Abstract
The aim of this study was to characterize the affinity and permeability patterns of the amino acid ester prodrugs of acyclovir (ACV), L-alanine-ACV (AACV), L-serine-ACV (SACV), L-serine-succinate-ACV (SSACV) and L-cysteine-ACV (CACV) on rabbit primary corneal epithelial cell culture (rPCEC) and on rabbit cornea. Amino acid prodrugs of acyclovir, AACV, SACV, SSACV and CACV were synthesized in our laboratory. Chemical hydrolysis in aqueous buffer, enzymatic hydrolysis in corneal homogenates and transport across freshly excised rabbit cornea of these prodrugs were studied. SSACV inhibited the uptake of [3H] L-alanine on rPCEC and across the intact rabbit cornea. Lineweaver-Burk plot transformation revealed competitive inhibition between L-alanine and SSACV. In corneal tissue homogenate, the half lives of SSACV, SACV and CACV (t1/2) were observed to be 3.5 ± 0.4, 9.2 ± 0.6 and 1.8 ± 0.1 hr respectively, whereas AACV was readily converted to the active parent drug acyclovir exhibiting complete degradation before 5 min. Interestingly translocation of SACV across cornea was inhibited in the presence of 5 mM arginine (~51%), a specific substrate for cationic transport system and in presence of BCH (~38%), a substrate specific for large neutral amino acid transport system (LAT) or cationic and neutral amino acid transport system (B0,+). SACV exhibited higher permeability across cornea along with excellent antiviral activity against herpes simplex virus (HSV-1) and varicella-zoster virus (VZV) in comparison to ACV. Recognition by multiple transporters, stability in corneal homogenate and changes in physico-chemical properties contributed to the increased permeability of SACV across cornea.
Keywords: prodrug, transporter, cornea, acyclovir, amino acid conjugates
Introduction
Ocular availability of the drugs is restricted due to physiological, pharmacokinetic, and pharmaceutical barriers. Prodrug strategy was employed to target the nutrient transporters by attaching the drug to the nutrient promoiety. Among the nutrient transporters, amino acid transporters are preferred for drug delivery due to their ubiquitous nature and overlapping substrate specificity.
Amino acid transporters have been classified on the basis of their functional differences such as sodium dependence and substrate specificity. In terms of specificity they can be divided into three types namely anionic, cationic and neutral.1 Small neutral amino acids, are transported predominantly by Na+-dependent transport system ASC (for Alanine-, Serine-, and Cysteine-preferring), system A (for Alanine-preferring) and B0,+ (neutral and cationic amino acids preferring) and also by Na+-independent transport system asc, and b0,+. Usually Na+ dependent transporters utilize the electrochemical gradients of Na+ to actively transport amino acids.1 One of the notable exception is system ASC, which is Na+-dependent but, does not utilize the Na+ gradient as a driving force, instead Na+ is bi-directionally co-transported along with the exchange of amino acids.2,3
Amino acid transport systems, b0,+, y+L (neutral and cationic amino acids preferring), and B0,+, translocate a wider range of substrates, including cationic and neutral amino acids, differing, however, in their interactions with inorganic monovalent ions such as Na+. System y+L exhibits a more complex pattern in its cation interaction. Transport of cationic amino acid (lysine, arginine) through system y+L is unaffected by Na+ replacement, but the affinity of system y+L toward neutral amino acids (alanine) is dramatically reduced in Na+ free buffer.4
Depending upon the affinity or the capacity of the transporter, these amino acid transporters have been known to transport not only naturally occurring amino acids but also amino acid–related drug compounds such as L-dopa, a therapeutic agent for Parkinsonism; melphalan,5 an anticancer Phe mustard; triiodothyronine6 and thyroxine,7 two thyroid hormones; and gabapentin,8 an anticonvulsant and valacyclovir,9 an anti-viral drug. A recent report suggests that the ability of B0,+ to transport valacyclovir is comparable to that of the peptide transporter PEPT1.9 These findings suggest that amino acid transporters can have significant potential as delivery targets for amino acid-based drugs and prodrugs.
Trifluorothymidine (TFT), used for the treatment of ocular herpes simplex virus (HSV) infections, is associated with severe cytotoxicity in long-term treatments. In comparison, acyclovir (ACV) exhibits excellent antiviral activity against HSV-1 and 2 and considerably less cytotoxic due to its selective mechanism of action. However, ACV cannot be formulated into 1%–3% eye drops due to its limited solubility.10 Main constraints to topical ocular delivery of ACV in the treatment of HSV-1 keratitis include rapid precorneal elimination, conjunctival absorption, nasolacrimal drainage, and poor corneal permeability due to its relative hydrophilic property.10 We have recently examined the possibility of delivering ACV by designing its water-soluble dipeptide ester prodrugs. These prodrugs showed excellent antiviral activity against HSV-1 with higher aqueous solubility, and enhanced corneal permeability than ACV.11,12 Although, the peptide transporters have remained a popular choice for drug targeting due to their robust and versatile nature, the utility of the amino acid transporters cannot be ruled out.
Recently, a significant amount of work has been published on the substrate specificities of membrane transporters expressed on the ocular barriers. A host of transporters have been discovered in the anterior segment, which could be targeted for drug delivery.13 Transport systems for peptide,14 amino acid,15–17 and nucleoside/nucleobase18,19 are such carriers that have been discovered on the corneal epithelium and utilized for targeted drug delivery in our laboratory.
In an earlier study from our laboratory, a Na+- dependent neutral amino acid transporter, ASCT1 (for Alanine-, Serine-, and Cysteine-preferring) was identified on the corneal epithelium. Therefore, water-soluble amino acid prodrugs of ACV, L-alanine-ACV (AACV), L-serine-ACV (SACV), L-serine-succinate-ACV (SSACV) and L-cysteine-ACV (CACV) were synthesized in order to target ASCT1 on the cornea. These prodrugs were also investigated for their affinity towards other amino acid transporters. Finally, the feasibility of utilizing the amino acid prodrugs of ACV for enhanced delivery and their antiviral activity against HSV viruses was also investigated.
Materials and Methods
Materials
Amino acid prodrugs of ACV, L-alanine-ACV (AACV), L-serine-ACV (SACV), L-serine-succinate-ACV (SSACV) and L-cysteine-ACV (CACV) were synthesized in our laboratory. [3H] alanine (66 Ci/mmol), [3H] phenylalanine (50 Ci/mmol), and [3H] arginine (42 Ci/mmol)) were obtained from NEN Biochemicals (Boston, MA, USA). The solvents used were of analytical grade and obtained from Fisher Scientific. The growth medium, minimum essential medium (MEM), non-essential amino acids (NEAA) and fetal bovine serum (FBS) were purchased from Gibco Life Technologies (Grand Island, NY). Penicillin, streptomycin, sodium bicarbonate, HEPES, amphotericin-B, polymixin-B and unlabeled amino acids were purchased from Sigma Chemical Company (St. Louis, MO). Culture flasks (75 cm2 growth area), 12 wells (3.8 cm2 growth area per well) were procured from Costar (Bedford, MA). All other chemicals were obtained from Sigma Chemical Company (St. Louis, MO) and were used without further purification.
Synthesis of prodrugs
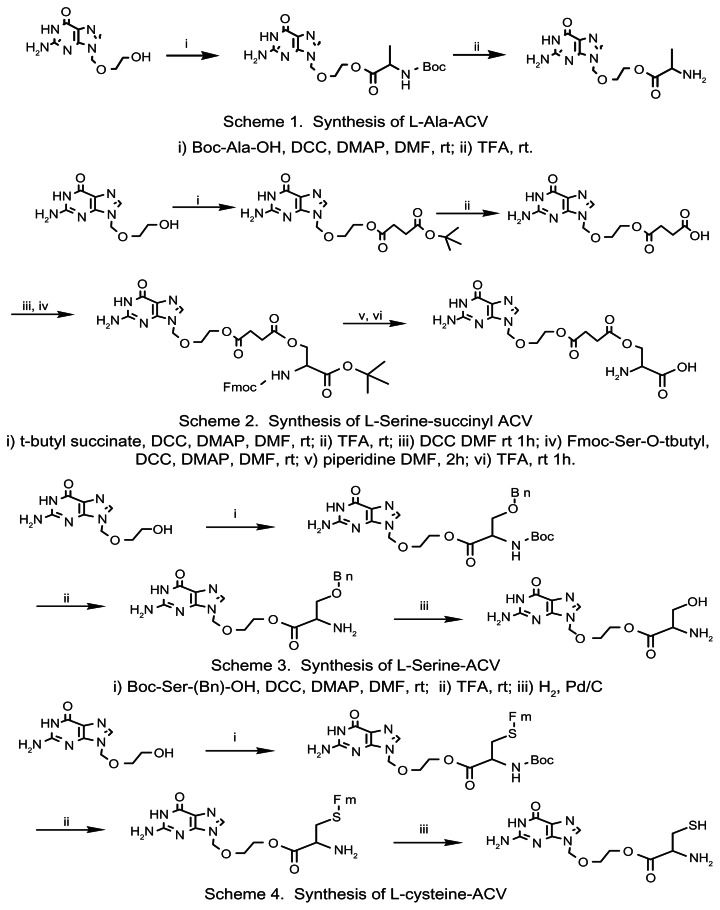
Preparation of AACV, SACV and CACV prodrugs of ACV involved (i) formation of protected amino acid anhydrides, (ii) coupling of the protected amino acid anhydride with ACV, and finally (iii) deprotecting the amino group of the amino acid ester of ACV. For SSACV prodrug where the β-hydroxyl was used to link to ACV, the mono-ACV succinate was synthesized first then conjugated with serine. The synthetic schemes for these prodrugs are shown in Figure 1.
Figure 1.
Synthetic schemes of AACV, SSACV, SACV, and CACV.
L-Alanine, L-Serine and L-Cysteine-ACV prodrugs
A mixture of protected amino acid (Boc-Alanine, Boc-Serine(Bn)-OH and Boc-Cysteine(Fm)-OH) and dicyclohexyl carbodiimide (DCC) in dimethylformamide (DMF) with ratio of 1:2 were stirred for 1 hour at room temperature under nitrogen atmosphere. A solution of ACV and 4-N,N (dimethylamino) pyridine (DMAP) was added to the above reaction mixture at 0 °C followed by stirring for 24 h at RT. The precipitate formed in the reaction was filtrated and then solvent was removed in vacuo. The residue was purified with a silica gel column. After the removal of the protection groups (by TFA, H2/Pd and piperidine, respectively), the prodrugs were obtained by adding the resultant solution drop wise to cold diethyl ether. The yields of AACV, SACV and CACV were 90, 80 and 59% respectively.
SSACV prodrug
Succinic acid was used as the linker between L-serine and ACV. Mono t-butyl succinic acid and DCC were dissolved in DMF with a molar ratio of 2:1 and stirred for 1 hour in nitrogen atmosphere. Then a solution of ACV and DMAP (molar ratio: 2:1) in DMF was added drop-wise at 0 °C. After raising the mixture to room temperature, it was continually stirred for 24 hours. DMF was evaporated in vacuo after the removal of precipitate. The mixture was purified with a silica gel column. After the removal of t-butyl group with TFA, succinyl ACV was obtained. Succinyl ACV was treated with TEA (triethylamine) for 5 mins, and then followed the similar procedure as mentioned in the above section. L-SSACV prodrug was obtained with the overall yield of 70%.
NMR and LC/MS spectroscopy
1H spectra was recorded on a Bruker AC 250-modified Tecmag DSPect Fourier transform NMR spectrometer at 250 MHz with DMSO-d6 solutions at 23 °C. Chemical shifts are expressed in ppm relative to tetramethylsilane (TMS), the internal standard. Thermo- Finnigan AQA LC/MS system coupled with ESI source was applied to obtain MS spectra. A Phenomenex 250 × 4.6 mm C18 column is utilized with a gradient elution of ACN/H2O at a flow rate of 0.6 ml/min.
1H NMR AACV: δ 1.4 (d, 3H, CH3), 3.7–4.1 (m, 5H, CH2CH2, CH ), 5.4 (s, 2H, NCH2O), 6.7 (s, 2H, NH2), 8.0 (s, 1H, NCHN), 8.4 (s, 2H, (CH)NH2 ), 10.9 (s, 1H, NH)
Calculated: C11H16N6O4, MS: 296.1; Measured: MS (MH+) 297.1.
1H NMR SACV: δ 3.8 (m, 4H, CH2CH2), 5.4 (s, 2H, NCH2O), 6.6 (s, 2H, NH2), 7.9 (s, 1H, NCHN), 8.4 (s, 2H, (CH)NH2), 10.8 (s, 1H, NH).
Calculated: C11H16N6O5, MS: 312.1; Measured: MS (MH+) 313.3.
1H NMR CACV: δ 2.9 (m, 1H, CH(NH2), 3.3–3.5 (m, 4H, CH2CH2), 5.4 (s, 2H, CH2O), 6.5 (b, 2H, NH2), 7.8 (s, 1H, NCHN).
Calculated: C11H16N6O4S, MS: 328.1; Measured MS (MH+) 329.0.
1H NMR SSACV: δ 2.5 (m, 4H, CH2CH2), 3.7 (m, 4H, OCH2CH2O), 4.3 (s, 1H, CH), 4.5 (m, 2H, CH2), 5.4 (s, 2H, NCH2O), 6.5 (s, 2H, NH2), 7.8 (s, 1H, NCHN), 8.3 (m, 2H, (CH)NH2), 10.8 (b, 1H, NH).
Calculated: C15H20N6O8, MS: 412.1; Measured: MS (MH+) 413.5.
Animals
Adult male New Zealand white (NZW) rabbits weighing between 2–2.5 kg were obtained from Myrtle’s rabbitry (Thompson Station, TN, USA). This research was conducted strictly according to the “Principles of Laboratory Animal Care” (NIH publication #85–23, revised 1985).
Prodrug stability in transport medium
The transport buffer, Dulbecco’s phosphate buffer saline (DPBS) was prepared at different pH values (5–7.4). Stock solution of the prodrug (1 mM) was prepared in DPBS buffer and used immediately. Aliquots (9.8 ml) of the buffer were placed in screw-capped vials and allowed to equilibrate at 34 °C. Prodrug stock solution (0.2 ml) was subsequently added to the buffer. The vials were placed in a constant shaker bath set at 34 °C and 60 rpm. Samples (0.1 ml) were collected at appropriate time intervals for up to 96 h. The samples were immediately stored at −80 °C until further analysis. All experiments were conducted at least in triplicate.
Corneal tissue hydrolysis
The corneal tissue hydrolysis was carried out as described previously.20 The method is described briefly as follows.
Preparation of corneal tissue
New Zealand albino male rabbits were used for this study. Animals were euthanized by a lethal injection of sodium pentobarbital through the marginal ear vein. Each eye was immediately enucleated and the ocular surface was rinsed with ice cold pH 7.4 Dulbecco’s phosphate buffer saline (DPBS) to remove any trace of blood. Cornea was removed after cutting along the scleral-limbus junction. The corneal tissue was homogenized in 5 ml chilled (4 °C) DPBS for about 4 min with a tissue homogenizer (Tissue Tearor Model 985–370) in an ice bath. The homogenate was centrifuged at 12,752 g for 25 min at 4 °C to remove cellular debris, and the supernatant was taken for the hydrolysis studies. Protein content of each supernatant was determined with a BioRad assay using bovine serum albumin as the standard.
Hydrolysis procedure
The supernatant was equilibrated at 34 °C for about 30 min prior to an experiment. Hydrolysis was initiated by the addition of 0.2 ml of a 1 mM prodrug solution to 1.3 ml of the supernatant. The control consisted of 1.3 ml of DPBS instead of the supernatant. Aliquots (50 μl) were withdrawn at appropriate time intervals for up to 24 h. The samples were immediately diluted with 50 μl chilled methanol to quench the reaction and stored at −80 °C until further analysis. Subsequently, these were thawed and centrifuged at 8,161 g for 10 min prior to analysis by HPLC for the intact ester prodrug and the regenerated parent acyclovir. Apparent first order rate constants were calculated and corrected for any chemical hydrolysis observed with the control.
In vitro antiviral testing
The in vitro potencies of the parent drug, ACV, and the prodrugs, AACV, SSACV, SACV, and CACV were determined against various herpes viruses. The compounds were screened against HSV-1, HSV-2, varicella-zoster virus (VZV), and Epstein-Barr virus (EBV). The in vitro antiviral testing was carried out as described previously.21 The method is described briefly as follows. Low-passage human fibroblast foreskin cells (HFF) or Daudi cells were used at a concentration of 2.5 × 106 cells per ml in 0.1 ml of minimum essential medium (MEM) supplemented with 10% fetal bovine serum. For HSV-1 and HSV-2, 1000 plaque forming units (PFU) per well were used. VZV was used at a concentration of 2500 PFU per well. All the studies were conducted by Dr. Earl Kern at the University of Alabama, Birmingham, Alabama under a contract from NIAID.
Uptake studies
The procedure for preparing primary corneal epithelial cell culture was reported previously from our laboratory.22 All uptake studies were conducted on cultured primary rabbit corneal cells after 10 to 12 days seeding. The medium was removed, and the cells were washed twice with DPBS (pH 7.4). In typical uptake experiments, cells were incubated with substrates ([3H] alanine (7.6 nM); [3H] phenylalanine (10 nM); [3H] arginine (11.9 nM)) prepared in DPBS for 10 minutes. Following incubation, cells were washed three times with ice-cold HEPES (4-(2- hydroxyethyl)-1-piperazine-ethanesulfonic acid) buffer to terminate the uptake experiment. Then cells were lysed overnight with 1 ml 0.05% (w/v) Triton X-100 in 1 N NaOH at room temperature. Aliquots (500 μl) from each well were transferred to scintillation vials containing 5 ml scintillation cocktail (Fisher Scientific, Fairlawn, NJ). Samples were then analyzed by the liquid scintillation spectrophotometry using scintillation counter (Beckman Instruments Inc., Model LS-6500) and the rate of uptake was normalized to the protein content of each well. The amount of protein in the cell lysate was measured by BioRad protein estimation kit using bovine serum albumin as the standard (BioRad Protein estimation Kit, Hercules, CA).
Corneal transport studies
Transport of ACV, AACV, SSACV, SACV and CACV (1 mM) across the freshly excised rabbit cornea was performed according to the method of Tak et al.23 Briefly, New Zealand albino rabbits weighing 2.0 to 2.5 kg were euthanized by an overdose of pentobarbital through a marginal ear vein. Eyes were then carefully enucleated and washed with ice-cold DPBS (pH 7.4). Subsequently, a small incision was made in the sclera and the cornea was carefully excised, leaving some portion of the sclera attached for mounting on the diffusion apparatus. The cornea was then mounted on a diffusion apparatus (side-by-side) maintained at 34 °C (in vivo corneal temperature). Prodrug/drug solutions (3 mL) were added on the epithelial side of the cornea (donor chamber). In the other half-chamber (receiver chamber), 3.2 mL of DPBS (pH 7.4) was added and solutions in both the chambers were stirred continuously using magnetic stirrers. Receiver chamber volume was maintained slightly higher to generate hydrostatic pressure to maintain the curvature of the cornea throughout the experiment. Transport experiments were conducted for a period of 3 h. 100 μl aliquots were removed from the receiver chamber at appropriate intervals and replaced with an equal volume of DPBS. Samples were stored at −80 °C until further HPLC analysis.
Na+-dependent transcorneal flux of SSACV
When the effect of Na+ on SSACV transport was studied, NaCl and Na2HPO4 in the buffer were substituted with equimolar quantities of choline chloride and K2HPO4, respectively.
Competitive inhibition studies
Transcorneal flux of prodrugs (1 mM) was studied across cornea in presence of various inhibitors, i.e. L-alanine, glycylsarcosine (gly-sar), L-arginine, L-glutamic acid, and BCH (2-aminobicyclo-[2,2,1]-heptane-2-carboxylic-acid) (5 mM).
Analytical procedures
All samples were assayed using HPLC. The HPLC system was comprised of a Rainin Dynamax Pump SD-200 and a Rainin Dynamax UV Detector UV-C at 254 nm. The column used was a C18 Luna column 4.6 × 250 mm (Phenomenex, Torrance, CA). Mobile phase consisted of a mixture of buffer and an organic modifier. The percentage of organic phase was varied in order to elute the compounds of interest. This method gave rapid and reproducible results. HPLC conditions for the various compounds have been summarized in Table 1.
Table 1.
HPLC assay conditions and retention times for the various ester prodrugs.
| Prodrug | Composition of aqueous phase (pH 2.5) | Composition of organic phase | Mobile phase Aq:Org | Retention timesa | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Parent drug | Intermediate | Prodrugb min | ||||
| ACV | 25 mM KH2PO4 | Acetonitrile | 99:1 | 13.7 | – | – |
| AACV | 25 mM KH2PO4 | Acetonitrile | 99:1 | 13.7 | – | 11.6 |
| SSACV | 25 mM KH2PO4 | Acetonitrile | 94:6 | 4.8 | 25.8 | 8.1 |
| SACV | 25 mM KH2PO4 | Acetonitrile | 99:1 | 13.7 | – | 7.3 |
| CACV | 25 mM KH2PO4 | Acetonitrile | 97:3 | 7.6 | – | 19.7 |
Notes:
UV detection at λmax = 254 nm;
Retention times noted when separately injected as a pure compound onto the column.
Data analysis
Permeability measurements
Steady state fluxes (SSF) were determined from the slope of the cumulative amount of drug transported vs. time plot and expressed per unit of corneal surface area as described by Eq. 1. The cumulative amount of drug transported is considered as the sum of the receptor cell prodrug and regenerated drug:
| (1) |
M is the cumulative amount of drug transported and A is the corneal surface area exposed to permeant. Corneal membrane permeabilities (CMP) are determined by normalizing the SSF to the donor concentration, Cd according to Eq. 2.
| (2) |
Determination of Km and Vmax
Uptake data of [3H] alanine in presence of SSACV was fitted to the classical Michaelis–Menten equation:
| (3) |
S is the concentration of the permeant, Vmax is the maximum rate of drug transport, and Km is the permeant concentration where half the maximal rate is reached. Km and Vmax for SSACV permeation across cornea were determined using a nonlinear least squares regression analysis program (KaleidaGraph V3.09). Quality of the fit was determined by evaluating the coefficient of determination (r2), standard error of parameter estimates, and by visual inspection of the residuals.
Affinity measurement
SSACV inhibited the uptake of [3H] alanine in a competitive manner, and the kinetics can be expressed according to Eq. (4)
| (4) |
In Eq. (4)I is the concentration of the SSACV and Ki is the affinity constant. Affinity (Ki) for the prodrug was calculated by fitting the data to Eq. (4). Ki for the prodrug can also be calculated by transforming the Michaelis–Menten Eq. (4) to a Lineweaver–Burk equation, which yields the linear Eq. (5) for competitive inhibition,
| (5) |
From a plot of 1/V versus 1/S, Vmax, Km, and hence Ki can be estimated by linear regression analysis.
Statistical analysis
All experiments were conducted at least in triplicate, and results are expressed as mean ± SD except in the case of Michaelis–Menten parameter Km, and Ki where the values are presented as mean ± SE. Student’s t test was used to detect statistical significance, and P < 0.05 was considered to be statistically significant.
Results
Stability studies
Stability in transport buffer
Aqueous stabilities of L-alanine, L-serine-succinate, L-serine and L-cysteine ester prodrugs of acyclovir were determined in the transport buffer, DPBS (pH 5–7.4) for a period of 96 h (Table 2). The pH of maximal stability within the range studied was 5.0. The half-lives for AACV and CACV in DPBS 7.4 were 2.88 ± 0.07 and 3.1 ± 0.3 hr respectively, indicating that chemical hydrolysis may cause regeneration of the parent drug during the course of a transport experiment (Table 2). The half-lives for SSACV and SACV in DPBS 7.4 were 38 ± 11.6 and 29.3 ± 2.7 hr respectively, indicating that SSACV and SACV were less prone to chemical hydrolysis compared to AACV and CACV.
Table 2.
First order hydrolysis rate constants of AACV, SSACV, SACV and CACV in buffers at pH 7.4, 6.0 and 5.0 and in cornea (protein content 0.25 mg/ml).
| Buffer | AACV | SSACV | SACV | CACV | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Rate (k* 104 min−1) | Half life (hr) | Rate (k* 104 min−1) | Half life (hr) | Rate (k* 104 min−1) | Half life (hr) | Rate (k* 104 min−1) | Half life (hr) | |
| DPBS 5.0 | 0.5 ± 0.2 | 273 ± 148 | a | – | 0.4 ± 0.2 | 353 ± 199 | 7.5 ± 0.9 | 15.5 ± 1.7 |
| DPBS 6.0 | 2.5 ± 0.2 | 47 ± 5 | 0.11 ± 0.03 | 1157 ± 369 | 0.5 ± 0.1 | 226 ± 52 | 17.4 ± 1.6 | 6.7 ± 0.6 |
| DPBS 7.4 | 39 ± 1 | 2.9 ± 0.1 | 3.2 ± 1 | 38 ± 12 | 3.9 ± 0.3 | 29 ± 3 | 37.1 ± 3.4 | 3.1 ± 0.3 |
| Cornea | b | – | 83 ± 8 | 3.5 ± 0.4 | 12.5 ± 0.8 | 9.2 ± 0.6 | 64.4 ± 5.5 | 1.8 ± 0.1 |
Notes: Cornea—Corneal hydrolysis in DPBS 7.4. Values are mean ± S.D. (n = 3).
No measurable degradation during the period of a 96 hr experiment;
Complete degradation before 5 min period.
Corneal tissue hydrolysis
Enzymatic hydrolysis of the prodrugs was assessed in the corneal tissue homogenates. L-alayl prodrug of acyclovir, AACV was readily converted to the active parent drug acyclovir exhibiting complete degradation before 5 min period (Table 2). The half lives of SSACV, SACV and CACV (t1/2) were observed to be 3.5 ± 0.4, 9.2 ± 0.6 and 1.8 ± 0.1 hr respectively indicating that the major contribution to the regeneration of the parent drug (acyclovir) in the receiver chamber during the course of a transport study (3 h) was due to enzymatic hydrolysis. Therefore, the total amount of drug permeating through the corneal membrane was taken as the sum of the prodrug and the regenerated parent drug.
In vitro antiviral screening
Several amino acid ester prodrugs of ACV exhibited excellent in vitro antiviral activity against HSV-1, and VZV. These prodrugs have to undergo hydrolysis to yield the active parent drug, acyclovir. SACV, CACV and AACV showed excellent antiviral activity against HSV-1 with an EC50 of 6.3, 5.4, and 6.6 μM respectively relative to 7.1 μM for ACV and 9.1 μM for VACV.11 The selectivity indices (SI) of SACV (48) and AACV (45) were better than VACV (30.3). In addition, SACV was also effective against VZV than the parent drug, ACV (Table 3).
Table 3.
In vitro antiviral activity of aminoacid prodrugs of ACV.
| Entity | HSV-1 (μM) CPE inhibition | HSV-2 (μM) CPE inhibition | VZV (μM) CPE inhibition |
|---|---|---|---|
| ACV | EC50 = 7.1 | EC50 = 6.6 | EC50 = 2 |
| AACV | EC50 = 6.6 | EC50 = 17.7 | EC50 > 300 |
| CC50 > 300 | CC50 > 300 | CC50 > 300 | |
| SI > 45 | SI > 17 | SI = 0 | |
| SACV | EC50 = 6.3 | EC50 = 38.4 | EC50 = 1.7 |
| CC50 > 300 | CC50 > 300 | CC50 > 300 | |
| SI > 48 | SI > 7.8 | SI > 176 | |
| SSACV | EC50 = 43.5 | EC50 = 245 | EC50 = 7.8 |
| CC50 > 300 | CC50 > 300 | CC50 > 300 | |
| SI > 6.9 | SI > 1.2 | SI > 39 | |
| CACV | EC50 = 5.4 | EC50 = 14.7 | Ec50 > 300 |
| CC30 > 300 | CC50 > 300 | CC50 > 300 | |
| SI > 55.5 | SI > 20 | SI > 0 |
Notes: EC50—concentration required to inhibit viral cytopathogenicity by 50%; CC50—concentration required to inhibit cell proliferation by 50%. SI (Selectivity Index) = CC50/EC50; CPE—Cytopathic Effect.
Uptake studies
Uptake of [3H] alanine, [3H] arginine, and [3H] phenylalanine in the presence of prodrugs
[3H] alanine, [3H] arginine, and [3H] phenylalanine uptake was carried out in the presence of 5 mM control, AACV, SSACV, SACV, and CACV. [3H] alanine uptake was significantly inhibited in the presence of 5 mM alanine, and SSACV. [3H] arginine uptake was significantly inhibited in the presence of 5 mM arginine, and partially inhibited in the presence of 5 mM SSACV. [3H] phenylalanine uptake was significantly inhibited in the presence of 5 mM phenylalanine, and partially inhibited in the presence of 5 mM SSACV (Table 4).
Table 4.
Uptake of [3H] alanine (7.6 nM), [3H] arginine (11.9 nM) and [3H] Phenylalanine (10 nM) by rPCEC in presence of 5 mM control, AACV, SSACV, SACV, and CACV.
| Fraction uptake/mg protein (% donor) | [3H] Arg inine | [3H] Phenylalanine | [3H] Alanine |
|---|---|---|---|
| Control | 12.3 ± 1.1 | 8.6 ± 1.3 | 19.2 ± 0.8 |
| 5 mM control | 0.8 ± 0.04** | 0.84 ± 0.09** | 2.6 ± 0.1** |
| 5 mM AACV | 11.7 ± 0.7 | 10.2 ± 0.7 | 18.8 ± 1.4 |
| 5 mM SSACV | 9.4 ± 0.1* | 6.4 ± 0.3* | 7.9 ± 0.5* |
| 5 mM SACV | 12.0 ± 0.5 | 10.7 ± 0.4 | 19.5 ± 1.8 |
| 5 mM CACV | 11.6 ± 0.8 | 9.7± 0.6 | 19.5 ± 1.8 |
Notes: Values are mean ± S.D. (n = 4 to 8).
Indicates P < 0.05;
Represents P < 0.01 from control.
Concentration Dependent Uptake of [3H] alanine in the Presence of SSACV
Uptake of [3H] alanine in rPCEC appeared to be concentration dependent and saturable at higher concentrations (Fig. 2A). Km and Vmax values were calculated to be 0.71 mM and 0.84 μmoles·min−1·mg protein−1, respectively. Concentration dependent uptake of [3H] alanine was also studied in presence of 5 mM SSACV (Fig. 2A). Km value increased in presence of 5 mM SSACV as Vmax remained similar, indicating competitive inhibition. Lineweaver–Burk transformation (Fig. 2B) also revealed a competitive inhibition of [3H] alanine uptake in presence of SSACV further confirming that the prodrug and the substrate share a common binding site on the transporter. Affinity constant (Ki) of SSACV towards this transporter was also calculated according to Eq. 5. and the Ki value for SSACV is 7.97 ± 0.61 mM.
Figure 2.
A) Concentration-dependent cellular uptake of [3H] alanine by rPCEC (R2 = 0.96) and in presence of 5 mM SSACV. Values are mean ± SE (n = 4). B) Lineweaver–Burk transformation of the concentration-dependent uptake of alanine (R2 = 0.96) in presence of 5 mM SSACV (R2 = 0.97). [1/Uptake of [3H] alanine, 1/V (μmoles−1. min. mg) vs. Concentration of alanine, 1/S (mM−1)]. Values are mean ± SE (n = 4).
Transcorneal flux experiments
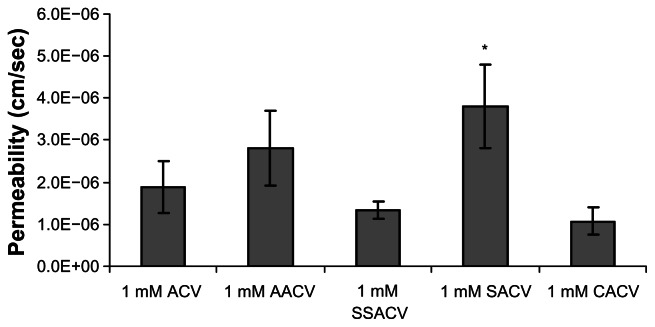
Corneal permeation of ACV, AACV, SACV, SSACV and CACV (1 mM) were studied with the isolated rabbit cornea. Estimated apparent permeability (Papp) values were ranked as SACV > AACV > ACV > S SACV > CACV (Fig. 3).
Figure 3.
Permeability values of 1 mM ACV, AACV, SSACV, SACV, and CACV across isolated rabbit cornea. Values are mean ± S.D. (n = 3 to 6).
Note: *Represents P < 0.05 from control.
Transcorneal flux of AACV in the presence of 5 mM alanine, BCH, and gly-sar
Translocation of AACV across isolated cornea was unaffected by the presence of gly-sar, a model peptide transporter substrate, alanine, a model ASCT transporter substrate and BCH, a model LAT or B0,+ transporter substrate. Instability of AACV was further confirmed by the presence of only parent drug, ACV in the receiver chamber (Fig. 4).
Figure 4.
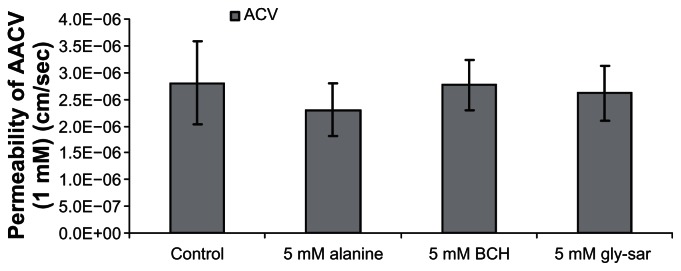
Permeability values of 1 mM AACV in presence of 5 mM alanine, BCH, and gly-sar across isolated rabbit cornea. Values are mean ± S.D. (n = 3 to 6).
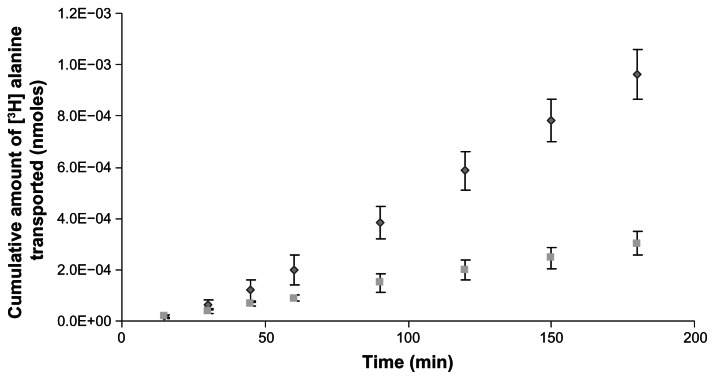
Cumulative amount of [3H] alanine transported in the presence of SSACV
Transport of [3H] alanine was studied in the presence of 10 mM SSACV across rabbit cornea and it was significantly inhibited in the presence of 10 mM SSACV, which is consistent with our uptake results (Fig. 5).
Figure 5.
Cumulative amount of [3H] alanine (7.6 nM) transported across isolated rabbit cornea in the absence (◆) and in presence of 10 mM SSACV (■). Values are mean ± S.D. (n = 3 to 6).
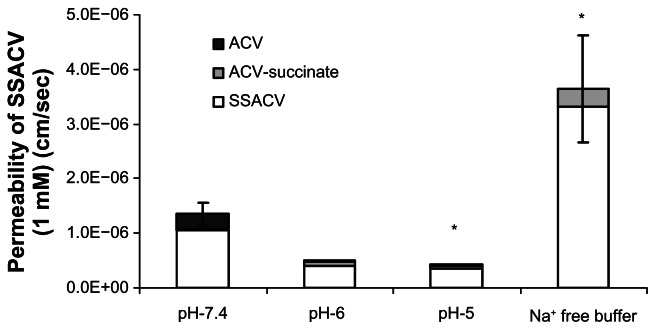
pH and Na+ dependent SSACV transport
pH effect on the transport of SSACV across isolated cornea was also examined. The buffer used was DPBS and the pH range studied was within 5–7.4. Transport of SSACV across cornea diminished with a lowering in pH values from pH-7.4 to 5.0. Surprisingly transport of SSACV was significantly elevated when Na+ in the DPBS buffer was replaced with K+ (Fig. 6).
Figure 6.
Permeability values of 1 mM SSACV in presence of Na+ free buffer and at pH ranging from 7.4–5.0 across isolated rabbit cornea. Values are mean ± S.D. (n = 3 to 6).
Note: *Represents P < 0.05 from control.
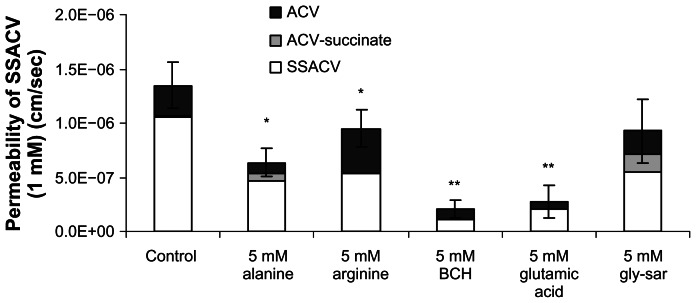
Competitive inhibition of SSACV transport
Inhibition of SSACV transport was studied in the presence of several inhibitors to further characterize the transcorneal flux mechanism (Fig. 7). SSACV permeation was significantly (P < 0.05) inhibited to a varying degree in the presence of various amino acids with the maximum inhibition observed in presence of BCH, a substrate specific for LAT or B0,+.4,24 Transcorneal permeability of SSACV was also attenuated in the presence of alanine and glutamic acid (Fig. 7).
Figure 7.
Permeability values of 1 mM SSACV in the presence of 5 mM alanine, arginine, BCH, glutamic acid, and gly-sar across isolated rabbit cornea. Values are mean ± S.D. (n = 3 to 6).
Notes: *Represents P < 0.05, **Represents P < 0.01 from control.
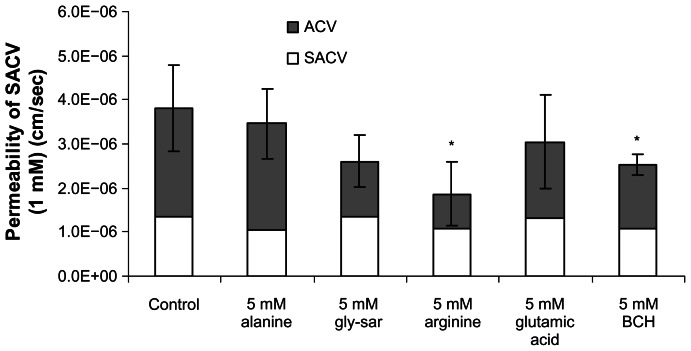
Transcorneal flux of SACV in the presence of inhibitors
Translocation of SACV across cornea was inhibited in the presence of 5 mM arginine (~51%), a specific substrate for cationic transport system and in presence of BCH (~38%), a substrate specific for LAT or B0,+. On otherwise alanine, gly-sar, and glutamic acid caused no measurable inhibition in the transport of SACV across cornea (Fig. 8).
Figure 8.
Permeability values of 1 mM SACV in the presence of 5 mM alanine, gly-sar, arginine, glutamic acid, and BCH across isolated rabbit cornea. Values are mean ± S.D. (n = 3 to 6).
Note: *Represents P < 0.05 from control.
Transcorneal flux of CACV in the presence of 5 mM gly-sar and alanine
Translocation of CACV across cornea was not affected by the presence of alanine and gly-sar in comparison to control. Alanine, a model ASCT transporter substrate and gly-sar, a model peptide transporter substrate produced no significant inhibition (Fig. 9).
Figure 9.

Permeability values of 1 mM CACV across rabbit cornea in the presence of 5 mM alanine and gly-sar. Values are mean ± S.D. (n = 3 to 6).
Log P values of ACV, AACV, SSACV, SACV and CACV
Log P values were calculated using VCC-Lab Program—which provides interactive on-line prediction of logP and aqueous solubility of compounds. This program also compares logP calculated with several different procedures. The Clog P values obtained were −2.42, −2.15, −3.89, −3.32, and −2.28 for ACV, AACV, SSACV, SACV and CACV respectively.
Discussion
Targeted prodrug design represents a new strategy for site directed and efficient drug delivery. Molecular targeting of drugs to transporters and receptors is emerging as a novel and clinically significant approach. In an earlier study, we have identified Na+ dependent neutral amino acid transporter, ASCT1 both on rPCEC cells and intact cornea.17 In this study, amino acid prodrugs of acyclovir were designed to target this neutral amino acid transporter and [3H] alanine was used as a model substrate. Carboxyl ester amino acid prodrugs of acyclovir, AACV, SACV and CACV did not inhibit the uptake of [3H] alanine, suggesting that the prodrugs did not interact with the alanine carrying system on the rPCEC cells (Table 4). On the other hand, carboxyl free amino acid prodrug of acyclovir, SSACV inhibited the uptake of [3H] alanine, indicating that free carboxyl group of amino acid is essential for substrate recognition by alanine carrying system. Even though SSACV inhibited the uptake of [3H] alanine in a competitive manner, SSACV exhibited low affinity towards the L-alanine carrying system relative to L-alanine as indicated by the Km values (Fig. 2A).
In hydrolysis studies with ocular tissues, it was evident that all the prodrugs hydrolyzed to the parent drug, ACV (Table 2). Therefore, the amino acid prodrugs of ACV are recognized by the ocular esterases and readily cleaved to the parent drug. The corneal half-lives of SSACV, SACV and CACV were 3.52 ± 0.36, 9.22 ± 0.58, and 1.8 ± 0.1 hrs respectively.
Although, the transcorneal flux of SACV was not inhibited in presence of 5 mM alanine, SACV exhibited highest permeability compared to all other prodrugs (Fig. 3). Transcorneal fluxes of CACV and AACV were also not affected in presence of 5 mM alanine, indicating that these prodrugs did not interact with the alanine carrying system present on the corneal epithelium (Table 4). Transcorneal flux of AACV was comparable to that of ACV however SSACV and CACV demonstrated a significantly lower permeability in comparison to ACV (Fig. 3). Further studies were conducted only with SSACV and SACV to delineate their interactions with other transporters.
To delineate the interaction of SSACV with neutral amino acid transporter (ASCT) on the cornea, transcorneal flux of SSACV was carried out by performing pH, and Na+ dependent transport experiments across isolated rabbit cornea. Transport of SSACV was found to be pH dependent (Fig. 7). Surprisingly, in the absence of Na+, transport of SSACV was found to be significantly (P < 0.05) enhanced (298% of control) (Fig. 6). These results suggest that SSACV interaction with the neutral amino acid transporter (ASCT1) was minimal as ASCT1 mediated transport process is relatively pH independent. Alteration of SSACV transport in Na+ free buffer may be due to the involvement of other inorganic monovalent cations like K+ and requires further studies to clarify this hypothesis.
Further functional characterization of SSACV and SACV was carried out by studying their transcorneal flux in the presence of BCH (an inhibitor of the transport system specific for LAT or B0,+),4,24 cationic (arginine), neutral (alanine), and anionic (glutamic acid) amino acids. Transcorneal flux of SSACV was significantly (P < 0.05) lower in the presence of BCH (81.2%), indicating the involvement of either LAT or B0,+ (Fig. 7). Transport of SSACV was also inhibited significantly (P < 0.05) in presence of neutral amino acid, alanine (44.8%); anionic amino acid, glutamic acid (76%); but not in presence of cationic amino acid, arginine (30%). SSACV inhibition with alanine further confirms its recognition by L-alanine carrying system but such inhibition effect is relatively minimal compared to inhibition with glutamic acid. The transport of ACV- succinate and succinate was also performed in the presence of 5 mM glutamic acid to delineate involvement of these intermediates. Glutamic acid did not cause any significant change in the transport of these intermediates (data not shown) so the marked inhibition of transcorneal transport of SSACV in the presence of glutamate was not due to succinate.
Inhibition patterns of the SACV were completely distinct from SSACV. Transcorneal flux of SACV was significantly (P < 0.05) inhibited in the presence of arginine (~51%), indicating the involvement of cationic amino acid transport system (Fig. 8). Interestingly, transcorneal flux of SACV was also inhibited in presence of BCH, a substrate specific for LAT or B0,+. Movement of SACV across cornea was not altered in the presence of alanine, gly-sar and an anionic amino acid, glutamic acid (Fig. 8). Control experiments measuring the transport of ACV were also performed in the presence of alanine, arginine and BCH (Table 5). This result confirms that inhibition pattern of these prodrugs was not due to parent drug, ACV.
Table 5.
Permeability values of 1 mM ACV in the presence of 5 mM alanine, arginine, and BCH across isolated rabbit cornea.
| Permeability of ACV (1 mM) (10−6 × cm/sec) | |
|---|---|
| Control | 2.33 ± 0.73 |
| 5 mM alanine | 2.06 ± 0.69 |
| 5 mM arginine | 2.7 ± 0.17 |
| 5 mM BCH | 2.03 ± 0.42 |
Note: Values are mean ± S.D. (n = 3 to 6).
Represents P < 0.05 from control.
Antiviral efficacy of these prodrugs was tested against HSV-1 and 2, and VZV (Table 3). The compounds were found to be highly effective against HSV-1 and VZV, as would be expected from a prodrug of the parent drug ACV, which also shows excellent activity against HSV and VZV. It should be noted that the activity of the prodrug is due to its regeneration to the parent drug, ACV, as the prodrugs are inactive themselves. The prodrug SACV appears to be a promising prodrug candidate due to its appreciable antiviral activity, long half-life and high permeability across the corneal membrane.
In conclusion, results of the current study indicate interaction of SSACV with the alanine carrying system on the rPCEC and rabbit cornea. Functional and molecular evidence of the presence of LAT1, B0,+, ASCT1 on the rabbit corneal epithelium were recently reported from our laboratory.15–17 Expression of amino acid transport systems on the rPCEC seems highly variable compared to corneal tissue and utilization of rPCEC as a cell culture model for amino acid transporters needs careful analysis. Differential transport capability of various transporters especially B0,+ compared to ASCT may also be playing an important role in the increased permeability of SACV. Even though various factors play a role in determining the permeability of the prodrugs, it appears that SACV offers significant improvement in the transport over ACV. Moreover all these prodrugs might still be useful due to their high aqueous solubility (>40 mg/ml) as compared to 2.5 mg/ml for ACV thus enabling formulation of 1% aqueous eye drops.10
Acknowledgments
The authors would like to acknowledge Dr. Earl R. Kern from Department of Pediatrics, University of Alabama at Birmingham, Birmingham, AL 35233, USA for conducting in vitro antiviral studies. This work was supported by NIH grants RO1 EY09171-12 and RO1 EY10659-10.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Christensen HN. Role of amino acid transport and counter transport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Arriza JL, Kavanaugh MP, Fairman WA, et al. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–32. [PubMed] [Google Scholar]
- 3.Zerangue N, Kavanaugh MP. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J Biol Chem. 1996;271:27991–4. doi: 10.1074/jbc.271.45.27991. [DOI] [PubMed] [Google Scholar]
- 4.Deves R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg GJ, Lam HY, Begleiter A, et al. Active carrier-mediated transport of melphalan by two separate amino acid transport systems in LPC-1 plasmacytoma cells in vitro. J Biol Chem. 1979;254:1057–64. [PubMed] [Google Scholar]
- 6.Blondeau JP, Beslin A, Chantoux F, Francon J, et al. Triiodothyronine is a high-affinity inhibitor of amino acid transport system L1 in cultured astrocytes. J Neurochem. 1993;60:1407–13. doi: 10.1111/j.1471-4159.1993.tb03302.x. [DOI] [PubMed] [Google Scholar]
- 7.Lakshmanan M, Goncalves E, Lessly G, Foti D, Robbins J, et al. The transport of thyroxine into mouse neuroblastoma cells, NB41 A3: the effect of L-system amino acids. Endocrinology. 1990;126:3245–50. doi: 10.1210/endo-126-6-3245. [DOI] [PubMed] [Google Scholar]
- 8.Su TZ, Lunney E, Campbell G, Oxender DL, et al. Transport of gabapentin, a gamma- amino acid drug, by system l alpha-amino acid transporters: a comparative study in astrocytes, synaptosomes, and CHO cells. J Neurochem. 1995;64:2125–31. doi: 10.1046/j.1471-4159.1995.64052125.x. [DOI] [PubMed] [Google Scholar]
- 9.Hatanaka T, Haramura M, Fei YJ, et al. Transport of amino acid-based prodrugs by the Na+- and Cl(−) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004;308:1138–47. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- 10.Hughes PM, Krishnamoorthy R, Mitra AK, et al. Effect of acylation on the ocular disposition of acyclovir. I: Synthesis, physicochemical properties, and antiviral activity of 2′-esters. J Ocul Pharmacol. 1993;9:287–97. doi: 10.1089/jop.1993.9.287. [DOI] [PubMed] [Google Scholar]
- 11.Anand B, Nashed Y, Mitra A, et al. Novel dipeptide prodrugs of acyclovir for ocular herpes infections: Bioreversion, antiviral activity and transport across rabbit cornea. Curr Eye Res. 2003;26:151–63. doi: 10.1076/ceyr.26.3.151.14893. [DOI] [PubMed] [Google Scholar]
- 12.Anand BS, Hill JM, Dey S, et al. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, val-val-acyclovir, against HSV-1 epithelial and stromal keratitis in the rabbit eye model. Invest Ophthalmol Vis Sci. 2003;44:2529–34. doi: 10.1167/iovs.02-1251. [DOI] [PubMed] [Google Scholar]
- 13.Dey S, Anand BS, Patel J, Mitra AK, et al. Transporters/receptors in the anterior chamber: pathways to explore ocular drug delivery strategies. Expert Opin Biol Ther. 2003;3:23–44. doi: 10.1517/14712598.3.1.23. [DOI] [PubMed] [Google Scholar]
- 14.Anand BS, Mitra AK. Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm Res. 2002;19:1194–202. doi: 10.1023/a:1019806411610. [DOI] [PubMed] [Google Scholar]
- 15.Jain-Vakkalagadda B, Dey S, Pal D, Mitra AK, et al. Identification and functional characterization of a Na+-independent large neutral amino acid transporter, LAT1, in human and rabbit cornea. Invest Ophthalmol Vis Sci. 2003;44:2919–27. doi: 10.1167/iovs.02-0907. [DOI] [PubMed] [Google Scholar]
- 16.Jain-Vakkalagadda B, Pal D, Gunda S, Nashed Y, Ganapathy V, Mitra AK, et al. Identification of a Na+-dependent cationic and neutral amino acid transporter, B(0,+), in human and rabbit cornea. Mol Pharm. 2004;1:338–46. doi: 10.1021/mp0499499. [DOI] [PubMed] [Google Scholar]
- 17.Katragadda S, Talluri RS, Pal D, Mitra AK, et al. Identification and characterization of a Na+-dependent neutral amino acid transporter, ASCT1, in rabbit corneal epithelial cell culture and rabbit cornea. Curr Eye Res. 2005;30:989–1002. doi: 10.1080/02713680500306439. [DOI] [PubMed] [Google Scholar]
- 18.Majumdar S, Gunda S, Mitra A, et al. Functional expression of a sodium dependent nucleoside transporter on rabbit cornea: Role in corneal permeation of acyclovir and idoxuridine. Curr Eye Res. 2003;26:175–83. doi: 10.1076/ceyr.26.3.175.14895. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar S, Tirucherai GS, Pal D, Mitra AK, et al. Functional differences in nucleoside and nucleobase transporters expressed on the rabbit corneal epithelial cell line (SIRC) and isolated rabbit cornea. AAPS PharmSci. 2003;5:E15. doi: 10.1208/ps050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dias CS, Anand BS, Mitra AK, et al. Effect of mono- and di-acylation on the ocular disposition of ganciclovir: physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J Pharm Sci. 2002;91:660–8. doi: 10.1002/jps.10072. [DOI] [PubMed] [Google Scholar]
- 21.Patel K, Trivedi S, Luo S, et al. Synthesis, physicochemical properties and antiviral activities of ester prodrugs of ganciclovir. Int J Pharm. 2005;305:75–89. doi: 10.1016/j.ijpharm.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, et al. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–18. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 23.Tak RV, Pal D, Gao H, Dey S, Mitra AK, et al. Transport of acyclovir ester prodrugs through rabbit cornea and SIRC-rabbit corneal epithelial cell line. J Pharm Sci. 2001;90:1505–15. doi: 10.1002/jps.1101. [DOI] [PubMed] [Google Scholar]
- 24.Sloan JL, Mager S. Cloning and functional expression of a human Na(+) and Cl(−)-dependent neutral and cationic amino acid transporter B(0,+) J Biol Chem. 1999;274:23740–45. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]