Abstract
Facioscapulohumeral muscular dystrophy (FSHD) is linked to the deletion of the D4Z4 arrays at chromosome 4q35. Recent studies suggested that aberrant expression of double homeobox 4 (DUX4) from the last D4Z4 repeat causes FSHD. The aim of this study is to determine transcriptomic responses to ectopically expressed DUX4 in human and mouse cells of muscle lineage. We expression profiled human rhabdomyosarcoma (RD) cells and mouse C2C12 cells transfected with expression vectors of DUX4 using the Affymetrix Human Genome U133 Plus 2.0 Arrays and Mouse Genome 430 2.0 Arrays, respectively. A total of 2267 and 150 transcripts were identified to be differentially expressed in the RD and C2C12 cells, respectively. Amongst the transcripts differentially expressed in the RD cells, MYOD and MYOG (2 fold, p<0.05), and six MYOD downstream targets were up-regulated in RD but not C2C12 cells. Furthermore, 13 transcripts involved in germline function were dramatically induced only in the RD cells expressing DUX4. The top 3 IPA canonical pathways affected by DUX4 were different between the RD (inflammation, BMP signaling and NRF-2 mediated oxidative stress) and the C2C12 cells (p53 signaling, cell cycle regulation and cellular energy metabolism). Amongst the 40 transcripts shared by the RD and C2C12 cells, UTS2 was significantly induced by 76 fold and 224 fold in the RD and C2C12 cells, respectively. The differential expression of MYOD, MYOG and UTS2 were validated using real-time quantitative RT-PCR. We further validated the differentially expressed genes in immortalized FSHD myoblasts and showed up-regulation of MYOD, MYOG, ZSCAN4 and UTS2. The results suggest that DUX4 regulates overlapped and distinct groups of genes and pathways in human and mouse cells as evident by the selective up-regulation of genes involved in myogenesis and gametogenesis in human RD and immortalized cells as well as the different molecular pathways identified in the cells.
Introduction
FSHD is an autosomal dominant disorder and the third most common inherited form of muscular dystrophy. The disease is characterized by a progressive and selective weakness and atrophy of the facial, scapular, and humeral muscles followed by weakness of muscles of the lower extremities. The weakness of muscles is often asymmetric. There are currently no pharmacologic therapies available to treat this disease [1]–[4]. FSHD1 (OMIM #158900) affects 95% of patients and is genetically linked to contractions of the D4Z4 repeat array at chromosome 4q35 from 11–150 repeat units in healthy individuals to 1–10 repeat units in patients with FSHD. Individuals without any repeat do not develop FSHD [1]–[4]. Each of the repeat units contains a conserved ORF for the double homeobox 4 (DUX4) gene, which is aberrantly transcribed from the last repeat in patients. FSHD2 (OMIM #158901) is not linked to contractions of the D4Z4 repeat array but to mutations in the SMCHD1 protein involved in chromatin structure [5]. DNA hypomethylation of the D4Z4 region is common to both FSHD1 and 2 and causes transcriptional de-repression, which allows the DUX4 gene to be transcribed. The FSHD permissive alleles further present a poly-adenylation signal in the pLAM region distal to the repeat array [6], which allows stabilization of the DUX4 transcripts derived from the last D4Z4 unit and their translation [6]–[14].
The DUX4 protein is a homeodomain transcription factor [6], [9]. The function of DUX4 has been primarily studied using mice [15], [16]. Previous studies showed that ectopic expression of human DUX4 in C2C12 cells induced genes involved in oxidative stress as well as suppressed MYOD pathways [15]. In addition, ectopic DUX4 expression induced p53-dependant muscle cell death both in vitro and in vivo [15], [16]. While some of the findings in these mouse studies agree with what has been reported in studies of human muscle biopsies and myoblasts, including the involvement of oxidative stress responses and cell apoptosis, others such as suppression of MYOD signaling did not agree with findings using patient samples [17]–[26]. Considering the differences between the mouse and human studies, and the fact that there is no orthologue of DUX4 in the mouse genome although two paralogues were reported [8], it is critical to know whether the transcription regulatory targets of human DUX4 are the same in mouse cells. The knowledge will allow us to determine whether DUX4-regulated pathways can be properly studied in mouse models.
In this study, we compared transcriptomic changes that are induced in response to ectopic DUX4 expression in human and mouse cell lines of muscle lineage. Expression profiling studies of human RD cells and mouse C2C12 cells transfected with DUX4 expression vectors were conducted. The C2C12 cell line is a mouse myoblast cell line that derived from skeletal muscles of C3H mice [27] and has since been commonly used to study cellular and molecular pathways in muscle [6], [15]. The human RD cell line is a rhabdomyosarcoma cell line that was derived from a human embryonal rhabdomyosarcoma [28]. This cell line expresses myogenic markers and has been used extensively for studying regulatory pathways in muscles [29], [30]. In this study the mRNA expression changes of the RD and C2C12 cells in response to ectopic DUX4 expression was studied and compared. Considering the RD cells are of neoplastic origin, we also validated our results using immortalized human myoblasts from patients with FSHD.
Methods
Cell Culture and Transfection
The cell culture and transfection experiments of both the RD from American Type Culture Collection (ATCC) and C2C12 cells (ATCC) were conducted in parallel under the same conditions. A total of 1×105 cells were seeded and cultured to 60% confluence in Dulbecco's modified Eagle's medium containing 10% heat inactivated fetal bovine serum (Sigma-Aldrich) and 1% penicillin-streptomycin in 25 cm2 flasks at 37°C, 5% CO2. The cells were transfected with 6.25 µg pCIneo-DUX4 [9] expression vector (n = 4) using Lipofectamine LTX (Life Technologies) according to the manufacturer's protocol, and cells collected 16 hours afterwards. Cells transfected with pCIneo insertless vector were used as controls. Transfection efficiency was determined using cells transfected with GFP expression vector. Percentages of GFP positive cells of 5 random fields were calculated and averaged. The transfection efficiency in C2C12 and RD cells were 91% (±3%) and 89% (±1%), respectively.
Immortalized human myoblasts were obtained from the Senator Paul Wellstone Muscular Dystrophy Cooperative Research Center at Boston Biomedical Research Institute. The patient myoblast cell line was derived from the biceps of a 42 years old male with mild muscle weakness (WS157) [31]. The control myoblasts were derived from the patient's 46 year old brother without FSHD (WS161) [31]. These cells were immortalized with expression vectors encoding hTERT that compensates for telomere loss and CDK4 that prevents growth arrest of CD56+ myogenic cells, when these cells are cultured in-vitro. We cultured these cells as described in previously published protocol [31], [32]. Briefly, proliferating immortalized myoblasts were cultured in a growth medium consisting of medium 199 and DMEM (Life Technologies) in a 1∶4 ratio with 0.8 mM sodium pyruvate (Life Technologies), 3.4 g/l sodium bicarbonate (Sigma-Aldrich), 15 % fetal bovine serum (Thermo Scientific), 0.03 µg/ml Zinc sulfate (Fisher), 1.4 µg/ml vitamin B12 (Sigma-Aldrich), 2.5 ng/ml recombinant human hepatocyte growth factor (Millipore), 10 ng/ml basic fibroblast growth factor (Biopioneer), 0.02 M HEPES, and (Life Technologies) at 37°C, 5% CO2. The culture dish was coated with 0.1 % gelatin (Sigma-Aldrich).
Expression Profiling and Data Analyses
Affymetrix Human Genome U133 Plus 2.0 and Mouse 430 Plus 2.0 arrays were used for profiling the RD and C2C12 cells, respectively. The procedures were conducted as previously described [6]. Briefly, total RNA was isolated from human RD and mouse C2C12 cells using TRIzol (Invitrogen) according to the manufacturer's protocol and purified using the RNeasy MinElute Cleanup Kit (Qiagen) according to the manufacturer's protocol. Four hundred nanograms of total RNA was converted into double stranded cDNA, then biotin labeled cRNA, which was subsequently fragmented. All of the steps were performed using the Affymetrix 3′-IVT Express Kit. The fragmented cRNA was hybridized to microarrays for 16 hours at 45°C. Following hybridization, the washing and staining steps were performed using Fluidics Station 450 as described in the Affymetrix protocol. The probe arrays were subsequently scanned using the Genechip Scanner 3000 to acquire images providing the raw data of gene expression. The microarray data generated is deposited to the Gene Expression Omnibus (GEO) database (accession number GSE45854).
The raw data were imported into GeneSpring GX 11.0 software (Silicon Genetics, CA, USA) for filtering and statistical analysis. The probe sets showing at least one Affymetrix ‘present’ calls out of a total of eight human or mouse arrays (∼10% Present calls), respectively, were selected for further statistical analysis. Welch's t test was performed to calculate the probabilities of significant gene expression changes (p<0.05) along with multiple testing correction using Benjamini Hochberg False Discovery Rate (5%).
The gene lists generated in Genespring were imported into Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Redwood, CA), which is a web based bioinformatics tool used to identify canonical pathways differentially regulated in microarray datasets. The significance of genes from the microarray data assigned to pathways by IPA is determined by the ratio of the number of genes in the dataset mapping to a specific pathway to the total number of genes in the IPA database mapping to that pathway. Fischer's exact test was used to calculate a p-value that determines whether the association between the gene and the pathway is significant. The pathways are subsequently ranked according to the p-value.
Real-time Quantitative Reverse Transcription Polymerase Chain Reaction (real-time qRT-PCR)
Real-time qRT-PCR was performed to validate microarray results as previously described [6], [33]. Briefly, total RNA (1 µg) from each sample was first subjected to DNAse I digestion (1 U) in 1× DNAse I reaction buffer (Promega) by incubating at 37°C for 30 minutes to remove genomic DNA contamination. The reaction was inactivated by adding 1 µl of stop solution (Promega) and heating for 10 minutes at 65°C. Subsequently, the RNA sample was reverse transcribed to cDNA using Superscript II (Life Technologies) and oligo dT primers. The cDNA thus generated was amplified in triplicates in SYBR Green PCR Master Mix (Life Technologies) using 1 µM of forward and reverse primers specific to each gene and 1 µl of cDNA template in a total volume of 50 µl. The thermal cycling conditions included 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of amplification using the condition of 95°C for 15 s then 60°C for 1 min. Primer sequences used for human myogenic differentiation 1 (MYOD) were (forward) 5′ -TGCTCCGACGGCATGATGGAC -3′ and (reverse) 5′-TCGACACCGCCGCACTCT -3′; urotensin 2 (UTS2) were (forward) 5′- AAGTTTCAGGATTTCTCTGGACAAGATCC -3′ and (reverse) 5′- CCAGAAGCAATCAGGAGTCTCACG-3′; myogenin (MYOG) (forward) were 5′-AACCCAGGGGATCATCTGCTCAC-3′ and (reverse) 5′-GTTGGGCATGGTTTCATCTGGGAAG-3′; zinc finger and SCAN domain containing 4 (ZSCAN4) were (forward) 5′-TGGAAATCAAGTGGCAAAAA-3′ and (reverse) 5′-CTGCATGTGGACGTGGAC-3′ [24]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control and the primers used were (forward) 5′- TGTCAAGCTCATTTCCTGGTA-3′ and (reverse) 5′- GTGAGGGTCTCTCTCTTCCTCTTGT-3′. Primer sequences used for mouse urotensin 2 (Uts2) were (forward) 5′-GAGGAAGGCTTTCGCTGGGCA-3′ and 5′-GGGCAGCCCCGTGTTGCTTA-3′. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as internal control and the primers used were (forward) 5′-CCAGGAGCGAGACCCCACTAACA-3′ and (reverse) 5′-TCAAGTGGGCCCCGGCCTT-3′. All primers were tested for nonspecific amplicons and primer dimers by visualizing PCR products on 1% agarose gels as well as melting curve analysis. The ΔΔCT method was used to determine expression values relative to GAPDH as well as fold differences relative to insertless vector. T-test was used (P<0.05) to determine statistical significance.
Results
DUX4 regulates distinct groups of transcripts in the RD cells
To determine the molecular responses to ectopic expression of DUX4 in human RD and mouse C2C12 cells, we expression profiled human RD and mouse C2C12 cells transfected with DUX4 expression vector. The cells were cultured and transfected in parallel and same statistical criteria were applied when the array data were analyzed. A total of 2267 transcripts were differentially expressed in RD cells (Table S1), while 150 differentially expressed transcripts were identified in C2C12 cells (Table S2). A total of 40 differentially expressed transcripts were shared between the two cell lines (Table S3). Among the shared genes, the direction of expression changes of the majority of the genes were the same suggesting these responses were truly shared between the RD and C2C12 cells. Molecular pathways affected by DUX4 in human RD cells and mouse C2C12 cells were further examined using Ingenuity Pathway Analysis (IPA). The results showed that top 3 canonical pathways affected by DUX4 expression in RD cells were those involved in Wnt-mediated immune responses, BMP signaling and NRF-2 mediated oxidative stress response, whereas in C2C12 cells were those involved in p53 signaling, cell cycle regulation, and cellular energy metabolism. The results showed that the most significantly affected molecular pathways by DUX4 are distinct in the RD cells and C2C12 cells while some expression changes were shared.
The Wnt-mediated inflammatory immune response pathway was found to be the top ranked pathway affected by DUX4 in the RD cells as evidenced by significant up-regulation of WNT5A (1.4 fold, p<0.05), and several frizzled family receptors, namely FZD1 (1.6 fold, p<0.01), FZD2 (1.5 fold, p<0.05), FZD4 (1.5 fold, p<0.01), and FZD7c (1.6 fold, p<0.01) (Table S4). However, the transcripts of interleukins IL6 (−1.8 fold, p<0.05), IL8 (−2.2 fold, p<0.01), and IL15 (−1.4 fold, p<0.05), which are downstream of WNT5A were down-regulated.
The BMP signaling pathway was found to be the second ranked pathway. Of the 39 transcripts that belong to this pathway in the IPA database, 17 transcripts were misregulated in response to ectopic DUX4 expression in the RD cells. Amongst the misregulated transcripts, 53% of the expression changes were shown to be involved in suppression of the BMP signaling pathway while 35% of the changes indicated activation of the pathway (Table S5).
The NRF-2 mediated oxidative stress response pathway was identified to be the third ranked pathway regulated by DUX4 in the RD cells. Thirty one of the 86 transcripts known to function in this pathway were found to be misregulated in this study. Among the 31 differentially expressed transcripts, 55% of the changes may potentially induce or contribute to oxidative stress, while 39% of them were reported to be involved in anti-oxidative stress responses (Table S6).
DUX4 significantly up-regulated genes involved in myogenesis and gametogenesis in the RD cells but not C2C12 cells
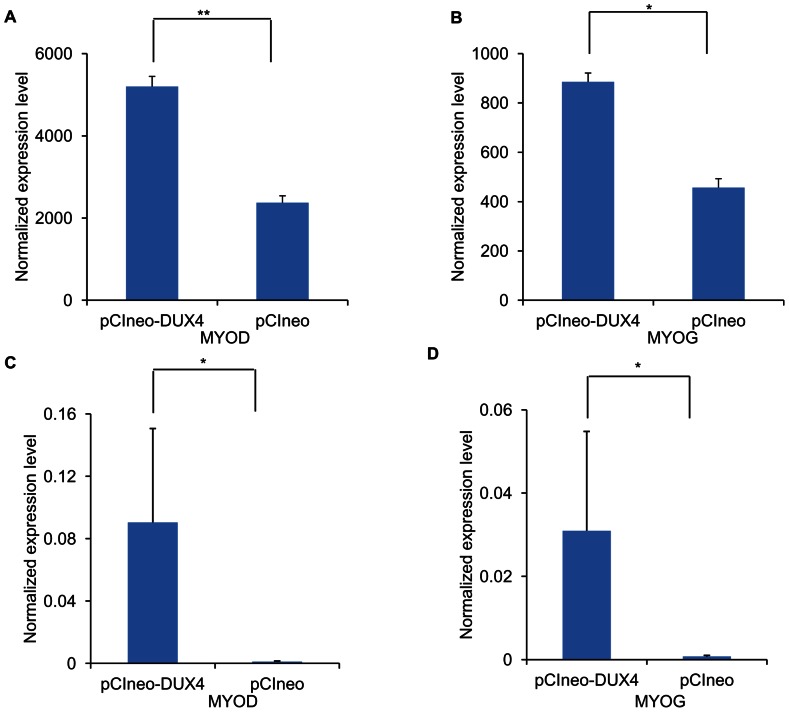
Since myogenesis factors and genes regulating cell cycle have previously been reported to be affected in primary FSHD myoblasts [19], [21], [26], we first looked up the expression changes of two major myogenic factors, MYOD and MYOG, in the profiling data. The results showed that MYOD (Figure 1A) and MYOG (Figure 1B) were 2 fold up-regulated in the RD cells (p<0.05) but not in the C2C12 cells ectopically expressing DUX4. Six transcripts reported as direct targets of MYOD, namely BIN1 (1.4 fold, p<0.05), HMGB3 (1.3 fold, p<0.05), SIX1 (1.5 fold, p<0.05), ACTC1 (1.3 fold, p<0.05), IGFBP5 (1.5 fold, p<0.05), and CHRNA1 (2.1 fold, p<0.05) [34], were also up-regulated only in the RD cells transfected with the DUX4 expression vector. In addition, several transcripts involved in cell cycle progression were shown down-regulated in the RD cells, including CCND1 (−1.3 fold, p<0.05), CCND2 (−1.3 fold, p<0.05), CDC6 (−1.3 fold, p<0.01) and E2F7 (−1.5-fold, p<0.05) but not in the C2C12 cells. To validate the significant up-regulation of MYOD and MYOG in the RD cells, we performed real-time qRT-PCR and confirmed that both MYOD (20 fold, p<0.05) and MYOG (12 fold, p<0.05) were up-regulated in the RD cells ectopically expressing DUX4 (Figure 1C–D).
Figure 1. Up-regulation of MYOD and MYOG in response to ectopic DUX4 expression in RD cells.
Expression levels of myogenic markers MYOD (A) and MYOG (B) were determined by expression profiling human RD cells transfected with an expression vector either encoding DUX4 or insertless (control), respectively. The differential expression of MYOD (C) and MYOG (D) were validated using real-time qRT-PCR (n = 4). Normalized expression levels of the transcripts were calculated using GAPDH as a reference. ** p<0.01, * p <0.05.
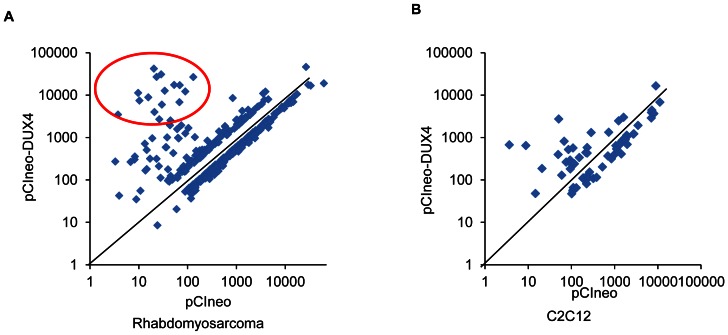
A small number of genes showed dramatic up-regulation (>100-fold) only in the RD cells ectopically expressing DUX4, which formed a cluster of transcripts (Figure 2A–B). These transcripts were MBD3L2 (2805 fold, p<0.01), TRIM43 (2060 fold, p<0.01), ZSCAN4 (1546 fold, p<0.01), RFPL1/RFPL2 (1231 fold, p<0.01), PRAMEF1/PRAMEF13/PRAMEF2 (1217 fold, p<0.01), PRAMEF12 (711 fold, p<0.01), TRIM48 (421 fold, p<0.01), TRIM49 (409 fold, p<0.05), RFPL2 (332 fold, p<0.01), KHDC1L (254 fold, p<0.01), RFPL3 (204 fold, p<0.01), SPRYD5 (171 fold, p<0.01), and PRAMEF11 (126 fold, p<0.01). Out of these, only Zscan4 was up-regulated (7.4 fold, p<0.01) in C2C12 cells ectopically expressing DUX4. While not expressing in normal skeletal muscle, these transcripts are expressed in germ cells, embryos during preimplantation and early embryogenesis. The genes were also reported to be up-regulated in immortalized human myoblasts ectopically expressing DUX4 [20].
Figure 2. Scatter plot analysis of transcripts regulated by ectopically expressed DUX4 in RD and C2C12 cells.
To clearly visualize transcripts highly induced by DUX4, only transcripts changed >2-fold were used for analysis. Log transformed expression levels of transcripts in cells transfected with the insertless vector was plotted against expression levels of transcripts in cells transfected with the DUX4 expression vector. A cluster of transcripts highly induced by DUX4 (circled) in RD (A.) but not C2C12 cells (B.) was observed.
DUX4 induced up-regulation of UTS2 in both the RD and C2C12 cells ectopically expressing DUX4
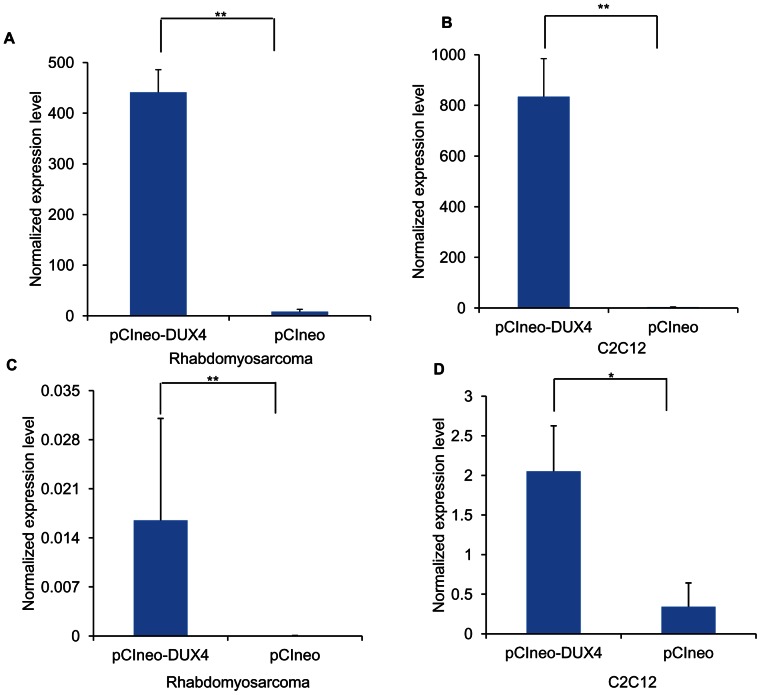
While the majority of the genes induced by DUX4 expression in RD and C2C12 cells were cell-specific, 40 transcripts were similarly regulated by DUX4 in both cell lines suggesting there are shared transcriptional targets in the cells (Table S3) and urotensin-2 (UTS2) was one of them. UTS2 was significantly up-regulated in the RD cells (76 fold, p<0.01) and C2C12 cells (224 fold, p<0.01) ectopically expressing DUX4 (Figures 3A–B). The finding were validated by real-time qRT-PCR in both the RD and C2C12 cells with fold changes of 130 fold (p<0.01) and 21 fold (p<0.05) respectively (Figure 3C–D).
Figure 3. Up-regulation of human UTS2 and mouse Uts2 was observed in RD and C2C12 cells ectopically expressing DUX4, respectively.
Expression levels of human UTS2 and mouse Uts2 were determined by expression profiling RD (A) and C2C12 (B) cells transfected with an expression vector either encoding DUX4 or insertless (control), respectively. The expression changes in RD (C) and C2C12 (D) were validated using real-time qRT-PCR (n = 4). Normalized expression levels of the transcripts were calculated using GAPDH and Gapdh as a reference in both cell lines. ** p<0.01, * p <0.05.
Transcripts upregulated by ectopic DUX4 expression were upregulated in immortalized FSHD myoblasts
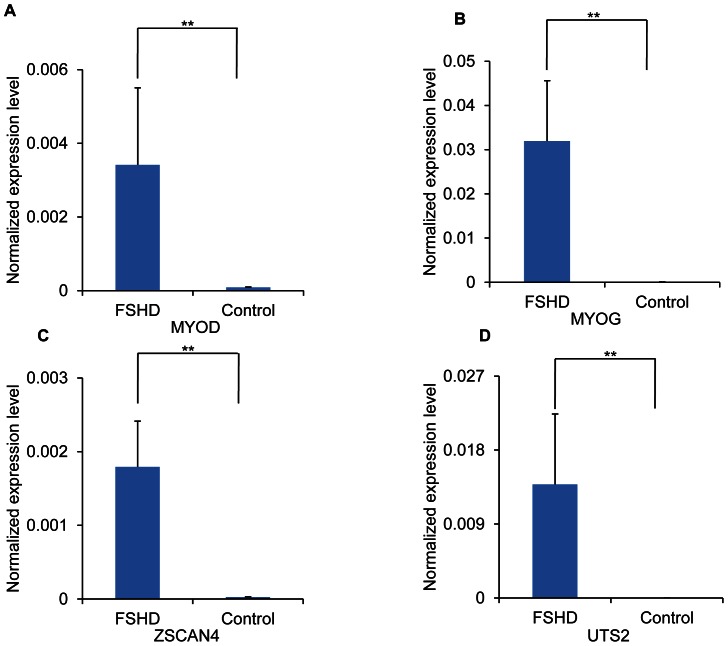
To determine whether the expression changes identified in the RD cells ectopically expressing DUX4 can be detected in the FSHD myoblasts, we performed real-time qRT-PCR and validated the significant up-regulation of MYOD (19 fold, p<0.01), MYOG (110 fold, p<0.01), ZSCAN4 (32 fold, p<0.01), and UTS2 (229 fold, p<0.01) (Figure 4A–D) in immortalized FSHD cells as compared to the control immortalized myoblasts.
Figure 4. Up-regulation of MYOD, MYOG, ZSCAN4, and UTS2 in FSHD immortalized cells.
MYOD (A.), MYOG (B.), ZSCAN4 (C.), and UTS2 (D.) levels were quantified in FSHD immortalized cells and control cells using real-time qRT-PCR (n = 4). Values representing expression levels of transcripts were calculated using GAPDH as a reference. ** p<0.01.
Discussion
Misregulation of genes and pathways involved in myogenesis, cell cycle regulation and oxidative stress in human FSHD myoblasts has been reported previously [13]–[23]. Recently genes involved in gametogenesis were shown to be up-regulated in immortalized FSHD myoblasts and muscle biopsies [20], [35], [36]. While some of the changes were also reported in studies conducted using C2C12 cells and animal models, such as increased susceptibility to oxidative stress and the induction of cell death, other findings did not agree with the human studies including how the myogenesis program was affected by DUX4 and the induction of the germline genes [15], [16], [35], [36]. To further investigate the genes and pathways regulated by DUX4 in human and mouse cells of muscle lineage, we analyzed mRNA transcripts affected by ectopically expressed DUX4 using expression profiling. Our results showed that while the RD cells expressing DUX4 recapitulated the molecular defects seen in human muscles and immortalized myoblasts, the C2C12 cells responded differently to the DUX4 expression. For example, the most dramatic expression changes of the germline genes were induced by DUX4 in both RD and immortalized FSHD myoblasts and observed in patients' muscles but not the C2C12 cells. Among these genes, only ZSCAN4 was mildly up-regulated in the C2C12 cells. In general, DUX4 regulated significantly greater number of genes (2267) in RD cells as compared with C2C12 cells (150) suggesting that DUX4 may have more direct regulatory targets in RD cells as compared with C2C12 cells. In addition, several pathways and genes previously reported to be misregulated in FSHD were shown affected in the RD cells but not in the C2C12 cells. The similarity among the human RD cells, the immortalized FSHD myoblasts and the primary myoblasts suggest that RD and immortalized FHSD myoblasts may be more suitable culture systems for studying DUX4 function than mouse C2C12 cells. Whether mouse or other animal models that carry human DUX4 in their genomes can be used to study DUX4 function or can be a suitable FSHD disease model need to be further investigated.
Previous studies showed up-regulation of MYOD and its downstream regulatory targets accompanied by a halt in cell cycle progression in human myoblasts and biopsies therefore a hypothesis that pre-mature activation of myogenesis program was involved in the pathological mechanisms of FSHD was proposed [19], [21], [26]. MYOD is a transcription factor that activates myogenesis through regulation of several transcriptional targets that facilitates the transformation of quiescent satellite cells (stem cells committed to muscle lineage) into proliferating myoblasts which are capable of undergoing differentiation [37]. While the activated myoblasts are essential for muscle maintenance and repair, maintaining a healthy number of satellite cells is critical for a continuous supply of myoblasts [38]. A pre-mature activation of myogenesis induced by DUX4 bares a risk to prematurely deplete satellite cells, which can potentially lead to diminished regenerative capacity of the adult skeletal muscle. Our profiling and real-time qRT-PCR data showed up-regulation of MYOD and MYOG as well as suppression of cell cycle progression in the RD cells expressing DUX4. The up-regulation of MYOD and MYOG was further validated using immortalized FHSD myoblasts. The findings are in concordance with previous studies in primary FSHD myoblasts and muscle biopsies [19], [21], [26]. Our data provide a direct link between DUX4 expression and the activation of myogenesis program as evidenced by activation of MYOD and its downstream target genes accompanied by a halt in cell cycle progression, a critical step prior to differentiation.
A recent study by Geng et al [20] showed activation of MYOG expression in response to ectopic expression of DUX4 in human immortalized myoblasts, which is consistent with activation of MYOD signaling since terminal differentiation involves increased MYOG and decreased MYOD expression levels, a step necessary for myoblasts to fuse and form mature myofibers [39], [40]. Since our results showed higher expression levels of both MYOD and MYOG by DUX4 overexpression, this indicates these cells are at an earlier stage of differentiation process. This could be explained by the fact that our study was conducted at an earlier time point (16 hours post-transfection) as compared to the study conducted by Geng et al (24 hours post-transfection). While the studies conducted using human muscle biopsies and myoblasts reported activation of MYOD pathways [19], [21], [26], studies conducted in C2C12 cells reported repression of MYOD pathways in response to ectopically expressed DUX4 as well as DUX4c [15], [41] The different conclusions from human and mouse studies can potentially be explained by the lack of DUX4 orthologue in mice; therefore human DUX4 does not regulate Myod and other regulatory target genes the same way.
In addition to activation of MYOD program, we also identified a suppression of BMP signaling pathways in RD cells expressing DUX4. The BMP pathways negatively regulate MYOD program and was identified as the second top ranked pathway in the RD cells ectopically expressing DUX4. BMP signaling has been shown to be activated during the proliferation stage of satellite cells. The BMP signaling is suppressed during differentiation by a BMP antagonist Noggin [42]. Our profiling data suggested a suppression of the BMP signaling with the BMP antagonist Noggin up-regulated by DUX4 (1.6 fold, p<0.05; Table S5). Overall, the data support our conclusions that DUX4 expression leads to induction of myogenesis through activation of MYOD signaling and increased expression of Noggin.
The top ranked pathway identified by IPA to be differentially regulated in RD cells ectopically expressing DUX4 are genes involved in innate immune response as evidenced by up-regulation of WNT5A, and several FZD receptors. Genomic studies have shown WNT5A, agonist of FZD receptors, to be up-regulated in T-cells, macrophages and dendritic cells exposed to pathogens as well as in pathologies involving inflammation such as rheumatoid arthritis. Previous studies suggest that Wnt5A is a positive regulator of immunity and inflammation [43]–[47]. Our data therefore indicate that DUX4 directly induces an inflammatory immune response, which could contribute to the T-cell mediated inflammation in FSHD reported previously [35], [48]. Interestingly, some interleukin genes functioning in lymphocyte activation and infiltration downstream of Wnt5A are downregulated in our RD data suggesting compensatory mechanisms to combat Wnt5A signaling. However, while WNT5A can be involved in regulating immune responses, it is also highly expressed in satellite cells and is involved in switching cells from proliferation to myogenic differentiation [49]. The up-regulation of Wnt signaling in the myoblasts can potentially contribute to increased myogenesis and not related to inflammation.
The top ranked pathway affected by DUX4 expression in the C2C12 cells was the p53 pathway. P53 signaling has been shown to be activated by DUX4 in mice ectopically expressing DUX4 in vivo and other animal models expressing DUX4 [16], [50]. In addition, it is shown to be up-regulated in human FSHD myoblasts during differentiation [24]. This pathway was also highly ranked among the pathways affected in the RD cells expressing DUX4 but not in the top 3. It should be noted that RD cells are of neoplastic origin and contain point mutations in the tumor suppressing p53 gene leading to its functional loss [51]–[53]. This could potentially explain the reasons for the p53 pathway not being amongst the top 3 ranked affected pathway by DUX4 in RD cells. While the activation of the p53 pathway was suggested based on the IPA, we did not observe obvious reduction of total cell numbers when we collected the cells. In addition, the amount of total RNA isolated from the cells was comparable between the cells transfected with the DUX4 and insertless vectors. We selected an earlier time point to collect cells in order to avoid profiling dying or dead cells. The cell death likely will occur at a later time point.
Previous studies showed that FSHD myoblasts and C2C12 cells expressing DUX4 were more vulnerable to oxidative stress [15], [17]–[19], [23]–[25], [54], while another study of FSHD and control myoblasts from relatives did not [31]. Our data showed that ectopic DUX4 expression in RD cells caused the misregulation of genes involved in the NRF2-mediated oxidative stress response pathway, which is involved in combating oxidative stress. Oxidative stress is caused when the rate of production of reactive oxygen species such as free radicals and peroxides, exceeds their rate of detoxification. Increases in levels of reactive oxygen species can be very damaging to the cell and trigger apoptotic responses. The NRF2 mediated oxidative stress response is the primary pathway involved in combating oxidative stress through the action of several detoxifying and anti-oxidant enzymes functioning in the pathway. Misregulation of transcripts involved in the NRF2 mediated oxidative stress response pathway has been reported in several FSHD studies [15], [17]–[19], [23]–[25], [54]. Our study again confirmed the link with DUX4. In addition, our data showed that more changes that are pro-oxidative stress were induced by DUX4. This pathway was also significant in C2C12 cells expressing DUX4 but was not in top 3.
A novel finding of this study is the dramatic induction of UTS2 in response to ectopic DUX4 expression in both the RD and C2C12 cells, which was also validated in the immortalized FHSD myoblasts. UTS2 is a powerful vasoconstrictor and has also been shown to be pro-angiogenic as evidenced by its ability to cause increased proliferation of endothelial cells as well as increased migration of vascular smooth muscle cells [55]–[59]. It has also been recently shown associated with diabetic retinopathy and atherosclerosis [60]; therefore, its induction in response to DUX4 provides a potential explanation for the retinal vasculopathy commonly observed in FSHD patients, who are found to exhibit symptoms similar to those exhibited by patients of Coats' disease, wherein abnormal vessels develop behind retina [61], [62]. Since the molecular mechanism of these retinal defects remains yet unknown, the up-regulation of UTS2 could potentially be involved and worth for further investigation.
Interestingly, rhabdomyosarcoma cells have often been used to study pharmacological properties of UTS2 and its receptors since these cells endogenously express UTS2 receptors. Moreover, UTS2 and its receptors have also been shown expressed at a significantly greater level in human skeletal cells and tissues compared with other organs such as pancreas, brain, liver, testis, placenta, lung, kidney, thymus, prostate, small intestine, colon, peripheral blood leukocytes, ovary and spleen. Our findings along with the findings reported in these studies indicate that overexpression of UTS2 in skeletal muscle could be particularly significant in contributing towards the skeletal muscular symptoms in FSHD patients [63]–[66].
Our study suggests that DUX4 can contribute to FSHD pathogenesis through several avenues including induction of MYOD pathways, induction of immune and inflammatory response, misregulation of genes involved in oxidative stress, and induction of germline genes. Our study also reported a dramatic induction of UTS2, a potent vasoconstrictor involved in angiogenesis and also reported preferentially expressed in skeletal muscle tissue in FSHD myoblasts, which could potentially explain the vasculopathy and skeletal muscular symptoms observed in FSHD patients. Furthermore, we showed that some of these critical changes were not observed in mouse C2C12 myoblasts while other changes overlapped, which suggest that a mouse model carrying human DUX4 gene may not fully recapitulate the human FSHD and needs to be evaluated carefully.
Supporting Information
Transcripts regulated by DUX4 in RD cells. RD cells transfected with DUX4 expression vector and insertless vector (control) were expression profiled and fold-changes of transcripts changed in response to ectopic DUX4 expression were calculated relative to control. Welch's t test was performed to calculate the probabilities of significant gene expression changes (p<0.05) along with multiple testing correction using Benjamini Hochberg False Discovery Rate (5%).
(XLSX)
Transcripts regulated by DUX4 in C2C12 cells. C2C12 cells transfected with DUX4 expression vector and insertless vector (control) were expression profiled and fold-changes of transcripts changed in response to ectopic DUX4 expression were calculated relative to control. Welch's t test was performed to calculate the probabilities of significant gene expression changes (p<0.05) along with multiple testing correction using Benjamini Hochberg False Discovery Rate (5%).
(XLSX)
Common transcripts regulated by DUX4 in RD and C2C12 cells. Genespring GX 11.0 was used to identify transcripts regulated by DUX4 in both RD and C2C12 cells (p<0.05).
(XLSX)
Transcripts functioning in immune response pathways regulated by DUX4 in RD cells. Transcripts identified in the top ranked canonical pathway regulated by DUX4 in RD cells were identified through IPA.
(XLSX)
Transcripts functioning in BMP signaling pathway regulated by DUX4 in RD cells. Transcripts functioning in BMP signaling pathway, the second ranked canonical pathway regulated by DUX4 in RD cells, were identified through IPA.
(XLSX)
Transcripts functioning in NRF2 mediated oxidative stress response pathway regulated by DUX4 in RD cells. Transcripts functioning in NRF2 mediated oxidative stress response pathway, the third ranked canonical pathway regulated by DUX4 in RD cells, were identified through IPA.
(XLSX)
Acknowledgments
We thank Dr. Kathryn Wagner for her critical review of the manuscript. We would like to thank the Wellstone Muscular Dystrophy Center at the Boston Biomedical Research Boston Biomedical Research Institute for providing us the immortalized myoblasts. V. S. is a pre-doctoral student in the Molecular Medicine Program of the Institute for Biomedical Sciences at the George Washington University. This work is from a dissertation to be presented to the above program in partial fulfillment of the requirements for the Ph.D. degree.
Funding Statement
Research reported in this publication was supported by the NIH/NIAMS under Award Number 1R01AR052027. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. V.S and N. H. are supported by NIH/NIAMS1R01AR052027. Y-W.C. is supported by NIH/NIAMS1R01AR052027, NIH/NICHD1R24HD050846 and DOD DOD W81XWH-10-1-0659. A.B. acknowledges the Global FSHD Foundation (Australia). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Emery AE (1991) Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord 1: 19–29. [DOI] [PubMed] [Google Scholar]
- 2. van der Maarel SM, Frants RR (2005) The D4Z4 repeat-mediated pathogenesis of facioscapulohumeral muscular dystrophy. Am J Hum Genet 76: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wijmenga C, Frants RR, Brouwer OF, Moerer P, Weber JL, et al. (1990) Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet 336: 651–653. [DOI] [PubMed] [Google Scholar]
- 4. Wijmenga C, Hewitt JE, Sandkuijl LA, Clark LN, Wright TJ, et al. (1992) Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet 2: 26–30. [DOI] [PubMed] [Google Scholar]
- 5. Lemmers RJ, Tawil R, Petek LM, Balog J, Block GJ, et al. (2012) Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet 44: 1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dixit M, Ansseau E, Tassin A, Winokur S, Shi R, et al. (2007) DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc Natl Acad Sci U S A 104: 18157–18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cabianca DS, Casa V, Bodega B, Xynos A, Ginelli E, et al. (2012) A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell 149: 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clapp J, Mitchell LM, Bolland DJ, Fantes J, Corcoran AE, et al. (2007) Evolutionary conservation of a coding function for D4Z4, the tandem DNA repeat mutated in facioscapulohumeral muscular dystrophy. Am J Hum Genet 81: 264–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gabriels J, Beckers MC, Ding H, De Vriese A, Plaisance S, et al. (1999) Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene 236: 25–32. [DOI] [PubMed] [Google Scholar]
- 10. Jones TI, Chen JC, Rahimov F, Homma S, Arashiro P, et al. (2012) Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: evidence for disease modifiers and a quantitative model of pathogenesis. Hum Mol Genet 21: 4419–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camano P, et al. (2010) A unifying genetic model for facioscapulohumeral muscular dystrophy. Science 329: 1650–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snider L, Asawachaicharn A, Tyler AE, Geng LN, Petek LM, et al. (2009) RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy. Hum Mol Genet 18: 2414–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snider L, Geng LN, Lemmers RJ, Kyba M, Ware CB, et al. (2010) Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene. PLoS Genet 6: e1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeng W, de Greef JC, Chen YY, Chien R, Kong X, et al. (2009) Specific loss of histone H3 lysine 9 trimethylation and HP1gamma/cohesin binding at D4Z4 repeats is associated with facioscapulohumeral dystrophy (FSHD). PLoS Genet 5: e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bosnakovski D, Xu Z, Gang EJ, Galindo CL, Liu M, et al. (2008) An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies. EMBO J 27: 2766–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wallace LM, Garwick SE, Mei W, Belayew A, Coppee F, et al. (2011) DUX4, a candidate gene for facioscapulohumeral muscular dystrophy, causes p53-dependent myopathy in vivo. Ann Neurol 69: 540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barro M, Carnac G, Flavier S, Mercier J, Vassetzky Y, et al. (2010) Myoblasts from affected and non-affected FSHD muscles exhibit morphological differentiation defects. J Cell Mol Med 14: 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Celegato B, Capitanio D, Pescatori M, Romualdi C, Pacchioni B, et al. (2006) Parallel protein and transcript profiles of FSHD patient muscles correlate to the D4Z4 arrangement and reveal a common impairment of slow to fast fibre differentiation and a general deregulation of MyoD-dependent genes. Proteomics 6: 5303–5321. [DOI] [PubMed] [Google Scholar]
- 19. Cheli S, Francois S, Bodega B, Ferrari F, Tenedini E, et al. (2011) Expression profiling of FSHD-1 and FSHD-2 cells during myogenic differentiation evidences common and distinctive gene dysregulation patterns. PLoS One 6: e20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geng LN, Yao Z, Snider L, Fong AP, Cech JN, et al. (2012) DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy. Dev Cell 22: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krom YD, Dumonceaux J, Mamchaoui K, den Hamer B, Mariot V, et al. (2012) Generation of isogenic D4Z4 contracted and noncontracted immortal muscle cell clones from a mosaic patient: a cellular model for FSHD. Am J Pathol 181: 1387–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandri M, El Meslemani AH, Sandri C, Schjerling P, Vissing K, et al. (2001) Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J Neuropathol Exp Neurol 60: 302–312. [DOI] [PubMed] [Google Scholar]
- 23. Tsumagari K, Chang SC, Lacey M, Baribault C, Chittur SV, et al. (2011) Gene expression during normal and FSHD myogenesis. BMC Med Genomics 4: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vanderplanck C, Ansseau E, Charron S, Stricwant N, Tassin A, et al. (2011) The FSHD atrophic myotube phenotype is caused by DUX4 expression. PLoS One 6: e26820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winokur ST, Barrett K, Martin JH, Forrester JR, Simon M, et al. (2003) Facioscapulohumeral muscular dystrophy (FSHD) myoblasts demonstrate increased susceptibility to oxidative stress. Neuromuscul Disord 13: 322–333. [DOI] [PubMed] [Google Scholar]
- 26. Winokur ST, Chen YW, Masny PS, Martin JH, Ehmsen JT, et al. (2003) Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation. Hum Mol Genet 12: 2895–2907. [DOI] [PubMed] [Google Scholar]
- 27. Yaffe D, Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727. [DOI] [PubMed] [Google Scholar]
- 28. McAllister RM, Melnyk J, Finkelstein JZ, Adams EC Jr, Gardner MB (1969) Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer 24: 520–526. [DOI] [PubMed] [Google Scholar]
- 29. Xu Q, Wu Z (2000) The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem 275: 36750–36757. [DOI] [PubMed] [Google Scholar]
- 30. Yang Z, MacQuarrie KL, Analau E, Tyler AE, Dilworth FJ, et al. (2009) MyoD and E-protein heterodimers switch rhabdomyosarcoma cells from an arrested myoblast phase to a differentiated state. Genes Dev 23: 694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Homma S, Chen JC, Rahimov F, Beermann ML, Hanger K, et al. (2012) A unique library of myogenic cells from facioscapulohumeral muscular dystrophy subjects and unaffected relatives: family, disease and cell function. Eur J Hum Genet 20: 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stadler G, Chen JC, Wagner K, Robin JD, Shay JW, et al. (2011) Establishment of clonal myogenic cell lines from severely affected dystrophic muscles - CDK4 maintains the myogenic population. Skelet Muscle 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen YW, Hubal MJ, Hoffman EP, Thompson PD, Clarkson PM (2003) Molecular responses of human muscle to eccentric exercise. J Appl Physiol 95: 2485–2494. [DOI] [PubMed] [Google Scholar]
- 34. Bergstrom DA, Penn BH, Strand A, Perry RL, Rudnicki MA, et al. (2002) Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol Cell 9: 587–600. [DOI] [PubMed] [Google Scholar]
- 35. Tasca G, Pescatori M, Monforte M, Mirabella M, Iannaccone E, et al. (2012) Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS One 7: e38779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rahimov F, King OD, Leung DG, Bibat GM, Emerson CP Jr, et al. (2012) Transcriptional profiling in facioscapulohumeral muscular dystrophy to identify candidate biomarkers. Proc Natl Acad Sci U S A 109: 16234–16239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, et al. (1988) MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science 242: 405–411. [DOI] [PubMed] [Google Scholar]
- 38. Schuster-Gossler K, Cordes R, Gossler A (2007) Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc Natl Acad Sci U S A 104: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, et al. (1993) Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364: 501–506. [DOI] [PubMed] [Google Scholar]
- 40. Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, et al. (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 41. Bosnakovski D, Lamb S, Simsek T, Xu Z, Belayew A, et al. (2008) DUX4c, an FSHD candidate gene, interferes with myogenic regulators and abolishes myoblast differentiation. Exp Neurol 214: 87–96. [DOI] [PubMed] [Google Scholar]
- 42. Ono Y, Calhabeu F, Morgan JE, Katagiri T, Amthor H, et al. (2011) BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ 18: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, et al. (2003) Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102: 672–681. [DOI] [PubMed] [Google Scholar]
- 44. Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, et al. (2002) Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci U S A 99: 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, et al. (2000) Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A 97: 2791–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghosh MC, Collins GD, Vandanmagsar B, Patel K, Brill M, et al. (2009) Activation of Wnt5A signaling is required for CXC chemokine ligand 12-mediated T-cell migration. Blood 114: 1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rauner M, Stein N, Winzer M, Goettsch C, Zwerina J, et al. (2012) WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J Bone Miner Res 27: 575–585. [DOI] [PubMed] [Google Scholar]
- 48. Frisullo G, Frusciante R, Nociti V, Tasca G, Renna R, et al. (2011) CD8(+) T cells in facioscapulohumeral muscular dystrophy patients with inflammatory features at muscle MRI. J Clin Immunol 31: 155–166. [DOI] [PubMed] [Google Scholar]
- 49. Otto A, Schmidt C, Luke G, Allen S, Valasek P, et al. (2008) Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci 121: 2939–2950. [DOI] [PubMed] [Google Scholar]
- 50. Wuebbles RD, Long SW, Hanel ML, Jones PL (2010) Testing the effects of FSHD candidate gene expression in vertebrate muscle development. Int J Clin Exp Pathol 3: 386–400. [PMC free article] [PubMed] [Google Scholar]
- 51. Felix CA, Kappel CC, Mitsudomi T, Nau MM, Tsokos M, et al. (1992) Frequency and diversity of p53 mutations in childhood rhabdomyosarcoma. Cancer Res 52: 2243–2247. [PubMed] [Google Scholar]
- 52. Taylor AC, Shu L, Danks MK, Poquette CA, Shetty S, et al. (2000) P53 mutation and MDM2 amplification frequency in pediatric rhabdomyosarcoma tumors and cell lines. Med Pediatr Oncol 35: 96–103. [DOI] [PubMed] [Google Scholar]
- 53. Miyachi M, Kakazu N, Yagyu S, Katsumi Y, Tsubai-Shimizu S, et al. (2009) Restoration of p53 pathway by nutlin-3 induces cell cycle arrest and apoptosis in human rhabdomyosarcoma cells. Clin Cancer Res 15: 4077–4084. [DOI] [PubMed] [Google Scholar]
- 54. Turki A, Hayot M, Carnac G, Pillard F, Passerieux E, et al. (2012) Functional muscle impairment in facioscapulohumeral muscular dystrophy is correlated with oxidative stress and mitochondrial dysfunction. Free Radic Biol Med 53: 1068–1079. [DOI] [PubMed] [Google Scholar]
- 55. Gendron G, Simard B, Gobeil F Jr, Sirois P, D'Orleans-Juste P, et al. (2004) Human urotensin-II enhances plasma extravasation in specific vascular districts in Wistar rats. Can J Physiol Pharmacol 82: 16–21. [DOI] [PubMed] [Google Scholar]
- 56. Matsusaka S, Wakabayashi I (2006) Enhancement of vascular smooth muscle cell migration by urotensin II. Naunyn Schmiedebergs Arch Pharmacol 373: 381–386. [DOI] [PubMed] [Google Scholar]
- 57. Shi L, Ding W, Li D, Wang Z, Jiang H, et al. (2006) Proliferation and anti-apoptotic effects of human urotensin II on human endothelial cells. Atherosclerosis 188: 260–264. [DOI] [PubMed] [Google Scholar]
- 58. Spinazzi R, Albertin G, Nico B, Guidolin D, Di Liddo R, et al. (2006) Urotensin-II and its receptor (UT-R) are expressed in rat brain endothelial cells, and urotensin-II via UT-R stimulates angiogenesis in vivo and in vitro. Int J Mol Med 18: 1107–1112. [PubMed] [Google Scholar]
- 59. Xu S, Wen H, Jiang H (2012) Urotensin II promotes the proliferation of endothelial progenitor cells through p38 and p44/42 MAPK activation. Mol Med Report 6: 197–200. [DOI] [PubMed] [Google Scholar]
- 60. Suguro T, Watanabe T, Kodate S, Xu G, Hirano T, et al. (2008) Increased plasma urotensin-II levels are associated with diabetic retinopathy and carotid atherosclerosis in Type 2 diabetes. Clin Sci (Lond) 115: 327–334. [DOI] [PubMed] [Google Scholar]
- 61. Fitzsimons RB, Gurwin EB, Bird AC (1987) Retinal vascular abnormalities in facioscapulohumeral muscular dystrophy. A general association with genetic and therapeutic implications. Brain 110 (Pt 3) 631–648. [DOI] [PubMed] [Google Scholar]
- 62. Padberg GW, Brouwer OF, de Keizer RJ, Dijkman G, Wijmenga C, et al. (1995) On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy. Muscle Nerve 2: S73–80. [PubMed] [Google Scholar]
- 63. Batuwangala MS, Calo G, Guerrini R, Ng LL, McDonald J, et al. (2009) Desensitisation of native and recombinant human urotensin-II receptors. Naunyn Schmiedebergs Arch Pharmacol 380: 451–457. [DOI] [PubMed] [Google Scholar]
- 64. Birker-Robaczewska M, Boukhadra C, Studer R, Mueller C, Binkert C, et al. (2003) The expression of urotensin II receptor (U2R) is up-regulated by interferon-gamma. J Recept Signal Transduct Res 23: 289–305. [DOI] [PubMed] [Google Scholar]
- 65. Douglas SA, Behm DJ, Aiyar NV, Naselsky D, Disa J, et al. (2005) Nonpeptidic urotensin-II receptor antagonists I: in vitro pharmacological characterization of SB-706375. Br J Pharmacol 145: 620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Douglas SA, Naselsky D, Ao Z, Disa J, Herold CL, et al. (2004) Identification and pharmacological characterization of native, functional human urotensin-II receptors in rhabdomyosarcoma cell lines. Br J Pharmacol 142: 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transcripts regulated by DUX4 in RD cells. RD cells transfected with DUX4 expression vector and insertless vector (control) were expression profiled and fold-changes of transcripts changed in response to ectopic DUX4 expression were calculated relative to control. Welch's t test was performed to calculate the probabilities of significant gene expression changes (p<0.05) along with multiple testing correction using Benjamini Hochberg False Discovery Rate (5%).
(XLSX)
Transcripts regulated by DUX4 in C2C12 cells. C2C12 cells transfected with DUX4 expression vector and insertless vector (control) were expression profiled and fold-changes of transcripts changed in response to ectopic DUX4 expression were calculated relative to control. Welch's t test was performed to calculate the probabilities of significant gene expression changes (p<0.05) along with multiple testing correction using Benjamini Hochberg False Discovery Rate (5%).
(XLSX)
Common transcripts regulated by DUX4 in RD and C2C12 cells. Genespring GX 11.0 was used to identify transcripts regulated by DUX4 in both RD and C2C12 cells (p<0.05).
(XLSX)
Transcripts functioning in immune response pathways regulated by DUX4 in RD cells. Transcripts identified in the top ranked canonical pathway regulated by DUX4 in RD cells were identified through IPA.
(XLSX)
Transcripts functioning in BMP signaling pathway regulated by DUX4 in RD cells. Transcripts functioning in BMP signaling pathway, the second ranked canonical pathway regulated by DUX4 in RD cells, were identified through IPA.
(XLSX)
Transcripts functioning in NRF2 mediated oxidative stress response pathway regulated by DUX4 in RD cells. Transcripts functioning in NRF2 mediated oxidative stress response pathway, the third ranked canonical pathway regulated by DUX4 in RD cells, were identified through IPA.
(XLSX)