Abstract
To determine the origin of peripheral blood mononulclear cells (PBMC) that activate regulatory T cells in anterior chamber-associated immune deviation (ACAID), fluorescein-labeled PBMC were intravenously injected into mice before the mice received an intracameral injection of antigen. Six-24 hr after intracameral injection, fluorescein-labeled PBMC increased in the iris. Twenty-four-48 hr labeled cells decreased in the iris and increased in the thymus and spleen. The entry of the labeled PBMC into the anterior chamber and subsequent production of PBMC that transfer ACAID required the expression of CCR2 by the PBMC and the production of the chemokine CCL2 by the recipient of the PBMC. The results suggest that the intracameral injection of antigen induces i) the infiltration of F4/80+ PBMC into the AC, ii) where these PBMC are converted to a regulatory phenotype, and iii) recirculate to activate T cells that suppress cell-mediated immunity.
Keywords: ACAID, monocytes, migration, chemokines, immunoregulation
Introduction
The “immune privilege” of the eye is conferred by cellular and environmental factors that limit the ability of innate and adaptive immune mechanisms to impose “collateral damage” on sensitive ocular tissue.1–4 Additionally, the injection of antigen into the anterior chamber induces a systemic suppression of both cell mediated immunity and the antigen-induced production of IgG2 (complement-fixing) antibodies.1–3 F4/80+ peripheral blood mononuclear cells (PBMC) and iris cells recovered from mice receiving the intracameral injection of antigen (AC-PBMC) i) induce anterior chamber-associated immune deviation (ACAID);1,2,4–10 ii) home to the thymus8 and the spleen5,6,10 where regulatory T cells that induce or effect the antigen-specific suppression of an immune response are generated.
Circulating, ACAID-inducing F4/80+ cells are thought to be derived from the iris and ciliary body.1,2,4,5 This is based on observations that F4/80+ iris cells recovered from mice that received an intracameral injection of antigen confer an immunosuppressive phenotype on systemic F4/80+ cells in vitro.5,9 Moreover, treatment of systemic F4/80+ cells with TGF-β and antigen or aqeuous humor in vitro induces an immunosuppressive phenotype similar to PBMC recovered from mice that received an intracameral injection of antigen.11,12 However, the outward passage of resident iris/ciliary body F4/80+ cells to the circulation has been questioned.13–15 Accordingly, the “delivery” of intracameral antigen to the periphery and the origin of the ACAID-inducing PBMC is not clear. Therefore, detailed information about the events that occur in the anterior chamber immediately post intracameral injection could provide insight into the nature of the generation of the ACAID-inducing “signal” that in turn influences systemic immunity. Because the egress of iris-resident F4/80+ cells has not been demonstrated, we reasoned that following an intracameral injection of antigen, there is an influx of circulating F4/80+ cells into the anterior chamber in response to the injection. These infiltrated cells would be exposed to TGF-β and to iris/ciliary body cells that provide antigen. Conceivably, the F4/80+ cells that infiltrated the anterior chamber would then reenter the circulation and home to the thymus and spleen where they interact with and induce regulatory T cells.
Here we demonstrate that an intracameral injection of antigen induces a CCR2-dependant entry of circulating F4/80+ cells into the anterior chamber that associate with the iris. These cells then re-enter the circulation and home to the thymus and spleen. The induction of the suppression of delayed-type hypersensitivity to the intracameral antigen by circulating monocytes recovered from mice receiving an intracameral injection of antigen is dependent on the influx of circulating F4/80+ cells to the anterior chamber and subsequent recirculation to the thymus and spleen. Therefore, we conclude that ACAID is a consequence of a response to the injection of antigen into the anterior chamber that utilizes environmental elements of ocular immune privilege to activate systemic immunoregulatory cells.
Matrials and Methods
Mice
Female BALB/c, and C57Bl/6 mice 6–8 wks old were purchased from Charles River Labor atories, Jackson, MA., Harlan Laboratories, Frederick, Maryland or Jackson Laboratories, Bar Harbor, Me. CCR2 −/− (B6.129S4-Ccr2tm1Ifc/J), CCR5 −/− (B6.129P2- Ccr5tm1Kuz/J) MCP-1(CCL2)−/− (B6.129S4- Ccl2tm1Rol/J) mice were purchased from Jackson Laboratories. The mice were maintained in the Center for Laboratory Animal Care of the University of Connecticut Health Center. All work with animals was approved previously by the University of Connecticut Health Center Animal Care Committee (ACC2007-369). All animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Antigens
2,4,6 trinitrobenzene sulphonic acid (TNP), bovine serum albumin (BSA) and ovalbumin (OVA) were purchased from the Sigma Chemical Company, (St Louis MO). Trinitrophenylated BSA (TNP-BSA) was prepared as described.7 Picryl chloride (PCl), an analogue for TNP, 2-chloro 1.3.5-trinitrobenzene was purchased as 2-chloro-5-triphtane from Chemical Alta Ltd (Edmonton, Alberta, Canada).
Immunization, elicitation and measurement of Delayed- Type Hypersensitivity (DTH)
Mice were sensitized by the subcutaneous (sc) injection of 200 μg TNP-BSA or ovalbumin (OVA) in 100 μl 1:1 phosphate-buffered saline (PBS, 7.2) and Complete Freund’s Adjuvant (CFA, Sigma, St Louis MO). DTH was measured 7 days after the mice were immunized. Sensitized or naïve Mice were anesthetized with ketamine/xylazine (please see anterior chamber injection for dose) and footpad thickness was measured in triplicate with a digital engineer’s micrometer (Mitatoyo, Tokyo, Japan) before the footpads were challenged with antigen or vehicle. TNP-BSA-sensitized or naïve mice receive an epicutaneous application of 15 μl 1% picryl chloride (PCl in acetone:olive oil 4:1) to one footpad, vehicle only to the other footpad and the thickness of the footpad receiving PCl and the non- antigen challenged footpad was measured 24 hr later. DTH to OVA was measured by the intradermal (id) injection of 100 μg OVA in 25 μl PBS. Micrometers of swelling were determined by computing the difference in thickness between the challenged and vehicle- challenged footpad before and after challenge.
Preparation of cells
Spleen cells, thymus cells
Mice were euthanized by cervical dislocation. Spleens and thymi are removed from the mice, pooled, diced and expressed through a 70-μm nylon mesh (BD Falcon, Bedford, MA, USA) with a 1-ml syringe plunger into RPMI-1640 medium (4 °C, Invitrogen, Carlsbad, CA). The cells were washed 2–3X and suspended in PBS. The cell suspension was washed 3X with PBS and centrifuged at 200Xg for 6–8 min. The cell pellet was dispersed with PharmLyse (BD Biosciences, San Jose, CA, USA) to lyse the erythrocytes according to the manufacturer’s protocol. The cells were then washed twice with PBS and re-suspended in PBS. Cells were counted with an Invtirogen automated cell counter.
Peripheral Blood Mononuclear Cells (PBMC)
Mice were bled via cardiac puncture with a Kendall Monojet Safety 1 mL syringe (Tyco Health Care, Mansfield, MA). Approximately 6–7 ml blood in a heparinized green top tube was collected. The blood was diluted 1:1 with PBS and 6 ml blood mixture layered on 4 ml Histopaque (Sigma, St Louis Mo). 24 °C) in two 15 ml tube. The cells were centrifuged (700Xg, 17 mins, 24 °C) no acceleration, no brake. The cells were washed 2X with PBS. @1300 rpm, 5 mins. The cells were resuspended in PBS.
Iris cells
Irides from at least 5 mice were recovered, incubated in collagenase/dispase, triturated and washed as described.7
Labeling of PBMC with Carboxyfluorescein Succinimidyl Ester (CFSE)
Two μl of 5 mM CFSE CellTrace™ CFSE Cell Proliferation Kit (Invitrogen) stock solution prepared according to the manufactures protocol was added to 1 ml PBS, 1% BSA (Sigma) containing 1–5 × 106 PBMC. This was held at 37 °C. for 10 min. The cells were then chilled on ice and washed 3X with PBS. CFSE-labeled cells were injected iv in 100 μl PBS.
Flow cytometry
Cells were incubated in staining buffer PBS, 1% fetal calf serum, 0.1% sodium azide with 0.5 mg/ml anti- CD16, CD32 blocking antibodies (Becton Dickinson) for 10 min at 4 °C. and then incubated for 30 min with 5–10 μl/1 × 106 cells phycoerythrin-labeled rat IgG1 isotype control or anti-F4/80 antibodies (Becton Dickinson Bioscience clone 4801). The cells were washed 3X with chilled PBS. The cells were analyzed by flow cytometry using a FACSCalibur Flow Cytometer and analyzed by CellQuest software (BD Biosciences). A total of at least 10,000 cells were acquired for each sample.
Injection of antigen into the Anterior Chamber (AC)
Naïve mice were anesthetized by intraperitoneal (ip) injection of ketamine (75 mg/kg)/xylazine (15 mg/kg). Under a dissecting microscope 3 μl PBS containing 4 μg TNP-BSA or 50 μg OVA was injected into the anterior chamber (AC) with manually-controlled microinjection with a 33 g needle on tubing attached to a Hamilton syringe (Stoelting Co, Woodale, Ill.). The mice recovered approximately 30 min after injected and exhibited no distress, ate and drank normally.
Polymerase Chain Reaction (PCR)
The irides of C57BL/6 mice were dissected at 16 hours post AC injection and stabilized in RNAlater™ Qiagen, (Valencia, CA). Total RNA was isolated from the cells using RNeasy plus mini kits (Qiagen), as described by the manufacturer. RNA (3 μg) was reverse-transcribed to cDNA using Super ScriptII (Invitrogen) and amplified using Platinum Taq DNA polymerase ( Invtrogen) and gene-specific primers. All PCR products were resolved in 1.5% agarose gels and visualized using ethedium bromide staining. Primers were designed using the NCBI Primer Blast software. Beta actin PCR was done using an initial 94 °C, 2 min denaturation followed by 30 cycles of 94 °C, 30 sec denaturation, 57 °C, 60 sec annealing step and 73 °C, 60 sec of extension followed by a final extension of annealing, 73 °C. 3 mins. CCR2 and CCR5 PCR conditions comprised an intitial denaturation step of 2 minutes at 94 °C. followed by 35 cycles of 94 °C, 30 sec, 56 °C. 30 sec and 72 °C. 30 sec. A 2 minute final extension was at 72 °C.
Primers
CCR5-F: ACACCCTGTTTCGCTGTAGG,
CCR5-R: GTTCTCCTGTGGATCGGGTA,
CCR2-F: TACGATGATGGTGAGCCTTG,
CCR2-R: CCTACAGCGAAACAGGGTGT,
Beta-actin-F: TCCACACCCGCCACCAGTTCGC CAT,
Beta-actin-R: TCCTCAGGGGCCACACGCAGCT CAT.
Statistics
Statistical significance was calculated by the one-way analysis of variance or Student’s t test. P values were determined by the Students-Neuman-Keuls test for the analysis of variance or by the t-test. P values < 0.05 were considered significant.
Results
F4/80+ PBMC migrate to the iris, thymus, and spleen after intracameral injection
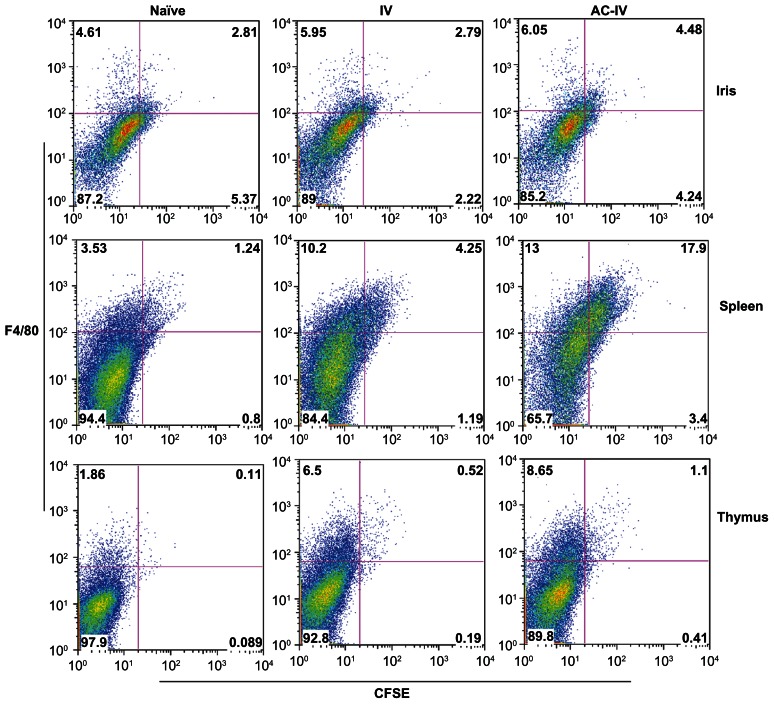
F4/80+ cells that transfer the suppression of DTH when injected into naïve or immunized mice have been recovered from the irides of mice that received an intracameral injection of antigen.4,6,7,9 To test our hypothesis that intracameral injection recruits F4/80+ PBMC to the anterior chamber, naïve mice received iv PBMC labeled with CFSE. These introduced, circulating CFSE-PBMC could be tracked to the iris, thymus and spleen following intracameral injection. Approximately 7.4% of the CFSE-labeled cells had a forward scatter (FSC) consistent with monocytes. The majority of the PBMC labeled well with CFSE and sixteen percent of the CFSE-labeled PBMC were stained by antibodies to F4/80 or CD11b (Fig. 1). Twenty-four hr after an intracameral injection of TNP-BSA, the mice were euthanized and single cell suspensions of irides, spleens and thymi stained with phycoerythrin-anti-F4/80 antibodies and analyzed by flow cytometry to detect and identify infiltrated cells. As shown in Figure 2, in a representative experiment the frequency of CSFE-labeled, F4/80+ cells increased approximately 29% in the iris in mice receiving only CFSE-labeled cells as compared to the background autofluorescent cells in naïve mice. However, CSFE/F4/80+ cells increased more than 60% in the iris in mice receiving an intracameral injection of TNP-BSA as compared to mice receiving iv CSFE-labeled cells but no intracameral injection of TNP-BSA. Most of the increased CFSE-labeled cells were F4/80+low. The frequency of CFSE-labeled, F4/80+ cells also increased in the spleen and thymus approximately 5 and 2-fold respectively in mice that received an intracameral injection of TNP-BSA in comparison to mice receiving iv CFSE-labeled PBMC only. Similar results were obtained when mice received an intracameral injection of antigen 1 or 24 hr after receiving iv CFSE-labeled PBMC.
Figure 1.
CFSE-labeling of PBMC. Blood from BALB/c mice was centrifuged over histopaque and the layer of cells at the buffer/histopaque interface was recovered. The cells were incubated with CFSE as described in Materials and Methods. An aliquot of the cells was stained with PE anti-F4/80 or CD11b.
Figure 2.
PBMC increase in the iris, thymus and spleen after the intracameral injection of antigen. 5 × 106 BALB/c PBMC labeled with CFSE were injected iv into naïve BALB/c mice. Twenty-four hr after the injection of CFSE-labeled cells some mice received an intracameral injection of TNP-BSA. Irides, thymi and spleens were removed 24 hr after the intracameral injection. Cell suspensions were stained with phycoerythrin anti-F4/80 antibody and the cells analyzed by flow cytometry. Cells counted left to right: iris cells:16220,18751,13963; spleen cells: 26858,46423,30500; thymus cells: 32612,42385,36102. Data is representative of 15 experiments.
Using the design shown in Figure 2 we combined the data of 15 experiments to compute the average percentage increase in CFSE-labeled cells relative to that observed in naïve mice or mice receiving CFSE-labeled PBMC only. This normalized the increase in CFSE-labeled, F4/80+ iris, spleen or thymus cells recovered from mice receiving an intracameral injection. Iris-associated, CSFE-labeled F4/80+ cells increased 10%–15% in mice receiving iv CFSE-PBMC cells but no intracameral injection compared to naïve mice. CFSE, F4/80+ cells increased approximately 90% in the spleens of mice receiving iv CFSE-labeled PBMC but did not receive an intracameral injection. In contrast, F4/80+, CFSE-labeled cells increased 180 or 230% in the iris, spleen and thymus respectively after an intracameral injection of antigen. There was no increase in the thymus of CFSE-labeled cells in the absence of an intracameral injection (Fig. 3A). CSFE-labeled, F4/80+ cells increased in the iris approximately 40% 24 hr after an insertion of the 30 g needle into the anterior chamber without an injection of PBS and a 54% increase with an intracameral injection of PBS. However, the intracameral injection of TNP-BSA induced approximately a 200% increase in F4/80+, CFSE+ PBMC in the iris (Fig. 3B). In addition, we observed a significant increase in unlabled, F4/80+ cells in the iris, thymus and spleen 24 hr after the intracameral injection of antigen (Fig. 3C) Although the intracameral injection of antigen induced an increase in CSFE-labeled, F4/80+ cells relative to naïve mice or mice receiving an iv injection of CFSE-labeled PBMC only, we used comparisons with mice receiving an iv injection of CFSE-labeled cells only.
Figure 3.
Quantifying infiltration of the iris, spleen and thymus by circulating F4/80+ PBMC after the intracameral injection of antigen. A) Six hr after 5–6 naïve BALB/c mice/group received an iv injection of 5 × 106 CFSE-labeled BALB/c PBMC, the mice received no injection (IV) or an intracameral injection into both eyes (IV-AC). Twenty-four hr after the intracameral injection of TNP-BSA, irides, thymi and spleens were removed and cell suspensions stained with PE-anti-F4/80. The cell suspensions were analyzed by FACS. The percent increase in F4/80+ CFSE-labeled cells is computed as: the % in CFSE/F4/80+ cells in mice receiving iv CFSE-PMBC but no intracameral injection of antigen/%fluorescent cells in naïve mice (IV) × 100; %CFSE.F4/80+ cells in mice receiving iv CFSE-PBMC and intracameral injection/%CFSE, F4/80+ cells in mice receiving iv CFSE-PBMC only (AC, IV) × 100. Data represents the mean percent increase ± S.E.M. of 9–15 experiments. * = P < 0.01. B) Twenty-four hr after the iv injection of 5 × 106 CFSE-labeled PBMC, 5–6 mice/group received an intracameral injection of TNP-BSA, PBS only or a needle stick without PBS or TNP-BSA. The percent increase in CFSE-labeled, F4/80+ cells is computed as the increase from mice receiving no intracameral injection. Data represents the mean ± S.E.M. of 6 experiments, 5–6 mice/group/experiment. C) Cells recovered from irides, thymi and spleens of mice receiving an intracameral injection (no CFSE-labeled PBMC) were stained with PE anti-F4/80 antibody. The percent of F4/80+ cells is compared to that of mice that did not receive an intracameral injection of OVA or TNP-BSA. Data is the mean percent ± SEM of 4 experiments.
Kinetics of the increase of F4/80+ PBMC in the iris, thymus, and spleen after the intracameral injection of antigen
To determine the duration of the increase in monocytes in the anterior chamber induced by the intracameral injection of antigen and a possible transit of these cells from the eye to the periphery, a time- dependent change in CFSE-labeled F4/80+ PBMC in the iris, thymus and spleen after the intracameral injection of antigen was investigated. Thymi, spleens and irides were recovered from mice receiving CFSE-labeled PBMC 1–168 hr after the intracameral injection of TNP-BSA. As shown in Figure 4, CFSE-labeled, F4/80+ PBMC increased 3-fold in the iris 6 hr after the intracameral injection of antigen. However, 6 hr after the intracameral injection there was an increase in CFSE-labeled cells in the iris and thymus but not the spleen compared to mice that received no intracameral injection. Twenty-four hr after the intracameral injection of antigen there was still a 3-fold increase in CFSE-labeled PBMC recovered from the iris but also a 4-fold increase in CFSE-labeled, F4/80+ cells in the thymus and the spleen. In contrast, CFSE-labeled, F4/80+ cells continued to increase in the thymus 72 hr after the intracameral injection of TNP-BSA. Three-5 days after the intracameral injection of TNP-BSA the number of CFSE-labeled PBMC in the thymus decreased.
Figure 4.
Kinetics of the migration of PBMC to the iris, thymus and spleen after the intracameral injection of antigen. Naïve Balb/c mice were injected iv with CFSE-labeled PBMC. Six or 24 hr after the iv injection of 3–5 × 106 PBMC some mice received an intracameral injection of TNP-BSA. At the noted times irides, thymi and spleens were removed and cell suspensions stained with phycoerythrin anti-F4/80 antibody. The cells were analyzed by flow cytometry. The percent increase of the infiltrated cells was computed as the %CFSE/F480+ cells in mice receiving an intracameral injection/%CFSE/F480+ cells in mice receiving only an iv injection of CFSE-PBMC. The data represents the mean ± S.E.M. of the results of 8 experiments.
Homing of F4/80+ PBMC to the iris after the intracameral injection of antigen is dependent on the chemokine receptor CCR2
To investigate the role of chemokines in the infiltration of PBMC to the anterior chamber after the intracameral injection of antigen, we first investigated the levels of chemokine receptors in the iris. Twenty-four hr after the intracameral injection of TNP-BSA, irides were recovered and RNA from the iris cells and cells from naïve mice were subjected to real-time PCR and probed for mRNA for the chemokine receptors CCR2, CCR5, known to participate in the recruitment of monocytes during inflammation.16 Mice receiving an intracameral injection of OVA showed a significant increase in mRNA for CCR2 and CCR5 (Fig. 5). Flow cytometry of circulating and infiltrated PBMC demonstrated that both populations of cells express CCR5 and CCR2. However, mRNA for CCR6 and CCR7 was not detected (data not shown). These results suggested that either the production of CCR2 and CCR5 was induced in iris cells after the intracameral injection of antigen or, monocytes expressing CCR2 and CCR5 infiltrated the iris.
Figure 5.
Recovery of increased mRNA for CCR2 and CCR5 from the iris after the intracameral injection of antigen. RNA was obtained from 10 irides of C57BL6 naïve mice and 16 hr after mice received an intracameral injection of OVA. RNA was reverse- transcribed using random hexamers. Primers were used to amplify CCR2, CCR5 and β–actin transcripts from cDNA. Serial doubling dilutions of the cDNA were done, left lane = 1:10. The final comparison was done in the linear range of dilutions of template DNA.
PBMC that transfer the suppression of DTH after the intracameral injection of antigen (AC-PBMC) are recovered from CCR5 −/− but not CCR2 −/− mice
A role for CCR5 or CCR2 chemokine receptors in the migration of F4/80+ PBMC to the iris was investigated by labeling PBMC from CCR2 −/− or CCR5 −/− mice with CFSE. These CFSE-PBMC were injected iv into C57BL/6 WT mice. One hr after the iv injection of the CFSE-labeled PBMC, the recipients received an intracameral injection of TNP-BSA. Irides, thymi and spleens were removed 20–24 hr after the intracameral injection of TNP-BSA. Intravenously injected F4/80+, CFSE-labeled PBMC from CCR5 −/− but not CCR2 −/− mice increased in the irides, thymi and spleens of mice that received an intracameral injection of TNP-BSA (Fig. 6). However, CFSE-labeled PBMC were detected in the spleens of wildtype (WT) mice that received an iv injection of CFSE-PBMC recovered from both CCR2 −/− or CCR5 −/− mice, However, there was no increase in the spleen in recipients of CFSE-labeled PBMC from CCR2 −/− mice. These results suggest that the increased migration of F4/80+ PBMC to the anterior chamber after the intracameral injection of antigen and subsequent increase in the spleen is dependent on the chemokine receptor CCR2. However, CFSE-labeled CCR2− PBMC do migrate to the spleen after iv injection only.
Figure 6.
The expression of CCR2 by PBMC is required for migration of PBMC to the iris after the intracameral injection of antigen. One hr after naïve C57BL/6 mice were injected iv with CFSE—labeled PBMC from naïve WT, CCR5 −/− or CCR2 −/− mice, the mice received an intracameral injection of TNP-BSA. Twenty-four hr after the intracameral injection irides were removed and cell suspensions stained with phycoerythrin anti-F4/80 antibody. The cells were analyzed by flow cytometry. % increase F4/80, CFSE+ = increase in F4/80, CFSE+ cells from iv only calculated as in Figure 3A. The data represents the mean ± S.E.M. of 3–5 experiments.
Note: *P < 0.01.
PBMC recovered 24 hr after the intracameral injection of antigen (AC-PBMC) induce the suppression of DTH when transferred to naïve or immunized recipients. 4–9 Since PBMC from CCR2 −/− mice do not infiltrate the anterior chamber after the intracameral injection of antigen (Fig. 6) we investigated whether AC-PBMC recovered from CCR2 −/− or CCR5 −/− mice transfer the induction of the suppression of DTH. PBMC recovered from CCR2 or CCR5 −/− mice (AC-PBMC) 24 hr after the donors received an intracameral injection of TNP-BSA were injected iv into naïve WT mice. One week after the injection of the AC-PBMC, the recipients were immunized with TNP-BSA/CFA. One week after immunizing, footpads of these mice and immunized mice that did not receive the AC-PBMC were challenged with PCl. DTH was significantly reduced in WT mice receiving AC-PBMC recovered from WT or CCR5 −/− mice but was not suppressed in WT mice receiving AC-PBMC from CCR2 −/− mice (Fig. 7). Therefore, CCR2 −/− PBMC do not infiltrate the anterior chamber after the intracameral injection of antigen and CCR2 −/− mice do not produce AC-PBMC that induce the suppression of DTH.
Figure 7.
The expression of CCR2 is required for the activation of AC-PBMC that transfer the suppression of DTH. PBMC recovered 24 hr after naïve WT, CCR5 −/− (A) or CCR2 −/− (B) mice received an intracameral injection of TNP-BSA were injected iv into naïve recipient C57BL/6 mice. The recipients were immunized with TNP-BSA and CFA one week later and one week pi a footpad was challenged with epicutaneous PCl. Swelling was measured 24 and 48 hr after challenge. Data represents the mean ± S.E.M/of eight mice/group, 3 experiments.
Note: *P < 0.02.
PBMC that transfer the suppression of DTH after the intracameral injection of antigen (AC-PBMC) are not produced by CCL2 −/− mice
Because AC-PBMC were not produced by CCR2 −/− mice that received an intracameral injection of antigen (Fig. 7) we investigated whether CCR2+ PBMC (WT) would migrate to the iris in mice lacking CCL2, one of the ligands for CCR2. Accordingly, CFSE-labeled WT PBMC were injected iv into CCL2 −/− or WT mice. One hour later the mice received an intracameral injection of TNP-BSA. Irides, spleens and thymi were recovered 24 hr after the intracameral injection. Cell suspensions were prepared and stained with PE anti-F4/80 antibody. Significantly fewer CFSE-labeled cells infiltrating the irides of AC-injected mice were observed in CCL2 −/− recipients (Fig. 8).
Figure 8.
CCL2 is required for the migration of PBMC to the iris after the intracameral injection of antigen. Naïve C57BL/6(129) WT or CCL2 −/− mice received an intracameral injection of TNP-BSA twenty-four hr after CFSE-labeled PBMC from naïve WT donors were injected iv. Irides were recovered 24 hr after the intracameral injection and cell suspensions stained with phycoerythrin anti-F4/80 antibody. Data represents the mean increase in the number of CFSE-labeled F4/80+ PBMC ± S.E.M. relative to mice receiving IV cells only in 4–8 replicates, 3 experiments.
Note: *P < 0.01.
To determine whether CCL2 −/− mice produce circulating AC-PBMC, PBMC were recovered from CCL2 −/− mice approximately 24 hr after the intracameral injection of OVA. These cells were injected iv into naïve WT mice. One week after the injection of AC-PBMC, these recipient mice and naïve mice were then immunized with OVA and CFA. DTH to OVA was determined one week after the mice were immunized. DTH to OVA was reduced significantly in OVA-immunized WT mice that received AC-PBMC from WT but not CCL2 −/− mice (Fig. 9). Similarly AC-PBMC recovered from CCL2 −/− mice receiving intracameral TNP-BSA did not induce the suppression of DTH to TNP when injected into mice subsequently immunized with TNP-BSA and challenged with epicutaneous PCl (data not shown).
Figure 9.
CCL2 −/− mice do not produce suppressive PBMC after the intracameral injection of antigen. PBMC were recovered from CCL2 −/− and WT (C57BL/6) mice 24 hr after the mice received an intracameral injection of OVA were injected iv into C57BL/6 WT mice. One week later the recipients of the PBMC and naïve mice were immunized with OVA. One week pi footpads of the mice were challenged with OVA. The increment in swelling was measured 24 and 48 hr after challenge. Data is the mean swelling ± S.E.M. for 4–8 mice/group.
Note: *P < 0.05.
Discussion
Although the phenomenon of ACAID has been known for 20+ years, events that occur in the eye that induce ACAID are not clear. Indeed, there are still questions as to the nature of the eye-derived immunosuppressive signal. Information on the ocular events required to generate the ACAID-inducing signal will illuminate the eye-systemic connection that induces ACAID.
Antigen is readily distributed to the circulation after the intracameral injection of the antigen, similar to antigen injected intravenously1,8,13–15 except that antigen injected into the anterior chamber is detected in the thymus within 24 hr after the intracameral injection8 Circulating antigen does not enter the thymus but may be delivered to the thymus by circulating monocytic cells.17 Moreover, the cell- associated antigen in the periphery may be “processed” antigen presented by MHC class II or Qa-1 or CD-1 that cannot be detected by antibodies or labeled antigen. Since the migration of monocytic cells resident in the iris and ciliary body has not been demonstrated, we hypothesized that circulating monocytic cells might enter the anterior chamber in response to the injection. Indeed, a monocytic infiltration into the anterior chamber after intracameral injection has been reported.18 Once these cells enter the anterior chamber they could interact with iris antigen presenting cells.11,13,19 These monocytic cells recover antigen at the iris and would be converted to a suppressive phenotype by TGF-β (in aqueous humor) as demonstrated in vitro.5 In other words, our results suggest that the induction of ACAID uses elements of ocular immune privilege to generate a systemic signal for the generation of regulatory T cells.
The intracameral injection of antigen induces an increase of TNF-α in aqueous humor20 suggesting that the injection induces a moderate inflammatory event in the anterior chamber. Moreover, the production of TNF- α is required to induce ACAID.20,21 Because the induction of ACAID by an intracameral injection of bovine serum albumin required TNF-α and was dependent on the gauge of the needle used for the intracameral injection20 these authors suggested that ACAID is the result of a response to the injection in the anterior chamber. Although monocytic F4/80+ cells resident in the iris present antigen,15,19 the ability of these cells to emigrate from the iris (and ciliary body) has not been demonstrated.13–15 It could be argued that since less than 100 F4/80+ cells treated with TGF-β and antigen in vitro induce the suppression of DTH,2 a small, undetectable number of resident antigen presenting cells in the iris could exit from the eye and induce the systemic suppression of DTH. Therefore, it is likely that circulating, F4/80+ cells that transmit the suppression of DTH are bearing the antigen injected into the anterior chamber.
We focused our phenotyping of the monocytic cells that infiltrated the anterior chamber on F4/80 because iris cells bearing F4/80 recovered from mice receiving an intracameral injection of antigen induce the suppression of DTH when transferred to immunized mice.4,7 In fact, the expression of F4/80 is required for regulatory macrophages to induce the suppression of DTH.22 To track the migration of circulating monocytes after the intracameral injection of antigen we injected CFSE-labeled PBMC iv before the intracameral injection. The migration of these CFSE-labeled cells provided direct evidence that circulating monocytes enter the anterior chamber after intracameral injection. A needle stick only, the injection of PBS only or antigen and PBS all increased the influx of F4/80+ PBMC into the anterior chamber. However, quantitatively, the influx of these cells after intracameral injection is: PBMC + antigen injection > PBS injection only > injection only. In addition, we observed an increase in unlabeled, F4/80+low cells in the iris, spleen and thymus. The increase of these cells in the blood, and spleen has been described.10 Therefore, the increased influx of PBMC is a likely response to the intracameral injection. However, this response to the injection is transient. Strong, persistent inflammation such as that generated by infection or a burn could prevent the induction of ACAID regulatory monocytic cells.23,24 A corneal transplant induces an unusual CD4+ regulatory T cell that has properties similar the CD8+ T regulatory T cell induced by ACAID.25 This cell may be induced by a chronic presence of antigen (even self antigen).26 However, a moderate inflammation- inducing infiltration of PBMC may also induce ACAID.
The more than 4-fold increase in CFSE-labeled, F4/80+ cells in the spleen after the intracameral injection of antigen is greater than the 60% increase in these cells in the spleen after intracameral injection described previously.10 The CFSE-labeled cells were introduced recently and represent a finite number of cells. We observed that the increase in splenic unlabeled F4/80+ spleen cells after intracameral injection was 60%, similar to that observed by Faunce et al.10 It is unlikely that the decrease in CFSE-labeled iris cells was due to a loss in fluorescence. The egress of these cells from the anterior chamber may be via the Canal of Schlemm and/or the uveoscleral route.14 Moreover, iris F4/80+ cells and PBMC recovered from the iris or blood (respectively) 24 hr after the intracameral injection of antigen also home to the thymus and spleen27 suggesting further that PBMC recruited to the iris after the intracameral injection of antigen recirculate over a three-day period.
The requirement for the expression of CCR2 by F4/80+ PBMC to infiltrate the anterior chamber after intracameral injection suggests that the F4/80+ cells are attracted by the CCR2 chemokine ligands CCL2 and/or CCL7. MCP-1 does attract inflammatory cells in uveitis and infection.28,29 Moreover, there is a marked reduction in the infiltration of CFSE-labeled PBMC into the anterior chamber after CCL2 −/− mice received an intracameral injection of antigen although CCR2 −/− monocytes do migrate to the spleen. Perhaps the migration of PBMC to the iris in CCL2 −/− mice receiving an intracameral injection of antigen is also due to CCL7 (MCP-3), another ligand for CCR2. PBMC recovered from CCR2 −/− or CCL2 −/− mice that received an intracameral injection of antigen fail to transfer the suppression of DTH to that antigen when injected iv into WT mice. Because these PBMC do not infiltrate the anterior chamber after the intracameral injection of antigen it is unlikely that these PBMC accessed antigen or were influenced by the immune-privileged environment of the anterior chamber. Although robust inflammation could diminish or prevent ACAID,2,23,24 the required infiltration of PBMC into the anterior chamber in response to the chemokine CCL2 suggests that ACAID may be a “by-product” of an ocular response to injury that induces a moderate, transient inflammation in response to the injection. Here elements of the peripheral inflammatory/adaptive immune system are influenced by factors in an immune-privileged environment, thereby extending an immunosuppressive influence to the periphery. Experiments are in progress to investigate this hypothesis.
Acknowledgements
This work was supported by grants EY 017289, EY017537A1 National Eye Institute, U.S.P.H.S and the Connecticut Lions Eye Research Foundation. We thank Dr. Irving Goldschneider, Department of Immunology, University of Connecticut Health Center, and Dr. Joel Pachter, Department of Cell Biology, University of Connecticut Health Center for valuable discussions.
Abbreviations
- AC
anterior chamber
- ACAID
anterior chamber-associated immune deviation
- BSA
bovine serum albumin
- CFA
complete Freund’s adjuvant
- CFSE
Carboxyfluorescein succinimidyl ester
- DNA
desoxy-ribonucleic acid
- DTH
delayed-type hypersensitivity
- FSC
forward scatter
- OVA
ovalbumin
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PCl
picryl chloride
- RNA
ribonucleic acid
- TNF
tumor necrosis factor
- TNP
trinitrophenol
- WT
wild type.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;11:13–46. [PubMed] [Google Scholar]
- 2.Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123–52. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 3.Cone RE, Chattopadhyay S, O’Rourke J. Control of delayed-type hypersensitivity by ocular- induced CD8+ regulatory T cells. Chem Immunol Allergy. 2008;94:138–49. doi: 10.1159/000154998. [DOI] [PubMed] [Google Scholar]
- 4.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID) II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991;146:3018–24. [PubMed] [Google Scholar]
- 5.Wilbanks GA, Streilein JW. Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Regional Immunology. 1992;4:130–7. [PubMed] [Google Scholar]
- 6.Wang Y, Goldschneider I, O’Rourke J, Cone RE. Blood mononuclear cells induce regulatory NK thymocytes in anterior chamber-associated immune deviation. J Leukoc Biol. 2001;69:741–6. [PubMed] [Google Scholar]
- 7.Li X, Shen S, Urso D, et al. Phenotypic and immunoregulatory characteristics of monocytic iris cells. Immunology. 2006;117:566–75. doi: 10.1111/j.1365-2567.2006.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Wang Y, Urso D, O’Rourke J, Cone RE. Thymocytes induced by antigen injection into the anterior chamber activate splenic CD8+ suppressor cells and enhance the production of IgG1 antibodies. Immunology. 2004;113:44–56. doi: 10.1111/j.1365-2567.2004.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streilein JW, Okamoto S, Hara Y, Kosiewicz M, Ksander B. Blood-borne signals that induce anterior chamber-associated immune deviation after intracameral injection of antigen. Investigative Ophthalmology and Visual Science. 1997;38:2245–54. [PubMed] [Google Scholar]
- 10.Faunce DE, Sonoda K-H, Stein-Streilein J. MIP-2 mediated recruitment of NKT cells to the spleen during tolerance induction. J Immunol. 2001;166:313–21. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 11.Wilbanks GA, Streilein JW. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-beta. European Journal of Immunology. 1992;22:1031–6. doi: 10.1002/eji.1830220423. [DOI] [PubMed] [Google Scholar]
- 12.Hara Y, Okamoto S, Rouse B, Streilein JW. Evidence that peritoneal exudate cells cultured with eye-derived fluids are the proximate antigen-presenting cells in immune deviation of the ocular type. J of Immunology. 1993;151:5162–71. [PubMed] [Google Scholar]
- 13.Crespo SM, Planck SR, Parker DC, Rosenbaum JT. APCs in the anterior uveal tract do not migrate to draining lymph nodes. J Immunol. 2002;172:6701–8. doi: 10.4049/jimmunol.172.11.6701. [DOI] [PubMed] [Google Scholar]
- 14.Camelo S, Kezic J, McMenamin P. Anterior chamber-associated immune deviation: a review of the anatomical evidence for the afferent arm of this unusual experimental model of ocular immune responses. Clin Exp Ophthalmology. 2005;33:426–32. doi: 10.1111/j.1442-9071.2005.01044.x. [DOI] [PubMed] [Google Scholar]
- 15.Dulforce PA, Garman KL, Seitz GW, et al. APCs in the anterior uveal tract do not migrate to draining lymph nodes. J Immunol. 2004;172:6701–8. doi: 10.4049/jimmunol.172.11.6701. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. Journal of Leukokcyte Biology. 2010;88:41–55. doi: 10.1189/jlb.1009671. [DOI] [PubMed] [Google Scholar]
- 17.Goldschneider I, Cone RE. A central role for peripheral dendritic cells in the induction of acquired thymic tolerance. Trends in Immunology. 2003;24:77–81. doi: 10.1016/s1471-4906(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 18.Sabari DR, Elder IA, Nguyen CQ, et al. Characterization of intraocular immunopathology following intracameral injection of antigen. Molecular Vision. 2008;14:615–24. [PMC free article] [PubMed] [Google Scholar]
- 19.Steptoe RJ, McMenamin PG, Holt PG. Resident tissue macrophages within the normal rat iris lack immunosuppressine activity and are effective antigen presenting cells. Ocul Immunol Inflamm. 2000;8:177–87. [PubMed] [Google Scholar]
- 20.Ferguson TA, Herndon JM, Dube P. The immune response and the eye: a role for TNF alpha in anterior chamber-associated immune deviation. Investigative Ophthalmology and Visual Science. 1994;35:2643–51. [PubMed] [Google Scholar]
- 21.Pais R, Chattopadhyay S, Lemire Y, et al. ACAID is initiated as a result of a moderate inflammatory insult to the anterior chamber. Abstract 4835. Association of Research in Vision and Ophthalmology annual meeting; Fort Lauderdale, FL. 2010. [Google Scholar]
- 22.Lin HH, Faunce DE, Stacey M, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. Jour Exp Med. 2005;20:1615–25. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo JS, Streilein JW. Analysis of immune privilege in eyes with Mycobacteria tuberculosis adjuvant-induced uveitis. Ocular Immunology and Inflammation. 2005;13:139–47. doi: 10.1080/09273940490912489. [DOI] [PubMed] [Google Scholar]
- 24.Qiao H, Lucas K, Stein-Streilein J. Retinal laser burn disrupts immune privilege in the eye. American Journal of Pathology. 2009;17:414–22. doi: 10.2353/ajpath.2009.080766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunnasamy K, Paunicka K, Reyes NJ, Yang W, Chen PW, Niederkorn JY. Orthotopic corneal allografts and alloantigens introduced into the anterior chamber induce two different regulatory T cell populations that promote corneal allograft survival. Investigative Ophthalmology and visual science. doi: 10.1167/iovs.10-6161. epub ahead of print 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregerson DS, Heuss ND, Lehmann U, McPherson SW. Peripheral induction of tolerance by retinal antigen expression. Journal of Immunology. 2009;183:814–22. doi: 10.4049/jimmunol.0803748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cone RE, Chattopadhyay S, Sharafieh S, Li Z, O’Rourke J, Goldschneider I. Intracameral injection of antigen attracts circulating monocytic cells associated with anterior chamber-associated immune deviation to the iris, blood, thymus and spleen; Association of Research in Vision and Ophthalmology Annual Meeting; 2008. Abstract 5208. [Google Scholar]
- 28.Crane IJ, McKillop-Smith S, Wallace CA, Lamont GR, Forrester JV. Expression of the chemokines MIP-1alpha, MCP-1, and RANTES in experimental autoimmune uveitis. Invest Ophthalmol and Vis Sci. 2001;42:1547–52. [PubMed] [Google Scholar]
- 29.Jia T, Serbina NV, Brandl K, et al. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. Journal of Immunology. 2008;180:6846–53. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]