Abstract
Cerebrotendinous xanthomatosis (CTX), also known as Van Bogaert-Scherer-Epstein disease is a rare autosomal recessive genetic disorder of the lipid metabolism. To date, there are less than 300 cases reported worldwide. We present a case of a 30 year old male who presented with mental retardation and swelling of ankles, with the a spectrum of CTX imaging findings. Imaging studies were performed which included plain X-ray, Ultrasound(US) and Magnetic Resonance Imaging(MRI) of both the brain and ankles. These pointed towards the diagnosis of CTX with the entire spectrum of findings which was confirmed with biopsy and laboratory findings. CTX is a potentially treatable condition with replacement therapy, and hence early diagnosis before neurological deterioration is important. This is aided by the imaging findings which are conclusive forte diagnosis of CTX.
Keywords: Cerebrotendinous xanthomatosis, CTX, Cholestanol, CYP27A1, Chenodeoxycholic acid
CASE REPORT
A 30 year old male presented to our surgical outpatient department with complaints of bilateral ankle swelling. As the patient also had late onset mental retardation, he was referred to our department for a MRI study of the brain. In addition, his past history included juvenile cataract which was operated on 10 years previously, recurrent diarrhoea from childhood, ataxia and recurrent jaundice.
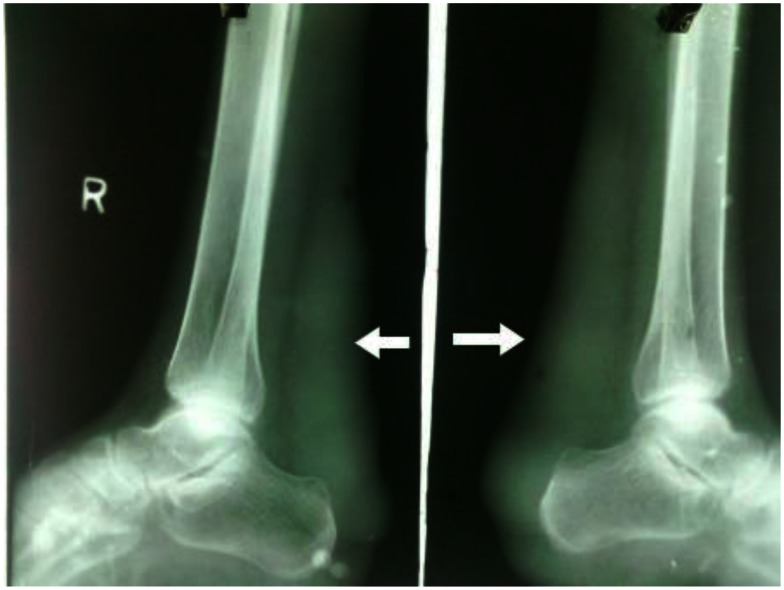
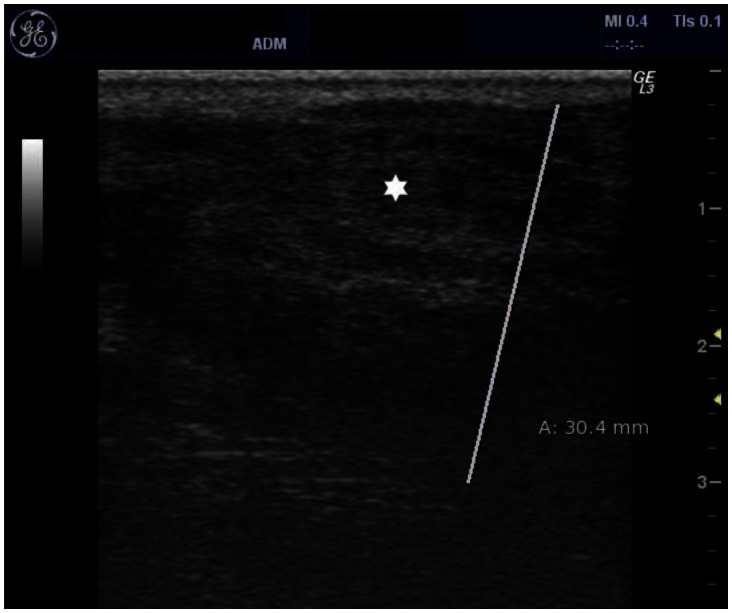
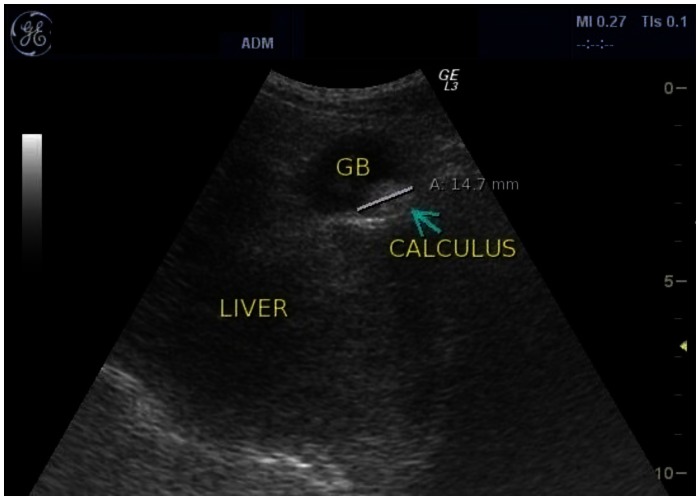
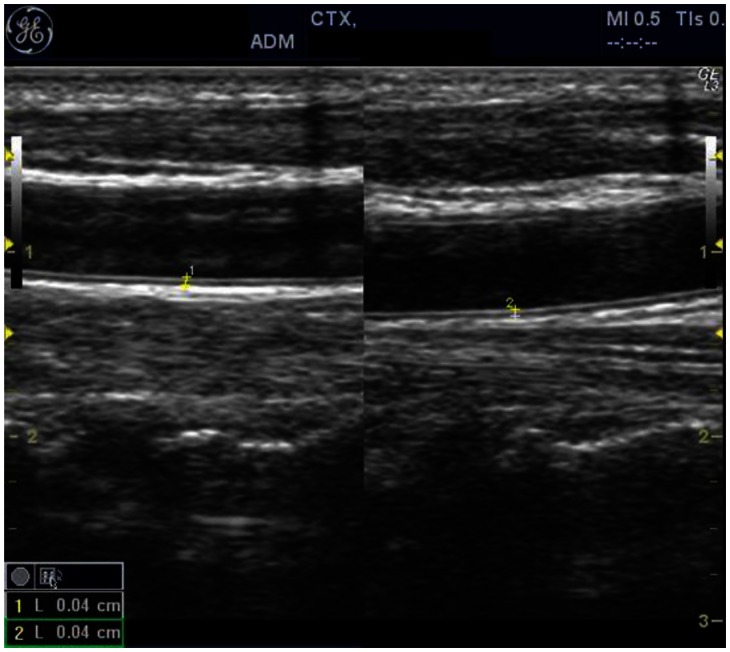
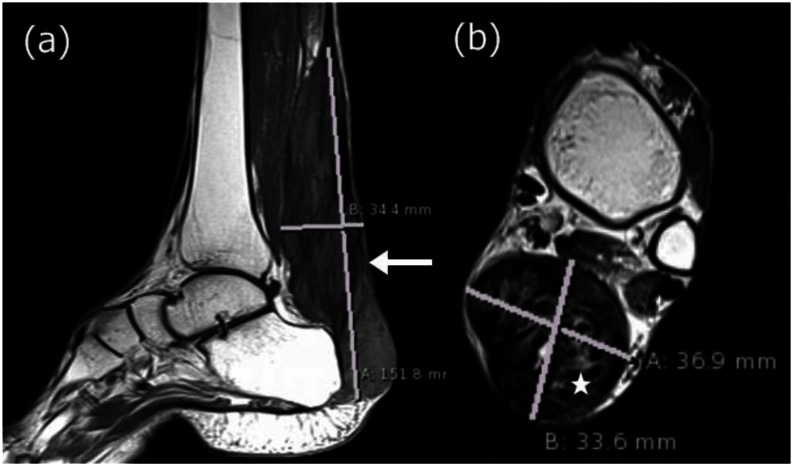
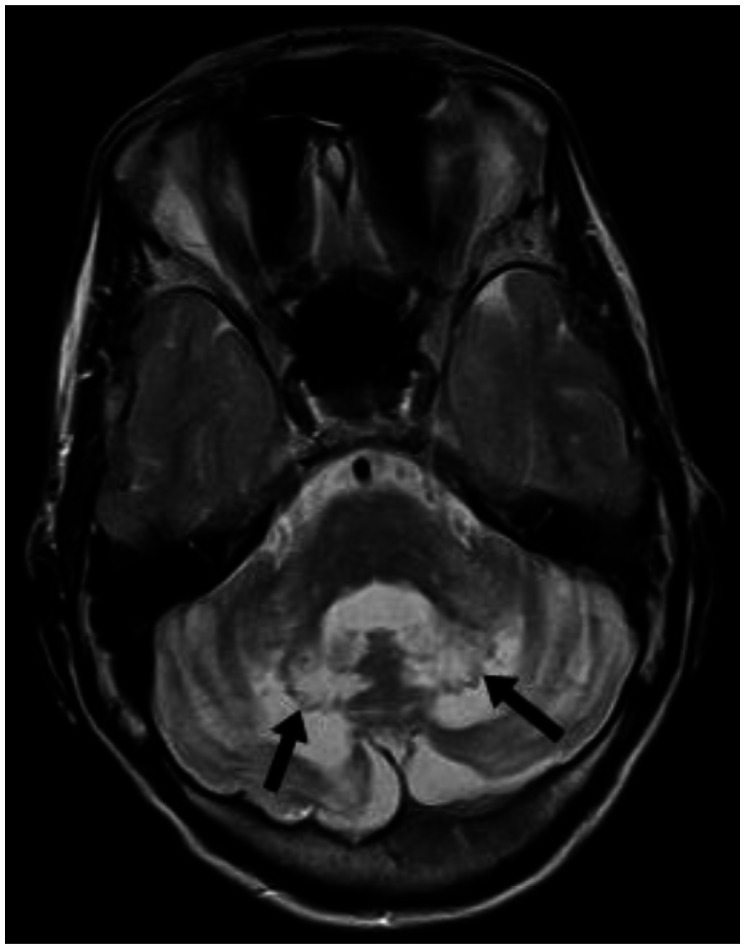
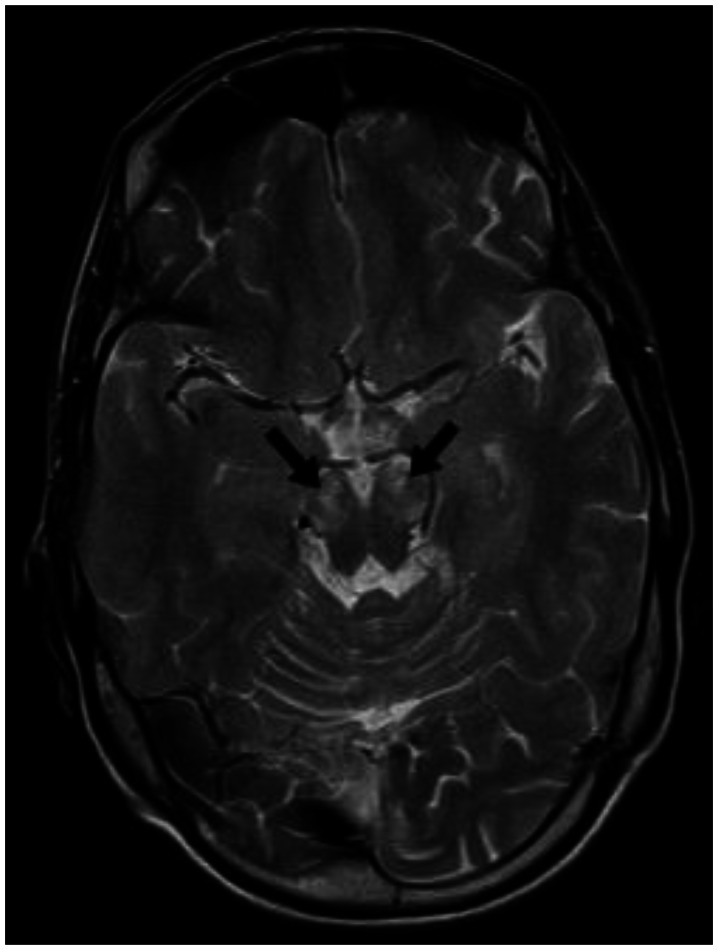
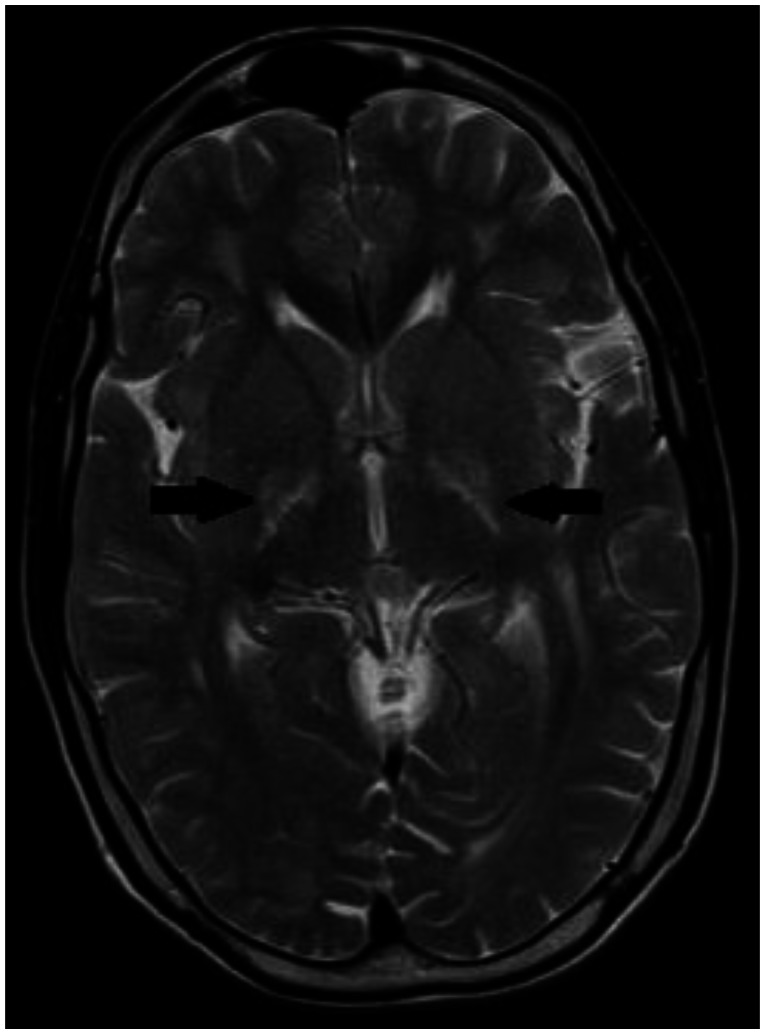
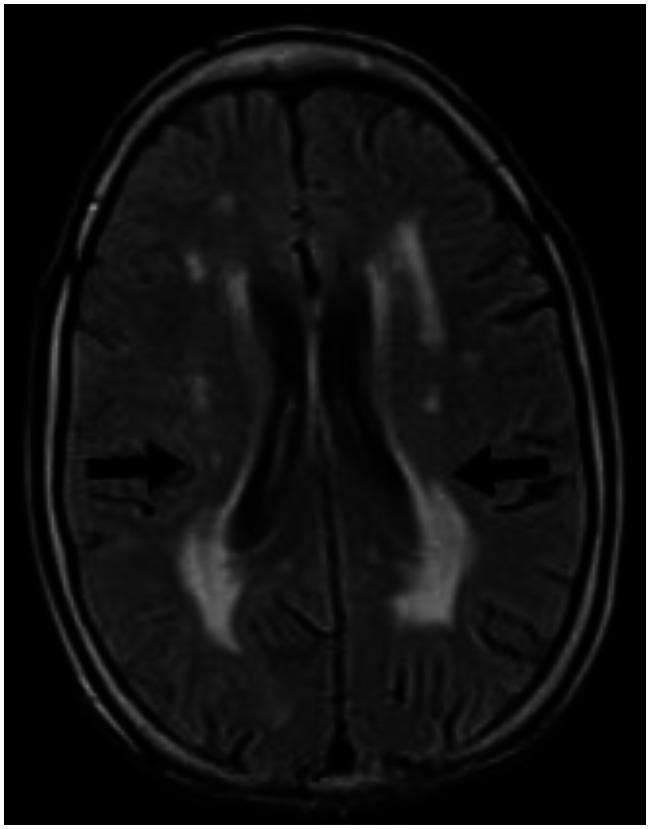
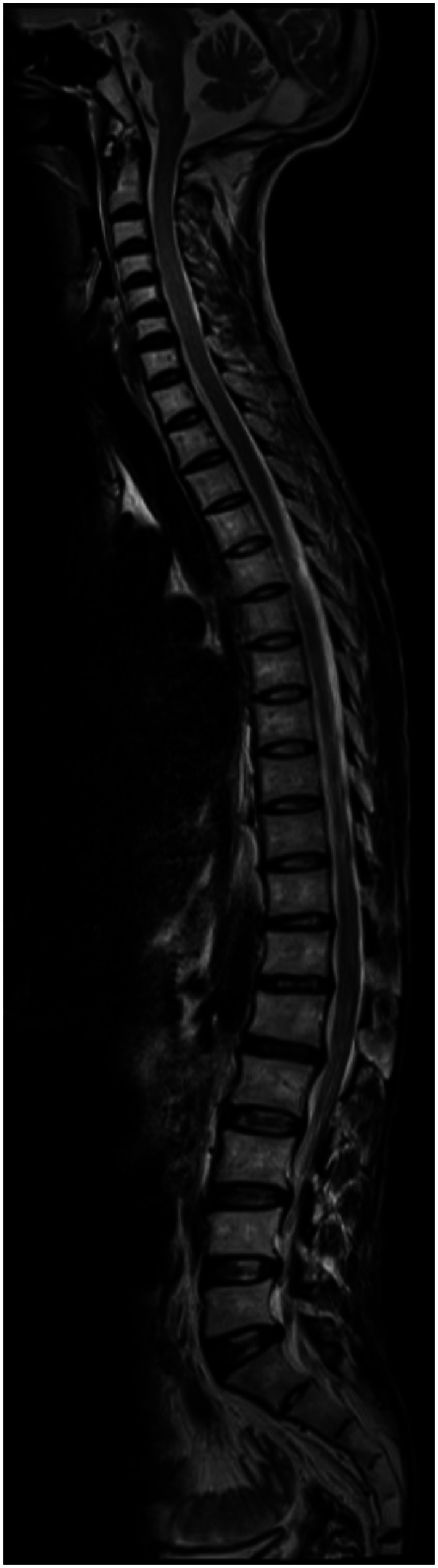
With the presenting complaint being bilateral ankle swelling, x-ray examination was performed which revealed a soft tissue opacity in the region of the Achilles tendon with normal bones (Figure 1). An US of the ankle swelling revealed a well defined, homogenous, hypoechoic mass completely replacing the Achilles tendon (Figure 2). US of the abdomen revealed cholelithiasis with a soft calculus seen in the GB lumen (Figure 3). The liver appeared normal and homogenous in echotexture. The rest of the abdominal viscera were normal on US. US of the carotid arteries revealed a normal intimo-medial thickness (IMT) without any evidence of atherosclerosis (Figure 4). MRI imaging of the brain and ankles was performed with a 1.5 T SIEMENS MAGNETOM AVANTO 8 CH system. MRI of the ankle showed a large T1 and T2 hypointense mass occupying the entire Achilles tendon (Figure 5). T2W and FLAIR images of the brain showed areas of high signal intensity in the cerebellum, predominantly in the bilateral Dentate nucleus and the cerebellar white matter which were hypointense on T1W images (Figure 6). There were also T2W and FLAIR hyperintense lesions seen in the bilateral substantia nigra, bilateral globuspallidus and the posterior limb of the internal capsule (Figures 7 and 8). There were periventricular white matter hyperintensities also seen in the T2W and FLAIR images (Figure 9). There was generalised atrophy of the brain. Screening of the spine was also performed which revealed no abnormality in our patient (Figure 10).
Figure 1.
30 year old male with bilateral Achilles tendon xanthomas. A plain radiograph of bilateral ankles (45 kV and 15 mAs, without grid, film-screen cassette) showing soft tissue radiodense opacity in the region of the Achilles tendon depicted with solid arrows. The visualized bones and joints in the x-ray appear normal. There is no evidence of any bony erosion seen in the calcaneum.
Figure 2.
30 year old male with bilateral Achilles tendon xanthomas. US image of Achilles tendon using a 5–12 MHz high frequency probe showing diffusely enlarged Achilles tendon showing smooth hypoechoic infiltration marked with an asterisk. The Achilles tendon is 3.0 cm thick.
Figure 3.
30 year old male with CTX and cholelithiasis. US using a 3–5 MHz convex probe showing an echogenic calculus with mild posterior acoustic shadowing in the GB lumen of the same patient. The calculus measures 1.4 cm in size. The liver appears normal in size and is homogenous in echotexture.
Figure 4.
30 year old male with CTX with normal intimo-medial thickness (IMT) of the bilateral carotid arteries. US using a 5–12 MHz linear probe was done that revealed an IMT of 0.04 cm on both carotid arteries. The IMT is measured by placing the callipers from the inner border of the echogenic intima to the outer border of the hypoechoic media. The intima of the carotid arteries appear smooth and there is no evidence of any intraluminal plaque is seen.
Figure 5.
30 year old male with bilateral Achilles tendon xanthomas. (a) Saggital T1W MRI (TR/TE - 400/10 ms, 3 mm slice thickness, 320×320 matrix) shows diffuse low intensity infiltration of the Achilles tendon which appears enlarged and is marked with a solid arrow. (b) Axial T2W MRI (TR/TE - 4380/84 ms, 4 mm slice thickness, 312×384 matrix) shows diffuse low intensity infiltration of the Achilles tendon with a few isointense areas marked with an asterisk. The xanthoma measures 15 cm in length, 3.4 cm in anteroposterior diameter and 3.7 cm in transverse dimension. The overall lesion is well defined with smooth margins and no infiltration into the adjacent soft tissue.
Figure 6.
30 year old male with cerebrotendinous xanthomatosis. Axial T2W MRI (TR/TE - 3800/91 ms, 5 mm slice thickness, 384×288 matrix) of the brain shows bilateral hyperintense lesions involving the dentate nuclei and the deep cerebellar white matter shown with arrows. The cerebellar cortex and the vermis appear normal in morphology and signal intensity. The brain stem appears normal in signal intensity.
Figure 7.
30 year old male with cerebrotendinous xanthomatosis. Axial T2W MRI (TR/TE - 3800/91 ms, 5 mm slice thickness, 384×288 matrix) of the brain shows hyperintense lesions in bilateral substantia nigra. The rest of the mid brain shows normal signal intensity. The bilateral temporal lobes are also seen which appear normal.
Figure 8.
30 year old male with cerebrotendinous xanthomatosis. Axial T2W MRI (TR/TE - 3800/91 ms, 5 mm slice thickness, 384×288 matrix) of the brain shows hyperintense lesions in the posterior limb of internal capsule. The head of caudate nucleus and putamen do not show any signal abnormality. Bilateral thalami appear normal. Both the lateral ventricles appear normal.
Figure 9.
30 year old male with cerebrotendinous xanthomatosis. Axial FLAIR MRI (TR/TE/TI - 7000/110/2000 ms, 5 mm slice thickness, 256×192 matrix) of the brain shows nonspecific hyperintense lesions in the periventricular white matter of the cerebrum. The cortical cerebral parenchyma appear normal. There is no evidence of any cerebral atrophy seen.
Figure 10.
30 year old male with cerebrotendinous xanthomatosis. Sagittal T2W MRI (TR/TE - 4000/113 ms, 4 mm slice thickness, 735×324 matrix) of the spine revealed no abnormality. Mild disc desiccation and few annular disc bulges are seen in the lower dorsal and upper lumbar discs. There are cases reported on literature with T2 hyperintense lesions in the cord.
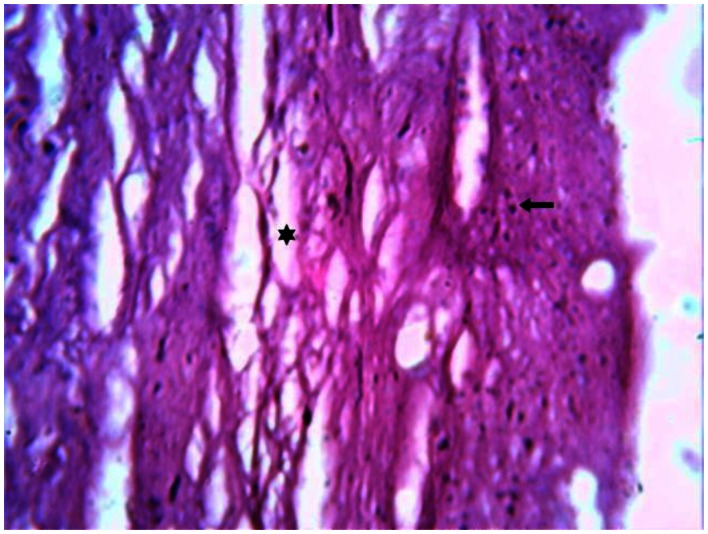
Laboratory investigations revealed mildly elevated total serum cholesterol. Other blood parameters were within normal limits with the exception of mild anaemia. A trucut biopsy of the Achilles tendon swelling was performed and histopathological examination revealed degenerated fibrocollagenous tissue interspersed with adipose cells and foam cells. The presence of Touton giant cells is a characteristic finding (Figure 11). The characteristic imaging findings together with histopathology confirms the diagnosis of Cerebrotendinous xanthomatosis. Though ursodeoxycholic acid & HMG-CoA reductase inhibitors were started, the patient showed no improvement in his neurological signs including ataxia and mental retardation on follow-up over six months. There was no history of diarrhoea or jaundice during the follow-up period.
Figure 11.
30 year old male with cerebrotendinous xanthomatosis. H-E stained section of the biopsy specimen (magnification 50x) from the Achilles tendon shows degenerated fibrocollagenous tissue marked with an asterisk interspersed with adipose cells and foam cells shown with a solid arrow. The presence of Touton giant cells is a characteristic finding.
DISCUSSION
CTX is a very rare genetic disorder, with only close to 300 cases reported worldwide[1]. It is also known as Van Bogaert-Scherer-Epstein disease[2]. CTX is autosomal recessive in inheritance pattern with a chronic course. It is characterized by accumulation of cholestanol and cholesterol in various tissues, predominantly within the central nervous system, tendons, liver, lung and kidneys[4]. The predominant clinical features of the disease are chronic diarrhoea, juvenile cataract, tendon xanthomas, and progression into cerebellar ataxia and mental retardation[5]. Early diagnosis is very important, as patients benefit from replacement therapy of chenodeoxycholic acid[3,6].
In patients with CTX, bile acid synthesis is abnormal because of a defect in the activity of the hepatic mitochondrial enzyme sterol 27-hydroxylase due to mutations in the CYP27A1 gene[4,7]. This enzyme works in the pathway that breaks down cholesterol to form chenodeoxycholic acid, a bile acid. As a result, there is accumulation of cholesterol and its by-product, cholestanol, which accumulates in tendons, nerve cells, other viscera and blood [7]. There is accumulation of sterols in the nerve cells along with a varying degree of demyelination [2,8]. Defective bile acid and bile salt production leads to increased incidence of cholelithiasis. CTX is associated with premature atherosclerosis, which is related to cholestanol accumulation in the vascular subendothelial space [8].
The pathophysiology of CTX has been well elucidated and treatment options are available. Replacement therapy with chenodeoxycholic acid has shown to cause negative feedback on cholestanol synthesis [3,9]. Also, HMG-CoA reductase inhibitors have been shown to be useful. Though somatic symptoms subside, early diagnosis and treatment is of utmost importance as there is a poor response of psychomotor symptoms to treatment once developed.
In our case, the patient is a 30 year old male, who presented with bilateral ankle swelling and mental retardation. Our patient also had most of the classic clinical features such as chronic childhood diarrhoea, juvenile cataracts and cerebellar ataxia [5]. Screening of the spine revealed no abnormality in our patient, though there are cases reported in the literature with T2 hyperintense lesions in the cord [11]. T2W hyperintensities in the Dentate nucleus, substantia nigra, and globuspallidus are due to the accumulation of sterols in the nerve cells along with varying degrees of demyelination. Changes on MRI include diffuse brain and cerebellar atrophy, white matter signal alterations, and bilateral focal cerebellar lesions. MR spectroscopy shows decreased n-acetylaspartate and increased lactate, indicative of widespread brain mitochondrial dysfunction [1].
Differential diagnosis for CTX includes disorders associated with xanthomas. Sitosterolemia is an inherited sterol storage disease characterized by tendon xanthomas and by a strong predisposition to premature atherosclerosis. Serum concentration of plant sterols (sitosterol and campesterol) is increased. Primary neurologic signs and cataracts are not present. Spastic paraparesis may occur as a result of spinal cord compression by multiple intradural, extramedullary xanthomas which are low intensity on T1W and T2W images. Hypercholesterolemia and hyperlipemia (especially type IIa), also present with xanthomas, but the plasma cholestanol level is normal. On imaging, there is no brain involvement.
Clinically, CTX resembles Marinesco-Sjogren syndrome, an autosomal recessive disorder characterized by the triad of cerebellar ataxia, congenital cataract, and mental retardation. Skeletal involvement on radiography includes scoliosis, shortening of the metacarpals, metatarsals and phalanges, coxa valga, pes planovalgus and pectus carinatum. On MRI, there is T2-hyperintensity in the cerebellar cortex, cerebellar atrophy predominantly involving the vermis and muscle tissue replacement with fat and connective tissue. The presence of tendon xanthomas helps differentiate CTX from this condition.
Myotonic dystrophy type I comprises the largest group of individuals with early-onset cataract and known neurologic disease followed by CTX [12]. There is diffuse cerebral and cerebellar T2 hyperintensity with hypoplasia of corpus callosum seen in Myotonic dystrophy. Mild myopathic changes and grouping of atrophic fast fibres are seen on histopathology.
Conservative management with ursodeoxycholic acid and HMG-CoA reductase inhibitors was started for this patient. The typical clinical history and imaging findings along with abnormal laboratory values are important in diagnosing this rare genetic disorder where institution of early treatment is necessary [7,13].
TEACHING POINT
Cerebrotendinous xanthomatosis or CTX is a rare genetic disorder. It is potentially treatable with replacement therapy and hence early diagnosis before neurological deterioration is important. The imaging findings are T2W hyperintensities in the Dentate nucleus, substantia nigra, globus pallidus and the presence of tendon xanthomas which are classic and should not be missed.
Table 1.
Summary table for Cerebrotendinous Xanthomatosis
| Etiology | Autosomal recessive genetic disorder |
| Incidence | Rare, 300 cases reported worldwide |
| Gender ratio | M:F–1:1 |
| Age predilection | 10–20 years |
| Risk factors | Genetic |
| Genetic defect | Mutations in the CYP27A1 gene |
| Enzyme defect | Hepatic mitochondrial enzyme sterol 27-hydroxylase |
| Pathophysiology | Accumulation of cholesterol and its by-product, cholestanol |
| Treatment | Replacement therapy with chenodeoxycholic acid |
| Prognosis | Good with early replacement therapy before neurological impairment |
| Prognosis if not diagnosed early | Progressive incapacitation and mental deterioration with pseudo bulbar palsies. Vascular abnormalities such as premature atherosclerosis can lead to stroke and myocardial infarction. |
| Imaging findings | T2W hyperintense lesions in the dentate nucleus, substantia nigra, globus pallidus and the presence of tendon xanthomas |
Table 2.
Differential diagnosis table for Cerebrotendinous Xanthomatosis
| DD | Clinical Features | X–ray | US | MRI | HPE/Serology |
|---|---|---|---|---|---|
| Cerebrotendinous xanthomatosis | chronic diarrhoea, juvenile cataract, tendon xanthomas, progressing into cerebellar ataxia and mental retardation | Soft tissue opacity in the region of the Achilles tendon | Smooth hypoechoic infiltration of the Achilles tendon | Diffuse low intensity infiltration of the Achilles tendon in both T1W & T2W images | Biopsy specimen from Achilles tendon shows degenerated fibrocollagenous tissue |
| Calculus in the gall bladder lumen | T2W hyperintense lesions in the dentate nucleus, substantia nigra, globus pallidus and periventricular white matter | intercepted by adipose cells, foam cells and Touton giant cells. Elevated serum cholestanol | |||
| Xanthoma | Swelling, restricted motion at joints | Soft tissue opacity in the region of the tendon involved | Smooth hypoechoic infiltration of the tendon | Diffuse low intensity infiltration of the tendon in both T1W & T2W images with varying degrees of increased intensity | Predominant adipose and foam cells |
| Myotonic dystrophy type I | Early onset cataract, mental retardation, high- stepping gait, muscle wasting and weakness | Not applicable | Not applicable | Diffuse cerebral and cerebellar T2 hyperintensity with hypoplasia of corpus callosum | Mild myopathic changes and grouping of atrophic fast fibres |
| Marinesco- Sjogren syndrome | Cerebellar ataxia, congenital cataract, mental retardation | Scoliosis; shortening of metacarpals, metatarsals and phalanges; coxa valga; pes planovalgus; and pectus carinatum | Not applicable | T2-hyperintensity in the cerebellar cortex, cerebellar atrophy predominantly involving the vermis, muscle tissue replacement with fat and connective tissue | atrophic fibers, fatty replacement, and rimmed vacuole formation in muscle fibres |
| Sitosterolemia | Tendon xanthomas, spastic paraparesis, primary neurologic signs and cataracts are not present | Not applicable | Not applicable | spinal cord compression by multiple intradural, extramedullary xanthomas which are low intensity on T1W and T2WI | Elevated serum sitosterol and campesterol |
ABBREVIATIONS
- CTX
Cerebrotendinous xanthomatosis
- HMG-CoA
3-hydroxy, 3-methyl, glutaryl CoA
- IMT
Intimo-medial thickness
- MRI
Magnetic resonance Imaging
- T1WI
T1 weighted image
- T2WI
T2 weighted image
- US
Ultrasound
REFERENCES
- 1.Barkhof F, Verrips A, Wesseling P, et al. Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology. 2000;217:869–876. doi: 10.1148/radiology.217.3.r00dc03869. [DOI] [PubMed] [Google Scholar]
- 2.Dotti MT, Federico A, Signorini E, et al. Cerebrotendinous xanthomatosis (van Bogaert-Scherer-Epstein disease): CT and MR ?ndings. AJNR Am J Neuroradiol. 1994;15:1721–1726. [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee G, Chakraborty I, Das TK, Datta C. Cerebrotendinous xanthomatosis in a family. Indian J Dermatol. 1999;44(3):153–6. [Google Scholar]
- 4.Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984;311:1649–52. doi: 10.1056/NEJM198412273112601. [DOI] [PubMed] [Google Scholar]
- 5.Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci. 2006;27:143–9. doi: 10.1007/s10072-006-0618-7. [DOI] [PubMed] [Google Scholar]
- 6.Moghadasian MH. Cerebrotendinous xanthomatosis: clinical course, genotypes and metabolic backgrounds. Clin Invest Med. 2004;27:42–50. [PubMed] [Google Scholar]
- 7.Cruysberg JR, Wevers RA, Tolboom JJ. Juvenile cataract associated with chronic diarrhea in pediatriccerebrotendinous xanthomatosis. Am J Ophthal. 1991;112 doi: 10.1016/s0002-9394(14)76874-6. [DOI] [PubMed] [Google Scholar]
- 8.Dotti MT, Lütjohann D, von Bergmann K, Federico A. Normalisation of serum cholestanol concentration in a patient with cerebrotendinous xanthomatosis by combined treatment with chenodeoxycholic acid, simvastatin and LDL apheresis. Neurol Sci. 2004;25:185–91. doi: 10.1007/s10072-004-0320-6. [DOI] [PubMed] [Google Scholar]
- 9.Kuriyama M, Tokimura Y, Fujiyama J, Utatsu Y, Osame M. Treatment of cerebrotendinous xanthomatosis: effects of chenodeoxycholic acid, pravastatin, and combined use. J Neurol Sci. 1994;125:22–8. doi: 10.1016/0022-510x(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 10.Berginer VM, Berginer J, Korczyn AD, Tadmor R. Magnetic resonance imaging in cerebrotendinous xanthomatosis: aprospective clinical and neuroradiological study. J NeurolSci. 1994;122:102–108. doi: 10.1016/0022-510x(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 11.Verrips A, Nijeholt GJ, Barkhof F, et al. Spinal xanthomatosis: a variant of cerebrotendinous xanthomatosis. Brain. 1999;122:1589–1595. doi: 10.1093/brain/122.8.1589. [DOI] [PubMed] [Google Scholar]
- 12.Federico A, Dotti MT. Cerebrotendinous xanthomatosis: clinical manifestations, diagnostic criteria, pathogenesis, and therapy. J Child Neurol. 2003 Sep;18(9):633–8. doi: 10.1177/08830738030180091001. [DOI] [PubMed] [Google Scholar]
- 13.Salen G, Berginer V, Shore V, et al. Increased concentrations of cholestanol and apolipoprotein B in the cerebrospinal ?uid of patients with cerebrotendinous xanthomatosis: effect of chenodeoxycholic acid. N Engl J Med. 1987 May 14;316(20):1233–8. doi: 10.1056/NEJM198705143162002. [DOI] [PubMed] [Google Scholar]