse in nature. CD is intensely enhanced following contrast administration. In MRI, lesions are typically heterogeneous with increased signal as compared to skeletal muscles on T1-weighted images and marked hyper intensities on T2–weighted images. Diffuse enhancement is common after intravenous gadolinium. CD is usually Fluorodeoxyglucose (FDG) avid on Positron emission tomography (PET), which can be used for staging; follow up of treatment and assessment of recurrence of disease.
The differential diagnosis of castleman's disease may include lymphoma, infection (abscess, tuberculosis), sarcoma and paraganglioma. Based on its CT appearance, the radiologic differential diagnosis for castleman's disease in our case study was lymphoma or tuberculosis. On CT, lymphoma is typically a mass of low attenuation, without significant enhancement or calcification while tuberculosis is typically seen on CT as a mass or multiple masses with necrotic, low-attenuation centres having peripheral enhancement. However retroperitoneal sarcomas may show enhancement and calcification, depending on histological type.
The treatment of CD depends upon the variety of disease. Unicentric form is generally curable using surgical resection (with or without radiotherapy) [2,7]. At present, there is no consensus on the optimal management strategy for MCD [4]. Successful treatment of MCD has been achieved using chemotherapy, with or without prednisone, given at the time of initial diagnosis. The chemotherapeutic agents used in the various case series that have yielded remissions include doxorubicin, vincristine, cyclophosphamide, melphalan and chlorambucil [2]. These have been used as single agents, together with steroids or in combination (e.g. CHOP). Azathioprine and bone marrow transplantation have also been attempted, especially following the failure of CHOP, but have yielded mixed results. Another treatment option includes monoclonal antibodies to IL-6 and suramin (reverse transcriptase inhibitor), which can cause resolution of symptoms and regression of lymphadenopathy; however, there is often recurrence with discontinuation of treatment.
TEACHING POINT
Castleman's Disease is a rare lymphoproliferative disease of uncertain etiology. The unicentric disease being more common in the 3rd and 4th decade and the multicentric form in 5th and 6th decade, with no specific sex predilection. HIV seropositive individuals appear to be at an increased risk for MCD at a younger age due to increased incidence of HHV-8 infection. The radiological findings of lymphoproliferative disease being nonspecific should always make the radiologist suspect CD in any lymphoproliferative disease of uncertain origin, which should always be confirmed by histopathology.
Table 1.
Summary table for Castleman's Disease
| Etiology | Uncertain etiology. Probably infective origin. |
| Incidence | Rare disorder, approximately 4 per 10,000 patient |
| Gender ratio | Male = Female |
| Age predilection | Unicentric – 3rd and 4th decade Multicentric – 5th and 6th decade |
| Risk factors | With AIDS in immunocompromised patients |
| Treatment | Unicentric--Surgical resection Multicentric Systemic therapy with corticosteroids and chemotherapeutic agents. |
| Prognosis | Unicentric--excellent, 100% survival Multicentric--poor due to development of disseminated infection and malignancy. |
| Findings on imaging | On USG--Enlarged retroperitoneal lymphnodes involving single or multiple groups of lymph nodes, depending upon type of disease, showing homogeneously hypo echoic echo texture without necrosis and with increased vascularity along with associated organomegaly in some cases. On non-enhanced CT--homogeneous (small size < 5cm) or heterogeneous (large size > 5 cm) mass of soft tissue density. Calcification is rare and typically central and coarse in nature. CD is intensely enhanced following contrast administration. Enhancement is homogeneous with small lesions and heterogeneous with large lesions. On MRI, lesions are typically heterogeneous with a low to intermediate signal compared to skeletal muscles on T1–weighted images and marked hyper intensities on T2–weighted images. Diffuse enhancement is common after intravenous gadolinium. |
Table 2.
Differential diagnosis table for Castleman's Disease
| Diseases | X-ray | USG | CT | MRI | Enhancement |
|---|---|---|---|---|---|
| Castleman’s disease | Abdomen X-ray: no abnormalities. Chest X-ray: widening of mediastinal shadow with enlargement of hilar shadows due to lymphadenopathy. |
Enlarged retroperitoneal lymphnodes involving single or multiple groups of lymph nodes, depending upon type of disease, showing homogeneously hypo echoic echo texture without necrosis and with increased vascularity along with associated organomegaly in some cases. | Homogeneous (small size, < 5cm) or heterogeneous (large size, > 5 cm) mass of soft tissue density. Calcification is rare and typically central and coarse in nature. | Lesions are typically heterogeneous with low to intermediate signal compared to skeletal muscles on T1 –weighted images and markedly hyper intense on T2 –weighted images. | CD is intensely enhanced following contrast administration. Enhancement is homogeneous with small lesions and heterogeneous with large lesions. |
| Tuberculous infection | Abdomen X-ray: features of intestinal obstruction may be present due to fibrosing stricture mainly ileum or IC junction. Chest X-ray: signs of pleuropulmonary tuberculosis. |
Edematous and inflamed bowel loops involving mesentery with multiple enlarged hypo echoic lymph nodes, a few of which show areas of necrosis. Lymphnodes may be matted forming a confluent mass without encasing vessels. | Multiple large (average size 2–3 cm) lymphnodes of low attenuation noted, degree of which depends upon central necrosis. | Low to intermediate signal compared to skeletal muscles on T1 weighted images and markedly hyperintense centre on T2 weighted images, depending upon central necrosis in active stage. | Peripheral rim enhancement noted with central nonenhancing necrotic areas. |
| Lymphoma | Abdomen X-ray: no abnormalities. Chest X-ray: widening of mediastinal shadow with enlargement of hilar shadows due to lymphadenopathy. |
Multiple enlarged lymphnodes with homogeneously hypo echoic echo texture, without necrosis and with associated organomegaly in some cases. | Nodal and extra nodal lymphomas are typically a mass of low attenuation, without significant enhancement or calcification. Confluent lymphadenopathy encasing vessels are characteristic. Lymphnodes are usually round to oval shaped. | Intermediate to high signal intensity on T1-weighted and high signal intensity on T2-weighted images relative to muscles, associated bone marrow, GIT, liver and spleen may be documented. Low signal on T2-weighted images occurs following successful treatment. | No significant enhancement |
ABBREVIATIONS
- AIDS
Acquired Immunodeficiency Syndrome
- CBC
complete blood count
- CD
Castleman’s Disease
- CRP
C-reactive protein
- CT
Computed Tomography
- ESR
Erythrocyte sedimentation rate
- FDG
Fludeoxyglucose
- GIT
Gastrointestinal tract
- Hb
haemoglobin
- HHV
Human herpes virus
- HIV
Human Immunodeficiency Virus
- IL
Interleukin
- KS
Kaposi sarcoma
- MCD
Multicentric Castleman’s Disease
- MRI
Magnetic Resonance Imaging
- PCR
Polymerase chain reaction
- PET
positron emission tomography
- USG
Ultrasonography
REFERENCES
- 1.Castleman B, Iverson I, Menendez VP. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer. 1956;9:822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant node hyperplasia of the mediastinium and other locations. Cancer. 1972;29:670–83. doi: 10.1002/1097-0142(197203)29:3<670::aid-cncr2820290321>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Martinez S, Mcadams HP, Erasmus JJ. Mediastinum. In: Haaga JR, editor. CT and MRI of whole body. 5th ed. Mosby; 2009. pp. 1016–17. [Google Scholar]
- 4.Gaba AR, Stein RS, Sweet DL, Variakojis D. Multicentric giant lymph node hyperplasia. Am J Clin Pathol. 1978;69:86–90. doi: 10.1093/ajcp/69.1.86. [DOI] [PubMed] [Google Scholar]
- 5.Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behaviour of localised and multicentric castleman's disease. Ann intern Med. 1998;128:657–62. doi: 10.7326/0003-4819-128-8-199804150-00010. [DOI] [PubMed] [Google Scholar]
- 6.Oskenhendler E, Durate M, Soulier J, et al. multicetric castleman's disease in HIV infection; a clinical and pathological study of 20 patients. AIDS. 1996;10:61–7. [PubMed] [Google Scholar]
- 7.Peterson BA, Frizzera G. multicentric castleman's disease, sem n oncol. 1993;20:636–47. [PubMed] [Google Scholar]
- 8.Rose C, Mahieu M, Hachullla E, et al. Le POEMS syndrome. Rev med interne. 1997;18:553–62. doi: 10.1016/s0248-8663(97)80807-7. [DOI] [PubMed] [Google Scholar]
- 9.Zietz C, Bogner JR, Goebel FD, Lohrs U. An unusual cluster of cases of Castleman's disease during highly active antiretroviral therapy for AIDS. N Engl J Med. 1999;340(24):1923–4. doi: 10.1056/NEJM199906173402415. [DOI] [PubMed] [Google Scholar]
- 10.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99(7):2331–6. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 11.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406–1. [PubMed] [Google Scholar]
- 12.Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95(1):56–61. [PubMed] [Google Scholar]
- 13.Meador TL, McLarney JK. CT Features of Castleman Disease of the Abdomen and Pelvis. AJR. 2000;175:115–118. doi: 10.2214/ajr.175.1.1750115. [DOI] [PubMed] [Google Scholar]